Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (23): 6000-6009.doi: 10.12307/2026.318
Previous Articles Next Articles
Sarcopenia and non-alcoholic fatty liver disease: analysis of the gut microbiota
Zhang Zheng1, Zhang Yibo1, Xu Bin2, Yan Shichao1, Guo Hui1
- 1Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China; 2Department of Hepatobiliary Surgery, First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China
-
Received:2025-05-06Accepted:2025-06-11Online:2026-08-18Published:2025-12-31 -
Contact:Xu Bin, MS, Professor, Master’s supervisor, Department of Hepatobiliary Surgery, First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China -
About author:Zhang Zheng, MS candidate, Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China Zhang Yibo, MS candidate, Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China Zhang Zheng and Zhang Yibo contributed equally to this work. -
Supported by:“Qihuang Engineering” High-level Talent Team Cultivation Project of Guangxi University of Chinese Medicine, No. 202411 (to XB)
CLC Number:
Cite this article
Zhang Zheng, Zhang Yibo, Xu Bin, Yan Shichao, Guo Hui. Sarcopenia and non-alcoholic fatty liver disease: analysis of the gut microbiota[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 6000-6009.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
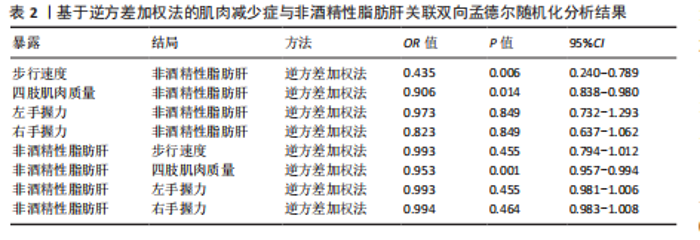
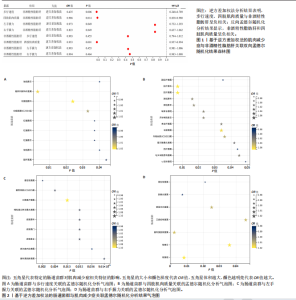
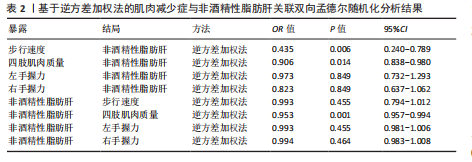
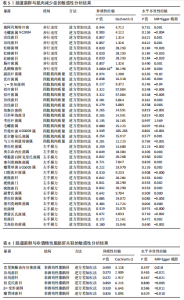
2.1 工具变量筛选结果 研究提取了与非酒精性脂肪肝及肌肉减少症关联的16个单核苷酸多态性作为工具变量。以肠道菌群作为暴露因素,筛选出1 055个非酒精性脂肪肝相关单核苷酸多态性,四肢肌肉质量、步行速度、左、右手握力相关的单核苷酸多态性分别为13 663,13 760,13 760,13 760个。 2.2 孟德尔随机化分析结果 2.2.1 肌肉减少症与非酒精性脂肪肝关联的孟德尔随机化分析结果 逆方差加权分析结果表明,步行速度的增加与非酒精性脂肪肝风险降低相关(P=0.006,OR=0.435,95%CI=0.240-0.789),四肢肌肉质量与非酒精性脂肪肝风险呈负相关(P=0.014,OR=0.906,95%CI=0.838-0.980),左、右手握力水平与非酒精性脂肪肝风险无显著相关性(左手握力:P=0.849,OR=0.973,95%CI=0.732-1.293;右手握力:P=0.849,OR=0.823,95%CI= 0.637-1.062),见表2、图1。基于逆方差加权法的反向孟德尔随机化结果显示,非酒精性脂肪肝和四肢肌肉质量呈负相关(P=0.001,OR=0.953,95%CI=0.957-0.994),非酒精性脂肪肝与步行速度(P=0.455,OR=0.993,95%CI=0.794-1.012)、左手握力(P= 0.455,OR=0.993,95%CI=0.981-1.006)、右手握力(P=0.464,OR=0.994,95%CI=0.983-1.008)无显著相关性,见表2、图1。"
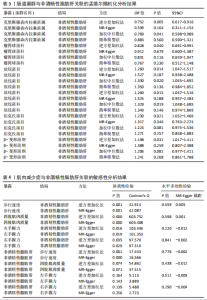
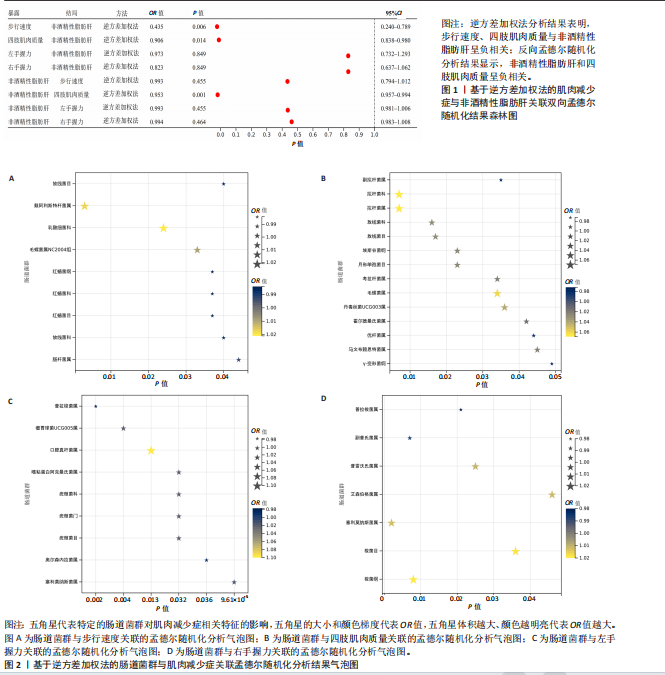
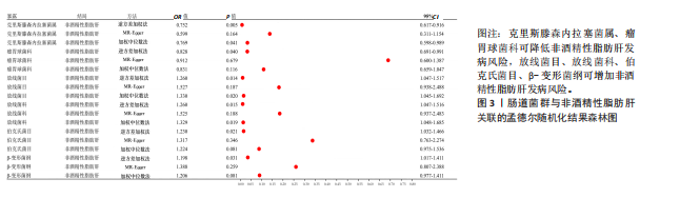
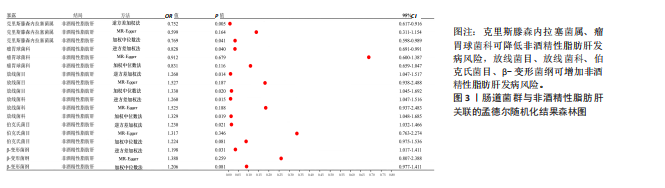
