Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (23): 5992-5999.doi: 10.12307/2026.350
Previous Articles Next Articles
Association between immune cells and cardiovascular disease risk: a genome-wide association study in European populations
Huang Zhe1, Shang Baoling2, 3, Yao Gengzhen2, 3, Pan Guangming2, 3
- 1Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; 2Guangdong Provincial Hospital of Chinese Medicine, Guangzhou 510120, Guangdong Province, China; 3The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510120, Guangdong Province, China
-
Received:2025-06-06Accepted:2025-08-12Online:2026-08-18Published:2025-12-31 -
Contact:Yao Gengzhen, MD, Associated chief physician, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou 510120, Guangdong Province, China; The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510120, Guangdong Province, China Corresponding author: Pan Guangming, MD, PhD, Chief physician, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou 510120, Guangdong Province, China; The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510120, Guangdong Province, China -
About author:Huang Zhe, MS candidate, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China -
Supported by:The Key Research Laboratory Construction Project of National Administration of Traditional Chinese Medicine (Lingnan TCM Academic School Inheritance), No. [2012]27-5 (to SBL [project participant]); Inheriting the Academic Experience of the Seventh Batch of National Veteran TCM Experts of National Administration of Traditional Chinese Medicine, No. [2022]76 (to YGZ); Key Discipline Construction Project for Talent Cultivation of National Administration of Traditional Chinese Medicine, No. 0102023703 (to SBL [project participant]); Project of Traditional Chinese Medicine Bureau of Guangdong Province, No. 20215004 (to SBL [project participant]); Guangzhou Science and Technology Plan Project, No. 2023A03J0230 (to YGZ); Traditional Chinese Medicine Academic School Inheritance Studio Construction Project of Guangdong Provincial Hospital of Chinese Medicine, No. [2013]233 (to SBL [project participant])
CLC Number:
Cite this article
Huang Zhe, Shang Baoling, Yao Gengzhen, Pan Guangming. Association between immune cells and cardiovascular disease risk: a genome-wide association study in European populations[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 5992-5999.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
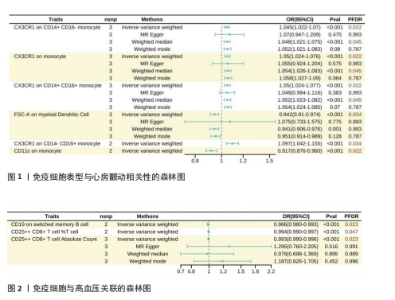
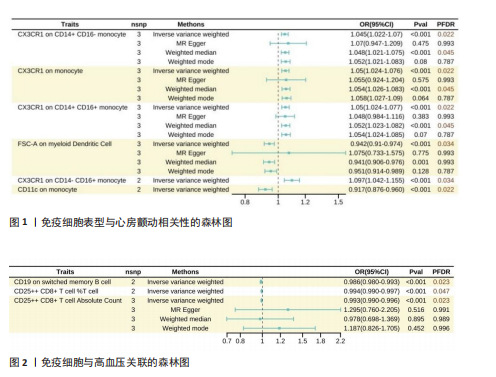
此次研究共纳入2 707个免疫表型相关单核苷酸多态性进行孟德尔随机化分析,分析过程中未使用代理单核苷酸多态性替换缺失变异,以确保工具变量的一致性。被纳入的所有单核苷酸多态性均为F > 10,适合作为强工具变量。 2.1 免疫表型与心房颤动的因果效应 固定效应逆方差加权法模型显示,43种免疫表型对心房颤动具有保护效应。经错误发现率校正(P < 0.05)后,有2种免疫细胞表型对心房颤动具有保护作用,分别是CD11c on monocytes(OR=0.917,95%CI:0.876-0.960,P=0.02)及FSC-A on myeloid dendritic cells(OR=0.942,95%CI:0.910-0.974,P=0.03);对于髓样树突状细胞FSC-A,加权中位数及加权模式分析趋势一致,P > 0.05;同时逆方差加权分析显示,CX3CR1 on CD14+ CD16- monocytes(OR=1.05,95%CI:1.024-1.077,P=0.02)、CX3CR1 on monocytes(OR=1.050,95%CI:1.024-1.076,P=0.02)及CX3CR1 on CD14+ CD16+ monocyte(OR=1.050,95%CI:1.024-1.077,P=0.02)水平的升高会增加心房颤动发病风险,运用Weighted mode法的分析结果类似,但MR-Egger及Weighted median法分析结果显示无显著差异(图1)。