2.2.2 肠道菌群与肌肉减少症关联的孟德尔随机化分析结果 逆方差加权法分析结果表明,39种肠道菌群与肌肉减少症发病具有显著相关性,见图2。其中,9种肠道菌群与步行速度显著相关,放线菌目(P=0.040,OR=0.986,95%CI=0.972-0.999)、放线菌科(P=0.040,OR=0.986,95%CI= 0.972-0.999)、红蝽菌纲(P=0.037,OR=0.984,95%CI=0.969-0.100)、红蝽菌科(P=0.037,OR=0.984,95%CI= 0.969-0.100)、红蝽菌目(P=0.037,OR=0.984,95%CI=0.969-0.999)、肠杆菌属(P=0.044,OR=0.993,95%CI= 0.986-0.100)与步行速度呈负相关,戴阿利斯特杆菌属(P=0.003,OR=1.018,95%CI=1.006-1.030)、乳酸细菌科(P=0.024,OR=1.021,95%CI=1.003-1.041)、毛螺菌属NC2004组(P=0.033,OR=1.010,95%CI=1.001-1.020)与步行速度呈正相关。 14种肠道菌群与四肢肌肉质量具有相关性,副拟杆菌属(P=0.035,OR=0.976,95%CI=0.954-0.998)、优杆菌属(P=0.044,OR=0.983,95%CI=0.967-0.100)、γ-变形菌纲(P=0.049,OR=0.972,95%CI=0.946-0.100)与四肢肌肉质量呈负相关性,拟杆菌科(P=0.007,OR=1.069,95%CI=1.019-1.122)、拟杆菌属(P=0.007,OR=1.069,95%CI=1.019-1.122)、放线菌科(P=0.016,OR= 1.029,95%CI=1.005-1.052)、放线菌目(P=0.017,OR=1.029,95%CI=1.005- 1.053)、埃斯谷菌纲(P=0.023,OR= 1.026,95%CI=1.004-1.049)、月形单孢菌目(P=0.023,OR=1.026,95%CI= 1.004-1.049)、考拉杆菌属(P=0.034,OR=1.022,95%CI=1.002-1.042)、毛螺菌属(P=0.034,OR=1.062,95%CI= 1.004-1.124)、丹毒丝菌UCG003属(P=0.036,OR=1.043,95%CI=1.003-1.085)、霍尔德曼氏菌属(P=0.042,OR=1.014,95%CI=1.001-1.028)、马文布赖恩特菌属(P=0.045,OR=1.023,95%CI=1.000-1.046)与四肢肌肉质量呈正相关性。 7种肠道菌群与右手握力之间存在因果关系,副普氏菌属(P=0.007,OR=0.987,95%CI=0.977-0.996)、普拉梭菌属(P=0.021,OR=0.980,95%CI=0.964-0.997)可降低右手握力,塞利莫纳斯菌属(P=0.002,OR=1.013,95%CI=1.005-1.021)、梭菌纲(P=0.008,OR=1.020,95%CI=1.005-1.036)、普雷沃氏菌属(P=0.025,OR=1.011,95%CI= 1.001-1.020)、梭菌目(P=0.036,OR=1.019,95%CI=1.001-1.036)、艾森伯格菌属(P=0.046,OR=1.012,95%CI= 1.000-1.023)可增加右手握力。 9种肠道菌群与左手握力之间存在因果关系,普拉梭菌属(P=0.002,OR=0.977,95%CI=0.963-0.992)、奥尔森内拉菌属(P=0.036,OR=0.988,95%CI=0.977-0.100)可降低左手握力;嗜黏蛋白阿克曼氏菌属(P=0.032,OR=1.020,95%CI=1.002-1.038)、塞利莫纳斯菌属(P=9.61×10-5,OR= 1.015,95%CI=1.007-1.023)、瘤胃球菌UCG005属(P=0.004,OR=1.020,95%CI=1.006-1.034)、口腔真杆菌属(P=0.013,OR=1.100,95%CI=1.002-1.017)、疣微菌科(P=0.032,OR=1.020,95%CI=1.002-1.038)、疣微菌门(P=0.032,OR=1.020,95%CI=1.002-1.038)、疣微菌目(P=0.032,OR=1.020,95%CI=1.002-1.038)可增加左手握力。 2.2.3 肠道菌群与非酒精性脂肪肝关联的孟德尔随机化分析结果 逆方差加权法分析表明,6种肠道菌群与非酒精性脂肪肝之间存在因果关系,见表3、图3。克里斯滕森内拉塞菌属(P=0.005,OR=0.752,95%CI= 0.617-0.916)、瘤胃球菌科(P=0.040,OR=0.828,95%CI=0.691-0.991)可降低非酒精性脂肪肝发病风险,放线菌目(P=0.014,OR=1.260,95%CI=1.047-1.517)、放线菌科(P=0.015,OR=1.260,95%CI=1.047-1.516)、伯克氏菌目(P= 0.021,OR=1.230,95%CI=1.032-1.466)、β-变形菌纲(P=0.031,OR= 1.198,95%CI=1.017-1.411)可增加非酒精性脂肪肝发病风险。 2.3 敏感性分析结果 2.3.1 肌肉减少症与非酒精性脂肪肝关联的敏感性分析结果 使用Cochran’s Q统计量测试异质性,逆方差加权分析表明,步行速度与非酒精性脂肪肝(P=0.001)、四肢肌肉质量与非酒精性脂肪肝(P=0.000)、左手握力与非酒精性脂肪肝(P=0.016)、右手握力与非酒精性脂肪肝(P=0.035)间的因果关系存在异质性,见表4。MR-Egger截距结果表明,步行速度与非酒精性脂肪肝(P=0.559)、四肢肌肉质量与非酒精性脂肪肝(P=0.598)、左手握力与非酒精性脂肪肝(P=0.220)、右手握力与非酒精性脂肪肝(P=0.841)间的因果关系均不存在水平多效性,见表4。 使用Cochran’s Q统计量测试异质性,逆方差加权分析表明,非酒精性脂肪肝与四肢肌肉质量(P=0.074)、非酒精性脂肪肝与左手握力(P=0.164)、非酒精性脂肪肝与右手握力(P=0.139)间的因果关系均不存在异质性,非酒精性脂肪肝与步行速度(P=0.001)间的因果关系存在异质性,见表4。MR-Egger截距结果表明,非酒精性脂肪肝与四肢肌肉质量(P=0.438)、步行速度(P=0.776)、左手握力(P=0.511)、右手握力(P=0.290)间的因果关系均不存在水平多效性,见表4。"
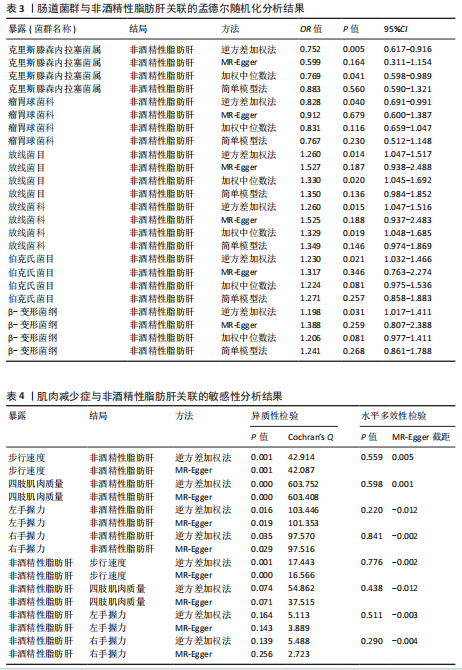
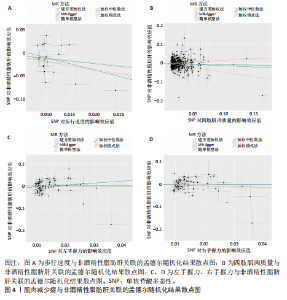
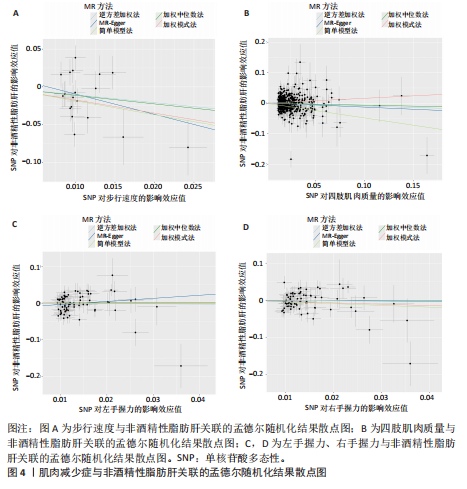
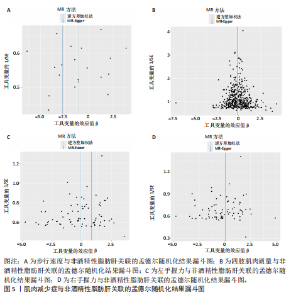
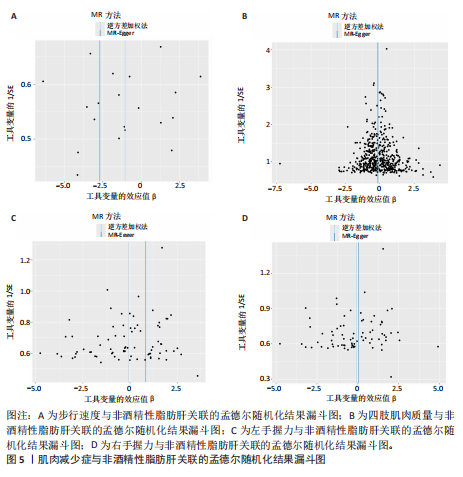
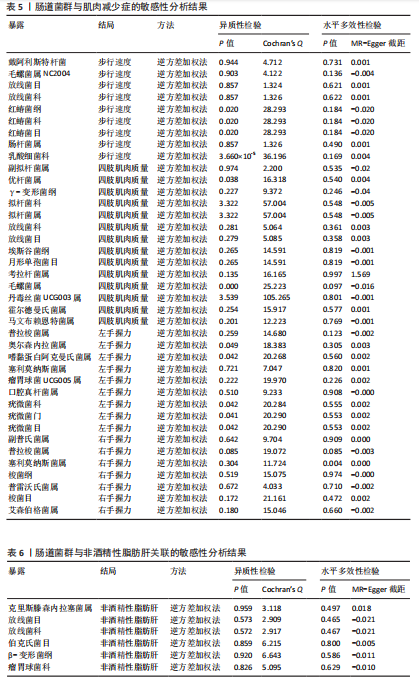
散点图通过不同方法显示了肌肉减少症与非酒精性脂肪肝之间的因果关系(图4),图中的每个黑点表示一个单核苷酸多态性,线条对应于使用不同孟德尔随机化方法时肌肉减少症对非酒精性脂肪肝的影响。漏斗图显示每个单核苷酸多态性的因果效应在逆方差加权法估计值周围对称分布,表明因果关联不受到潜在偏差的影响(图5)。 2.3.2 肠道菌群与肌肉减少症敏感性分析结果 使用Cochran’s Q统计量测试异质性,逆方差加权分析表明,9种与步行速度相关的肠道菌群中,乳酸细菌科(P=3.66×10-5)、红蝽菌目(P=0.020)、红蝽菌纲(P=0.020)、红蝽菌科(P=0.020)与步行速度间的因果关系存在潜在异质性,其余5种肠道菌群与步行速度间的因果关系均不存在异质性(P > 0.05);采用MR-Egger法的截距项对水平多效性进行评估,9种肠道菌群与步行速度间的因果关系均不存在水平多效性(P > 0.05),见表5。 14种肠道菌群与四肢肌肉质量间的因果关系均不存在水平多效性,其中埃斯谷菌纲(P=0.036)、丹毒丝菌UCG003属(P=3.54×10-15)、γ-变形菌纲(P=0.000)与四肢肌肉质量间的因果关系存在潜在异质性,其余9种肠道菌群与四肢肌肉质量间的因果关系无异质性。7种肠道菌群与右手握力间的因果关系均不存在水平多效性及异质性;9种肠道菌群与左手握力间的因果关系均不存在水平多效性,其中嗜黏蛋白阿克曼氏菌属(P=0.042)、疣微菌科(P=0.042)、疣微菌门(P=0.042)、疣微菌目(P=0.042)与左手握力间的因果关系存在潜在异质性,其余5种肠道菌群与左手握力间的因果关系未发现异质性的证据,见表5,表明结果具有稳健性。 2.3.3 肠道菌群与非酒精性脂肪肝关联的敏感性分析结果 使用Cochran’s Q统计量测试异质性,6种肠道菌群与非酒精性脂肪肝间的因果关系均不存在异质性,克里斯滕森内拉塞菌属(逆方差加权法P=0.959,Q=3.118;MR-Egger检验P=0.956,Q=2.611)、放线菌目(逆方差加权法P=0.573,Q=2.909;MR-Egger检验P=0.530,Q=2.212)、放线菌科(逆方差加权法P=0.572,Q=2.917;MR-Egger检验P=0.527,Q=2.226)、伯克氏菌目(逆方差加权法P=0.859,Q=6.215;MR-Egger检验P=0.803,Q=6.147)、β-变形菌纲(逆方差加权法P=0.920,Q=6.643;MR-Egger检验P=0.899,Q=6.330)、瘤胃球菌科(逆方差加权法P=0.826,Q=5.095;MR-Egger检验P=0.774,Q=4.843),见表6。采用MR-Egger法的截距项对水平多效性进行评估,克里斯滕森内拉塞菌属(P=0.497,MR-Egger截距=0.018)、放线菌目(P=0.465,MR-Egger截距= -0.021)、放线菌科(P=0.467,MR-Egger截距=-0.021)、伯克氏菌目基于(P=0.800,MR-Egger截距=-0.005)、β-变形菌纲(P=0.586,MR-Egger截距=-0.011)、瘤胃球菌科(P=0.629,MR-Egger截距=-0.010)与非酒精性脂肪肝间的因果关系无水平多效性,见表6。"
| [1] DOS SANTOS ALS, ANASTÁCIO LR. The Impact of L-Branched-Chain Amino Acids and L-Leucine on Malnutrition, Sarcopenia, and Other Outcomes in Patients With Chronic Liver Disease. Expert Rev Gastroenterol Hepatol. 2021;15(2):181-194. [2] PETERMANN-ROCHA F, BALNTZI V, GRAY SR, et al. Global Prevalence of Sarcopenia and Severe Sarcopenia: A Systematic Review and Meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):86-99. [3] HUANG DQ, EL-SERAG HB, LOOMBA R. Global Epidemiology of NAFLD-related HCC: Trends, Predictions, Risk Factors and Prevention. Nat Rev Gastroenterol Hepatol. 