基于Cochran’s Q及MR-Egger截距,上述5项关联均未发现水平多效性。 2.2 免疫表型对高血压的因果效应 逆方差加权结果提示37种免疫表型与高血压风险相关,OR=0.987-1.010。经错误发现率校正(P < 0.05)后,CD19 on switched memory B cells水平较高的患者高血压患病率为对照组的0.986倍(95%CI:0.980-0.993,P=0.02);CD25++ CD8+ T cell百分比(OR=0.993,95%CI:0.990-0.997,P=0.047)及绝对计数(OR=0.993,95%CI:0.989-0.996,P=0.02)亦对高血压起到保护作用(图2)。敏感性分析均未发现异质性或水平多效性。 2.3 免疫表型与其他5种心血管疾病的因果效应 逆方差加权结果提示以下免疫表型与冠状动脉粥样硬化性心脏病具有一定相关性:CD24+CD27+ B cell百分比(OR=0.899,95%CI:0.810-0.998,P=0.04)、SSC-A on granulocytes(OR=1.07,95%CI:1.013-1.133,P=0.01)及FSC-A on myeloid dendritic cells(OR=1.64,95%CI:1.004-1.127,P=0.03)。 对于扩张型心肌病,固定效应逆方差加权分析显示4种免疫表型具有保护作用:CD19 on memory B cells(OR=0.649,95%CI:0.432-0.976,P=0.04)、CD45 on CD33-HLADR-(OR=0.807,95%CI:0.657-0.990,P=0.04)、CD19 on CD24+ CD27+ B cells(OR=0.665,95%CI:0.452-0.979,P=0.04)及FSC-A on monocyte(OR=0.680,95%CI:0.479-0.967,P=0.03)。另外,有2种免疫表型与扩张型心肌病发病风险增加相关:CD8 on CD39+ CD8+ T cells (OR=1.347,95%CI:1.102-1.647,P=0.03)及Naive CD8+ T cell绝对计数(OR=1.729,95%CI:1.002-2.981,P=0.04)。 在24种与心力衰竭存在因果关联的免疫表型中,18种亚型具有保护作用(OR=0.905-0.982),6种增加心力衰竭发病风险(OR=1.002-1.105)。在肥厚型心肌病人群中,9种免疫表型降低肥厚型心肌病发病风险(OR=0.227-0.734),8种增加肥厚型心肌病发病风险(OR=1.153-2.642)。在瓣膜性心脏病的孟德尔随机化分析中,固定效应逆方差加权分析显示14种免疫表型具有保护作用(OR=0.827-0.999),另有3种免疫表型可增加瓣膜性心脏病发病风险(OR=1.028-1.036)。 在以上分析中,Cochran’s Q及MR-Egger的P均> 0.05,表明使用随机效应逆方差加权法的因果估计可信,同时上述关联无异质性或多效性。然而经错误发现率校正(检验水平P < 0.05)后,免疫细胞表型与以上5种心血管疾病在统计学上无显著关联。"
| [1] JOSEPH P, LEONG D, MCKEE M, et al. Reducing the global burden of cardiovascular disease, part 1. Circ Res. 2017;121(6):677-694. [2] ARNETT DK, BLUMENTHAL RS, ALBERT MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11): 596-646. [3] MACH F, SMULDERS YM, CARBALLO D, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur J Prev Cardiol. 2022;29(1):5-115. [4] TSAO CW, ADAY AW, ALMARZOOQ ZI, et al. Heart disease and stroke statistics—2022 update: a report from the american heart association. Circulation. 2022;145(8):153-639. [5] TOWNSEND N, KAZAKIEWICZ D, LUCY WRIGHT F, et al. Epidemiology of cardiovascular disease in Europe. Nat Rev Cardiol. 2021;19(2):133-143. [6] WANG H, ZHANG H, ZOU Z. Changing profiles of cardiovascular disease and risk factors in China: a secondary analysis for the Global Burden of Disease Study 2019. Chin Med J. 2023;136(20):2431-2441. [7] 刘明波,何新叶,杨晓红,等.《中国心血管健康与疾病报告2023》概要(心血管疾病流行及介入诊疗状况)[J].中国介入心脏病学杂志,2024,32(10):541-550. [8] MENG X, YANG J, DONG M, et al. Regulatory T cells in cardiovascular diseases. Nat Rev Cardiol. 2015;13(3):167-179. [9] LIEVENS D, HUNDELSHAUSEN PV. Platelets in atherosclerosis. Thromb Haemost. 2011; 106(11):827-838. [10] LIBBY P, LICHTMAN AH, HANSSON GK. Immune Effector Mechanisms Implicated in Atherosclerosis: From Mice to Humans. Immunity. 2013;38(6):1092-1104. [11] 李婧玉,李琦,陈畅.免疫细胞在动脉粥样硬化进程中作用的研究进展[J].药学进展,2023,47(7):542-550. [12] 胡科丹,寇艳,邢文静,等.颈动脉超声参数、B淋巴细胞与冠心病病人斑块稳定性的关系及其对预后的评估价值[J].中西医结合心脑血管病杂志,2024,22(20): 3742-3748. [13] IMIELA AM, MIKOŁAJCZYK TP, SIEDLIŃSKI M, et al. The Th17/treg imbalance in patients with primary hyperaldosteronism and resistant hypertension. Pol Arch Inter Med. 2021;132(3): 16171. [14] RIDKER PM, EVERETT BM, THUREN T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Eng J Med. 2017;377(12):1119-1131. [15] EVERETT BM, CORNEL JH, LAINSCAK M, et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139(10):1289-1299. [16] BOWDEN J, HOLMES MV. Meta‐analysis and mendelian randomization: a review. Res Synth Methods. 