2021;18(4):223-238. [4] ALSAQAL S, HOCKINGS P, AHLSTRÖM H, et al. The Combination of MR Elastography and Proton Density Fat Fraction Improves Diagnosis of Nonalcoholic Steatohepatitis. J Magn Reson Imaging. 2022;56(2):368-379. [5] JEE JJ, LIM J, PARK S, et al. Gut Microbial Community Differentially Characterizes Patients With Nonalcoholic Fatty Liver Disease. J Gastroenterol Hepatol. 2022; 37(9):1822-1832. [6] HARRING M, GOLABI P, PAIK JM, et al. Sarcopenia Among Patients With Nonalcoholic Fatty Liver Disease (NAFLD) is Associated with Advanced Fibrosis. Clin Gastroenterol Hepatol. 2023;21(11): 2876-2888. [7] NICOLETTI A, PONZIANI FR, BIOLATO M, et al. Intestinal Permeability in the Pathogenesis of Liver Damage: From Non-alcoholic Fatty Liver Disease to Liver Transplantation. World J Gastroenterol. 2019;25(33):4814-4834. [8] DENG KQ, HUANG X, LEI F, et al. Role of Hepatic Lipid Species in the Progression of Nonalcoholic Fatty Liver Disease. Am J Physiol Cell Physiol. 2022;323(2):C630-C639. [9] HU H, LIN A, KONG M, et al. Intestinal Microbiome and NAFLD: Molecular Insights and Therapeutic Perspectives. J Gastroenterol. 2020;55(2):142-158. [10] JENNISON E, BYRNE CD. The Role of the Gut Microbiome and Diet in the Pathogenesis of Non - alcoholic Fatty Liver Disease. Clin Mol Hepatol. 2021;27(1):22-43. [11] GOMAA EZ. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek. 2020; 113(12):2019-2040. [12] ALBILLOS A, DE GOTTARDI A, RESCIGNO M. The Gut-Liver Axis in Liver Disease: Pathophysiological Basis for Therapy. J Hepatol. 2020;72(3):558-577. [13] HU H, LIN A, KONG M, et al. Intestinal Microbiome and NAFLD: Molecular Insights and Therapeutic Perspectives. J Gastroenterol. 2020;55(2):142-158. [14] EMDIN CA, KHERA AV, KATHIRESAN S. Mendelian Randomization. JAMA. 2017; 318(19):1925-1926. [15] XU HQ, SUN JQ, LIU Y, et al. Cutpoints for Muscle Mass and Strength Derived from Weakness or Mobility Impairment and Compared with Other Diagnostic Criteria in Community - Dwelling Elderly People. Calcif Tissue Int. 2021;108(3):324-345. [16] SKINNER J, SHEPSTONE L, HICKSON M, et al. Alcohol Consumption and Measures of Sarcopenic Muscle Risk: Cross - Sectional and Prospective Associations Within the UK Biobank Study. Calcif Tissue Int. 2023; 113(2):143-156. [17] CRUZ-JENTOFT AJ, BAHAT G, BAUER J, et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing. 2019;48(1):16-31. [18] SUDLOW C, GALLACHER J, ALLEN N, et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015;12(3):e1001779. [19] PETERMANN-ROCHA F, GRAY SR, FORREST E, et al. Associations of Muscle Mass and Grip Strength With Severe NAFLD: A Prospective Study of 333,295 UK Biobank Participants . J Hepatol. 2022;76(5):1021-1029. [20] KURILSHIKOV A, MEDINA-GOMEZ C, BACIGALUPE R, et al. Large-Scale Association Analyses Identify Host Factors Influencing Human Gut Microbiome Composition. Nat Genet. 2021;53(2):156-165. [21] HARTWIG FP, DAVIES NM, HEMANI G, et al. Two-Sample Mendelian Randomization: Avoiding the Downsides of a Powerful, Widely Applicable but Potentially Fallible Technique. Int J Epidemiol. 2016;45(6):1717-1726. [22] ZHANG L, ZHANG C, ZHANG J, et al. A Bidirectional Mendelian Randomization Study of Sarcopenia - Related Traits and Knee Osteoarthritis. Clin Interv Aging. 2023;18:1577-1586. [23] JOO SK, KIM W. Interaction Between Sarcopenia and Nonalcoholic Fatty Liver Disease. Clin Mol Hepatol. 2023;29(Suppl): S68-S78. [24] DENG C, OU Q, OU X, et al. Association between Non-alcoholic Fatty Liver Disease and Risk of Sarcopenia: A Systematic Review and Meta-analysis. BMJ Open. 2024;14(5): e78933. [25] SAMUEL VT, SHULMAN GI. The Pathogenesis of Insulin Resistance: Integrating Signaling Pathways and Substrate Flux. J Clin Invest. 2016;126 (1):12-22. [26] JAMIALAHMADI T, NEMATY M, JANGJOO A, et al. The Predictive Role of Parathyroid Hormone for Non - alcoholic Fatty Liver Disease Based on Invasive and Non - invasive Findings in Candidates of Bariatric Surgery. Eat Weight Disord. 2022;27(2):693-700. [27] NACHIT M, DE RUDDER M, THISSEN JP, et al. Myosteatosis Rather Than Sarcopenia Associates With Non - alcoholic Steatohepatitis in Non - alcoholic Fatty Liver Disease Preclinical Models. J Cachexia Sarcopenia Muscle. 2021;12(1):144-158. [28] YAMASANDHI PG, DHARMALINGAM M, BALEKUDURU A. Fetuin - A in Newly Detected Type 2 Diabetes Mellitus as a Marker of Non-alcoholic Fatty Liver Disease. Indian J Gastroenterol. 