2019;10(4):486-496. [17] SHEEHAN NA, DIDELEZ V, BURTON PR, et al. Mendelian Randomisation and Causal Inference in Observational Epidemiology. PLoS Med. 2008;5(8):e177. [18] SMITH GD, HEMANI G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89-R98. [19] SKRIVANKOVA VW, RICHMOND RC, WOOLF BAR, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA. 2021;326(16):1614. [20] ORRÙ V, STERI M, SIDORE C, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet. 2020;52(10):1036-1045. [21] NIELSEN JB, THOROLFSDOTTIR RB, FRITSCHE LG, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. 2018;50(9):1234-1239. [22] SAKAUE S, KANAI M, TANIGAWA Y, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. 2021;53(10):1415-1424. [23] SHAH S, HENRY A, ROSELLI C, et al. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. 2020;11(1):163. [24] HEMANI G, ZHENG J, ELSWORTH B, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018;7:e34408. [25] GAO N, KONG M, LI X, et al. The Association Between Psoriasis and Risk of Cardiovascular Disease: A Mendelian Randomization Analysis. Front Immunol. 2022;13:918224. [26] LEID J, CARRELHA J, BOUKARABILA H, et al. Primitive embryonic macrophages are required for coronary development and maturation. Circ Res. 2016;118(10):1498-1511. [27] KRISHNASAMY K, LIMBOURG A, KAPANADZE T, et al. Blood vessel control of macrophage maturation promotes arteriogenesis in ischemia. Nat Commun. 2017;8(1):952. [28] SATTLER S, FAIRCHILD P, WATT FM, et al. The adaptive immune response to cardiac injury—the true roadblock to effective regenerative therapies? NPJ Regen Med. 2017;2(1):19. [29] DOMÍNGUEZ-ANDRÉS J, DOS SANTOS JC, BEKKERING S, et al. Trained immunity: adaptation within innate immune mechanisms. Physiol Rev. 2023;103(1):313-346. [30] FERRARI I, VAGNOZZI RJ. Mechanisms and strategies for a therapeutic cardiac immune response. J Mol Cell Cardiol. 2021;158:82-88. [31] RANJIT N. Psychosocial Factors and Inflammation in the Multi-Ethnic Study of Atherosclerosis. Arch Inter Med. 2007; 167(2):174. [32] FROSTEGÅRD J. Immunity, atherosclerosis and cardiovascular disease. Trends Immunol. 2001;22(4):180-181. [33] WOOLLARD KJ, GEISSMANN F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7(2):77-86. [34] TALEB S. Inflammation in atherosclerosis. Arch Cardiovas Dis. 2016;109(12):708-715. [35] MURAKATA Y, YAMAGAMI F, MURAKOSHI N, et al. Electrical, structural, and autonomic atrial remodeling underlies atrial fibrillation in inflammatory atrial cardiomyopathy. Front Cardiovas Med. 2023;9:1075358. [36] XU L, DAI PERRARD X, PERRARD JL, et al. Foamy Monocytes Form Early and Contribute to Nascent Atherosclerosis in Mice With Hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2015;35(8): 1787-1797. [37] WU H, GOWER RM, WANG H, et al. Functional Role of CD11c+ Monocytes in Atherogenesis Associated With Hypercholesterolemia. Circulation. 2009; 119(20):2708-2717. [38] DEVALAPALLI AP, LESHER A, SHIEH K, et al. Increased levels of IgE and autoreactive, polyreactive IgG in wild rodents: implications for the hygiene hypothesis. Scand J Immunol. 