2021;40(6):556 -562. [29] CHADT A, AL-HASANI H. Glucose Transporters in Adipose Tissue, Liver, and Skeletal Muscle in Metabolic Health and Disease. Pflugers Arch. 2020;472(9):1273-1298. [30] LIU L, HU R, YOU H, et al. Formononetin Ameliorates Muscle Atrophy by Regulating Myostatin -mediated PI3K/Akt/FoxO3a Pathway and Satellite Cell Function in Chronic Kidney Disease. J Cell Mol Med. 2021;25(3):1493-1506. [31] OTA T. Molecular Mechanisms of Nonalcoholic Fatty Liver Disease (NAFLD)/Nonalcoholic Steatohepatitis (NASH). Adv Exp Med Biol. 2021;1261:223-229. [32] ANAND AC. Nutrition and Muscle in Cirrhosis. J Clin Exp Hepatol. 2017;7(4):340-357. [33] KHAN S, BENJAMIN J, MAIWALL R, et al. Sarcopenia Is the Independent Predictor of Mortality in Critically Ill Patients With Cirrhosis. J Clin Transl Res. 2022;8(3):200-208. [34] CHEN L, CHANG S, CHANG H, et al. Probiotic Supplementation Attenuates Age-Related Sarcopenia via the Gut-Muscle Axis in SAMP8 Mice. J Cachexia Sarcopenia Muscle. 2022;13(1):515-531. [35] WANG Y, ZHANG Y, LANE NE, et al. Population-based Metagenomics Analysis Reveals Altered Gut Microbiome in Sarcopenia: Data from the Xiangya Sarcopenia Study. J Cachexia Sarcopenia Muscle. 2022;13(5):2340-2351. [36] DAS S, PREETHI B, KUSHWAHA S, et al. Therapeutic Strategies to Modulate Gut Microbial Health: Approaches for Sarcopenia Management. Histol Histopathol. 2024; 39(11):1395-1425. [37] VELÁZQUEZ KT, ENOS RT, BADER JE, et al. Prolonged High-Fat-Diet Feeding Promotes Non-alcoholic Fatty Liver Disease and Alters Gut Microbiota in Mice. World J Hepatol. 2019;11(8):619-637. [38] SUK KT, KIM DJ. Gut Microbiota: Novel Therapeutic Target for Nonalcoholic Fatty Liver Disease. Expert Rev Gastroenterol Hepatol. 2019;13(3):193-204. [39] ALIWA B, HORVATH A, TRAUB J, et al. Altered Gut Microbiome, Bile Acid Composition and Metabolome in Sarcopenia in Liver Cirrhosis. J Cachexia Sarcopenia Muscle. 2023;14 (6):2676-2691. [40] ŞANLIBABA P, TOPRAK ZTT, TEZEL BU. The Role of Probiotics on Slowing Down the Aging Process. Acta Sci Pol Technol Aliment. 2022;21(1):53-66. [41] YAN D, CAI X, XUE H, et al. Recent Advances in the Bioproduction of Mannitol. Sheng Wu Gong Cheng Xue Bao. 2024;40(8):2626-2643. [42] DAHL WJ, HUNG WL, FORD AL, et al. In Older Women, a High-Protein Diet Including Animal-sourced Foods Did Not Impact Serum Levels and Urinary Excretion of Trimethylamine-N- oxide. Nutr Res. 2020; 78:72-81. [43] ALIWA B, HORVATH A, TRAUB J, et al. Altered Gut Microbiome, Bile Acid Composition and Metabolome in Sarcopenia in Liver Cirrhosis. J Cachexia Sarcopenia Muscle. 2023;14(6):2676-2691. [44] KRAUTKRAMER KA, FAN J, BÄCKHED F. Gut Microbial Metabolites as Multi-kingdom Intermediates. Nat Rev Microbiol. 2021;19(2):77-94. [45] DODD D, SPITZER MH, VAN TREUREN W, et al. A Gut Bacterial Pathway Metabolizes Aromatic Amino Acids Into Nine Circulating Metabolites. Nature. 2017;551(7682):648-652. [46] DELEAVAL P, LUAIRE B, LAFFAY P, et al. Short-Term Effects of Branched-Chain Amino Acids-Enriched Dialysis Fluid on Branched-Chain Amino Acids Plasma Level and Mass Balance: A Randomized Cross - Over Study. J Ren Nutr. 2020;30(1):61-68. [47] 方志杰,马抢平,董万涛,等.肠道菌群与骨质疏松症的遗传关系:来自英国数据库211个肠道微生物群分析[J].中国组织工程研究,2025,29(18):3941-3947. |
| [1] | Zhou Jian, Zhang Tao, Zhou Weili, Zhao Xingcheng, Wang Jun, Shen Jie, Qian Li, Lu Ming. Effects of resistance training on quadriceps mass and knee joint function in patients with osteoporosis and sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1081-1088. |
| [2] | Rong Xiangbin, , Zheng Haibo, Mo Xueshen, Hou Kun, Zeng Ping, . Plasma metabolites, immune cells, and hip osteoarthritis: causal inference based on GWAS data from European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1028-1035. |
| [3] | Gu Fucheng, Yang Meixin, Wu Weixin, Cai Weijun, Qin Yangyi, Sun Mingyi, Sun Jian, Geng Qiudong, Li Nan. Effects of Guilu Erxian Glue on gut microbiota in rats with knee osteoarthritis: machine learning and 16S rDNA analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1058-1072. |
| [4] | Yin Xingxiao, Jiang Yang, Song Yanping, Yao Na, Shen Zhen, Li Yanqi, Song Yueyu, Peng Hao, Chen Qigang. Association between sarcopenia and osteoporosis: a genome-wide data analysis in European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 6030-6039. |
| [5] | Li Yiguang, Guo Haonan, Ding Xiaotao, Yuan Mengyao, Jiang Lijin, Fan Xinfeng, Feng Yan. Visual analysis of research hotspots in the field of gut microbiota in the elderly at home and abroad [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 6071-6080. |
| [6] | Yan Wei, Kong Lingjun, He Tianxiang, Zhu Qingguang, Xi Xiaobing, Fang Min. The relationship between inflammatory cytokines and frozen shoulder: a large-sample analysis of the European population based on the FinnGen GWAS database [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5867-5875. |