2006;64(2):125-136. [39] IMAI T, HIESHIMA K, HASKELL C, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91(4): 521-530. [40] FONG AM, ROBINSON LA, STEEBER DA, et al. Fractalkine and CX3CR1 Mediate a Novel Mechanism of Leukocyte Capture, Firm Adhesion, and Activation under Physiologic Flow. J Exp Med. 1998;188(8): 1413-1419. [41] LUDWIG A, WEBER C. Transmembrane chemokines: versatile ‘special agents’ in vascular inflammation. Thromb Haemost. 2007;97(5):694-703. [42] TEUPSER D, PAVLIDES S, TAN M, et al. Major reduction of atherosclerosis in fractalkine (CX3CL1)-deficient mice is at the brachiocephalic artery, not the aortic root. Proc Nati Acad Sci. 2004;101(51):17795-17800. [43] MCALLISTER TW, SPARLING MB, FLASHMAN LA, et al. Differential working memory load effects after mild traumatic brain injury. NeuroImage. 2001;14(5):1004-1012. [44] MCDERMOTT DH, FONG AM, YANG Q, et al. Chemokine receptor mutant CX3CR1-M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. J Clin Invest. 2003;111(8):1241-1250. [45] METCALF TU, WILKINSON PA, CAMERON MJ, et al. Human monocyte subsets are transcriptionally and functionally altered in aging in response to pattern recognition receptor agonists. J Immunol. 2017;199(4):1405-1417. [46] KASHIWAGI M, IMANISHI T, TSUJIOKA H, et al. Association of monocyte subsets with vulnerability characteristics of coronary plaques as assessed by 64-slice multidetector computed tomography in patients with stable angina pectoris. Atherosclerosis. 2010;212(1):171-176. [47] IKEJIMA H, IMANISHI T, TSUJIOKA H, et al. Upregulation of Fractalkine and Its Receptor, CX3CR1, is Associated With Coronary Plaque Rupture in Patients With Unstable Angina Pectoris. Circul J. 2010;74(2):337-345. [48] HERRERO-FERNANDEZ B, GOMEZ-BRIS R, SOMOVILLA-CRESPO B, et al. Immunobiology of atherosclerosis: a complex net of interactions. Int J Mol Sci. 2019;20(21):5293. [49] CHANG W, ZHU F, ZHENG H, et al. Glucagon-like peptide-1 receptor agonist dulaglutide prevents ox-LDL-induced adhesion of monocytes to human endothelial cells: an implication in the treatment of atherosclerosis. Mol Immunol. 2019;116:73-79. [50] RAZEGHIAN-JAHROMI I, KARIMI AKHORMEH A, RAZMKHAH M, et al. Immune system and atherosclerosis: hostile or friendly relationship. Int J Immunopathol Pharmacol. 2022;36: 3946320221092188 [51] SHAYA GE, LEUCKER TM, JONES SR, et al. Coronary heart disease risk: low-density lipoprotein and beyond. Trends Cardiovasc Med. 2022;32(4):181-194 [52] WALSH R, OFFERHAUS JA, TADROS R, et al. Minor hypertrophic cardiomyopathy genes, major insights into the genetics of cardiomyopathies. Nat Rev Cardiol. 2021; 19(3):151-167. [53] ZENG Z, WANG K, LI Y, et al. Down-regulation of microRNA-451a facilitates the activation and proliferation of CD4+ T cells by targeting Myc in patients with dilated cardiomyopathy. J Biol Chem. 2017; 292(14):6004-6013. [54] WU J, SUN P, CHEN Q, et al. Metabolic reprogramming orchestrates CD4+ T-cell immunological status and restores cardiac dysfunction in autoimmune induced-dilated cardiomyopathy mice. J Mol Cell Cardiol. 