| [7] | Chai Jinlian, Liang Xuezhen, Sun Tiefeng, Li Shudong, Li Wei, Li Guangzheng, Yu Huayun, Wang Ping. Mechanistic insights into how Cervi Cornus Colla regulates the intestinal flora-bile acid metabolic pathway to alleviate steroid-induced osteonecrosis of the femoral head in a rat model [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4568-4581. |
| [8] | Wu Yilin, Tian Hongying, Sun Jiale, Jiao Jiajia, Zhao Zihan, Shao Jinhuan, Zhao Kaiyue, Zhou Min, Li Qian, Li Zexin, Yue Changwu. Intervention effect and mechanism of Compound Herba Gueldenstaedtiae in a mouse model of breast hyperplasia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4377-4389. |
| [9] | Zou Yuxiong, Liu Xiaomeng, Liu Ying, Zhu Yue, Li Shuming, Guo Fangyang, Yu Xinyu, Nie Heyun, Liu Qian, Ao Meiying. Cerebral palsy decoction improves cerebral palsy in male and female young rats: mechanisms based on the “gut-brain-muscle” axis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4054-4066. |
| [10] | Sun Long, Wu Haiyang, Tong Linjian, Liu Rui, Yang Weiguang, Xiao Jian, Liu Lice, Sun Zhiming. Regulatory mechanism of leptin in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(12): 3100-3108. |
| [11] | Wang Tao, Min Youjiang, Wang Min, Wang Shunpu, Li Le, Zhang Chen, Xiao Weiping, Yu Yiping. Causal relationship between gut microbiota and amyotrophic lateral sclerosis: sample analysis from the IEU Open GWAS Database [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(12): 3182-3189. |
| [12] | Wu Fangjia, Lei Senlin, Li Xianhui, Yang Yang. Aerobic and resistance exercise interventions in a mouse model of nonalcoholic fatty liver disease: correlation between gut microbiota and irisin [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(12): 3029-3043. |
| [13] | Zou Yuxi, Chen Yanyan, Jiang Peng, Chen Ting, Ding Lingling. Critical role of lysosomal enzymes in metabolic diseases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2823-2833. |
| [14] | Qiu Xueli, Cui Hao, Wu Chenyang, Tao Lide, Yao Yuqian, Tian Bo, Bai Jinyu, Zhang Yingzi. Gut microbiota tryptophan metabolite indole-3-propionic acid alleviates inflammatory bowel disease-related osteoporosis in a mouse model [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2413-2421. |
| [15] | Zhang Yibo, Lu Jianqi, Mao Meiling, Pang Yan, Dong Li, Yang Shangbing, Xiao Xiang. Exploring the causal relationship between rheumatoid arthritis and coronary atherosclerosis: a Mendel randomized study involving serum metabolites and inflammatory factors [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||