2019;135:134-148. [55] SHINTANI Y, NAKAYAMA T, MASAKI A, et al. Clinical impact of the pathological quantification of myocardial fibrosis and infiltrating T lymphocytes using an endomyocardial biopsy in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2022;362:110-117. [56] KÖRÖSKÉNYI K, JUBA F, VAJDA GY. Human vascular antigen complement consumption test of hypertensive patients (preliminary report). Experientia. 1961;17(2):91-92. [57] KIRABO A, FONTANA V, DE FARIA APC, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124(10):4642-4656. [58] ITANI HA, MCMASTER WG, SALEH MA, et al. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension. 2016;68(1):123-132. [59] DINGWELL LS, SHIKATANI EA, BESLA R, et al. B-cell deficiency lowers blood pressure in mice. Hypertension. 2019;73(3):561-570. [60] CAILLON A, PARADIS P, SCHIFFRIN EL. Role of immune cells in hypertension. Br J Pharmacol. 2018;176(12):1818-1828. [61] YOUN JC, YU HT, LIM BJ, et al. Immunosenescent CD8+ T Cells and C-X-C Chemokine Receptor Type 3 Chemokines Are Increased in Human Hypertension. Hypertension. 2013;62(1):126-133. [62] HICKEY JW. Organization of the human intestine at single-cell resolution. Nature. 2023;619(7970):572-584. [63] DRUMMOND GR, VINH A, GUZIK TJ, et al. Immune mechanisms of hypertension. Nat Rev Immunol, 2019;19(8):517-532. [64] HOFMANN U, BEYERSDORF N, WEIRATHER J, et al. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125(13):1652-1663. [65] FRIELER RA, MORTENSEN RM. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation. 2015;131(11):1019-1030. [66] NAHRENDORF M, SWIRSKI FK. Innate immune cells in ischaemic heart disease: does myocardial infarction beget myocardial infarction? Eur Heart J. 2015;37(11):868-872. [67] LIU L, WANG Y, CAO Z, et al. Up‐regulated TLR 4 in cardiomyocytes exacerbates heart failure after long‐term myocardial infarction. J Cell Mol Med. 2015;19(12):2728-2740. [68] MANN DL, MCMURRAY JJ, PACKER M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the randomized etanercept worldwide evaluation (RENEWAL). Circulation. 2004; 109(13):1594-1602. [69] TORRE-AMIONE G, ANKER SD, BOURGE RC, et al. Results of a non-specific immunomodulation therapy in chronic heart failure (ACCLAIM trial): a placebo-controlled randomised trial. Lancet. 2008; 371(9608):228-236. [70] BARTOLI-LEONARD F, ZIMMER J, AIKAWA E. Innate and adaptive immunity: the understudied driving force of heart valve disease. Cardiovasc Res. 2021;117(13): 2506-2542. |
| [1] | Ye Qianqian, Pan Hang, Tian Chuan, Zhu Xiangqing, Ye Li, Zhao Xiaojuan, Shu Liping, Pan Xinghua. Effects of highly active umbilical cord mesenchymal stem cells on structure and function of thymus in elderly tree shrews [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1720-1729. |
| [2] | Wu Zhilin, , He Qin, Wang Pingxi, Shi Xian, Yuan Song, Zhang Jun, Wang Hao . DYRK2: a novel therapeutic target for rheumatoid arthritis combined with osteoporosis based on East Asian and European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1569-1579. |
| [3] | Liu Hongtao, Wu Xin, Jiang Xinyu, Sha Fei, An Qi, Li Gaobiao. Causal relationship between age-related macular degeneration and deep vein thrombosis: analysis based on genome-wide association study data [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1602-1608. |
| [4] | Guo Ying, Tian Feng, Wang Chunfang. Potential drug targets for the treatment of rheumatoid arthritis: large sample analysis from European databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1549-1557. |
| [5] | Gao Zengjie, , Pu Xiang, Li Lailai, Chai Yihui, Huang Hua, Qin Yu. Increased risk of osteoporotic pathological fractures associated with sterol esters: evidence from IEU-GWAS and FinnGen databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1302-1310. |
| [6] | Liu Fengzhi, Dong Yuna, Tian Wenyi, Wang Chunlei, Liang Xiaodong, Bao Lin. Gene-predicted associations between 731 immune cell phenotypes and rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1311-1319. |
| [7] | Zhang Cuicui, Chen Huanyu, Yu Qiao, Huang Yuxuan, Yao Gengzhen, Zou Xu. Relationship between plasma proteins and pulmonary arterial hypertension and potential therapeutic targets [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1331-1340. |
| [8] | Zeng Hao, Sun Pengcheng, Chai Yuan, Huang Yourong, Zhang Chi, Zhang Xiaoyun. Association between thyroid function and osteoporosis: genome-wide data analysis of European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1019-1027. |
| [9] | Rong Xiangbin, , Zheng Haibo, Mo Xueshen, Hou Kun, Zeng Ping, . Plasma metabolites, immune cells, and hip osteoarthritis: causal inference based on GWAS data from European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1028-1035. |
| [10] | He Qiwang, , , Chen Bo, Liang Fuchao, Kang Zewei, Zhou Yuan, Ji Anxu, Tang Xialin, . Relationship between Alzheimer’s disease and sarcopenia and body mass index: analysis of GWAS datasets for European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1036-1046. |
| [11] | Ding Yu, Chen Jingwen, Chen Xiuyan, Shi Huimin, Yang Yudie, Zhou Meiqi, Cui Shuai, . Circulating inflammatory proteins and myocardial hypertrophy: large sample analysis of European populations from GWAS Catalog and FinnGen databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1047-1057. |
| [12] | Zhao Feifan, Cao Yujing. An artificial neural network model of ankylosing spondylitis and psoriasis shared genes and machine learning-based mining and validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 770-784. |
| [13] | Liu Chu, Qiu Boyuan, Tong Siwen, He Linyuwei, Chen Haobo, Ou Zhixue. A genetic perspective reveals the relationship between blood metabolites and osteonecrosis: an analysis of information from the FinnGen database in Finland [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 785-794. |
| [14] | Zhang Zheng, Zhang Yibo, Xu Bin, Yan Shichao, Guo Hui. Sarcopenia and non-alcoholic fatty liver disease: analysis of the gut microbiota [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 6000-6009. |
| [15] | Yin Xingxiao, Jiang Yang, Song Yanping, Yao Na, Shen Zhen, Li Yanqi, Song Yueyu, Peng Hao, Chen Qigang. Association between sarcopenia and osteoporosis: a genome-wide data analysis in European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 6030-6039. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||