Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (12): 3029-3043.doi: 10.12307/2026.656
Previous Articles Next Articles
Aerobic and resistance exercise interventions in a mouse model of nonalcoholic fatty liver disease: correlation between gut microbiota and irisin
Wu Fangjia1, Lei Senlin2, Li Xianhui3, Yang Yang1
- 1College of Physical Education and Health, Guangxi Science & Technology Normal University, Laibin 546100, Guangxi Zhuang Autonomous Region, China; 2College of Physical Education, 3College of Medicine, Jishou University, Jishou 416000, Hunan Province, China
-
Received:2025-06-06Accepted:2025-07-29Online:2026-04-28Published:2025-09-29 -
Contact:杨阳,博士,讲师,广西科技师范学院体育与健康科学学院,广西壮族自治区来宾市 546100 -
About author:伍方佳,男,1991年生,湖南省衡阳市人,硕士,讲师,主要从事体育教学、运动健康促进研究。 并列第一作者:雷森林,男,1997年生,河南省信阳市人,吉首大学在读博士,主要从事运动慢病防治研究。 -
Supported by:the National Natural Science Foundation of China, No. 81860636 (to LXH); Sports and Health Industry Research Team, No. GXKS2024QNTD14
CLC Number:
Cite this article
Wu Fangjia, Lei Senlin, Li Xianhui, Yang Yang. Aerobic and resistance exercise interventions in a mouse model of nonalcoholic fatty liver disease: correlation between gut microbiota and irisin[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(12): 3029-3043.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
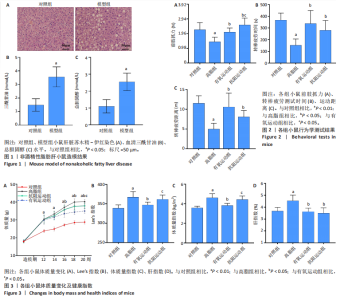
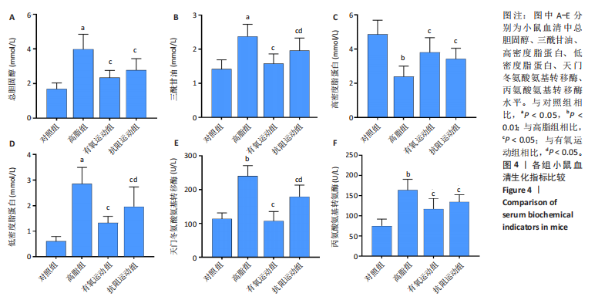
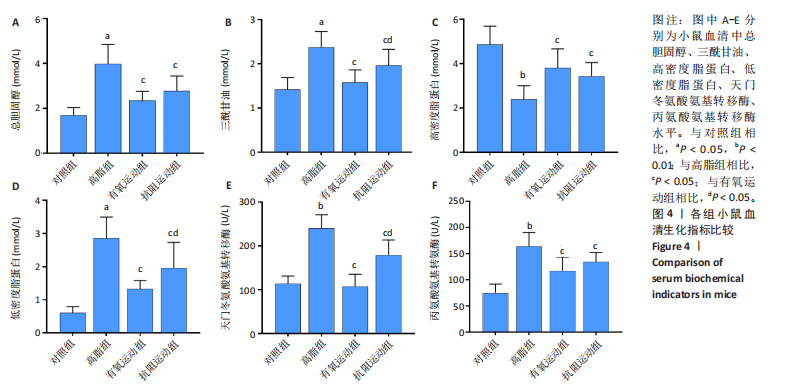
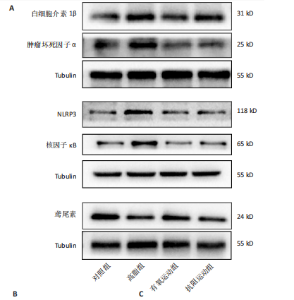
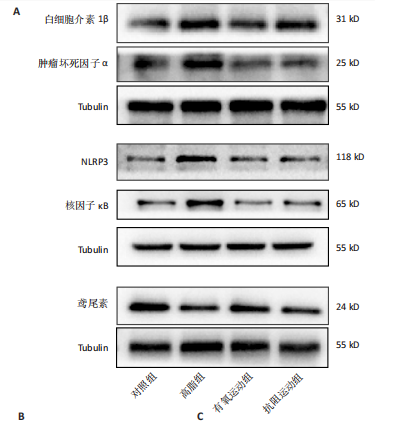
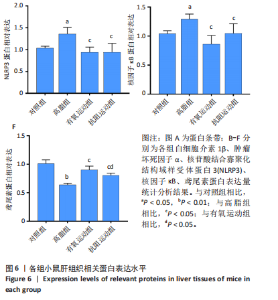
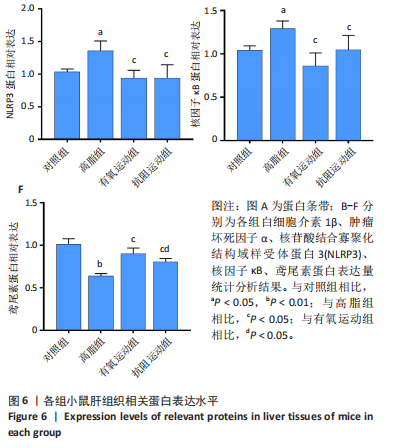
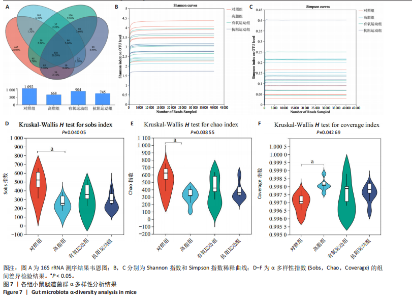
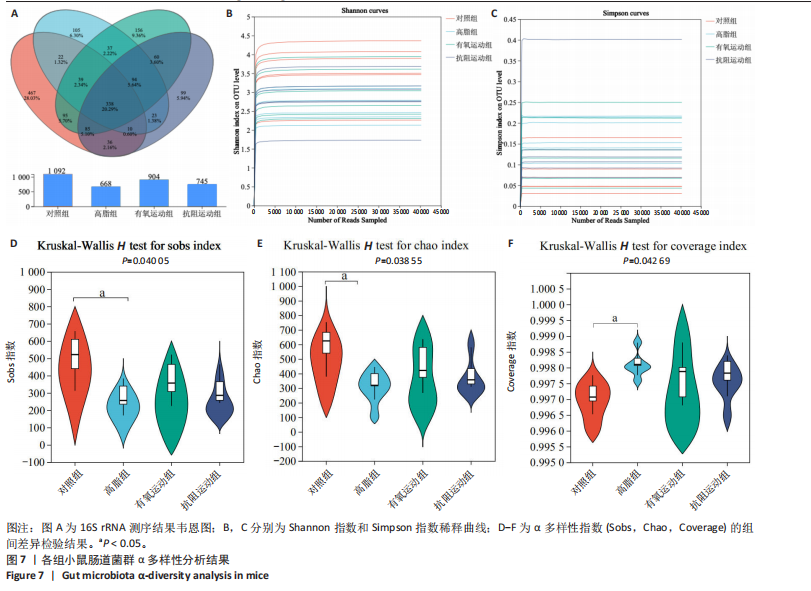
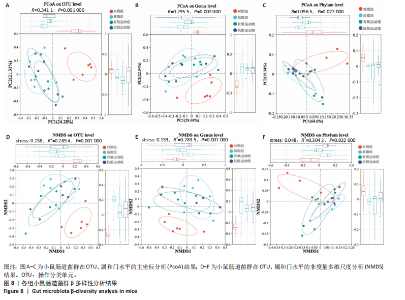
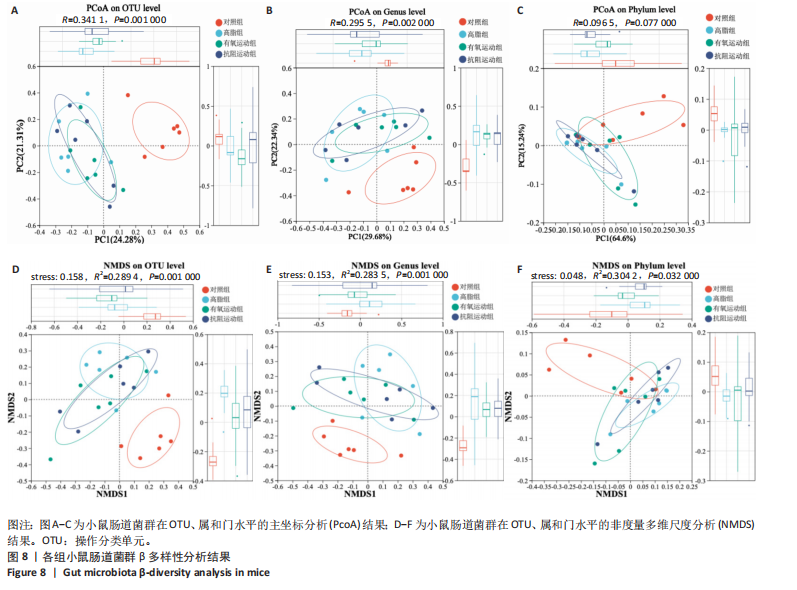
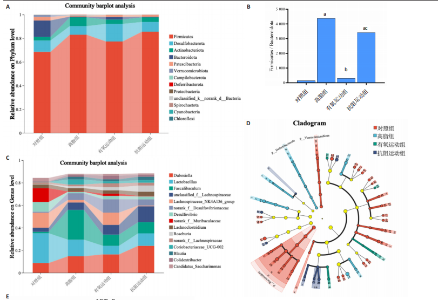
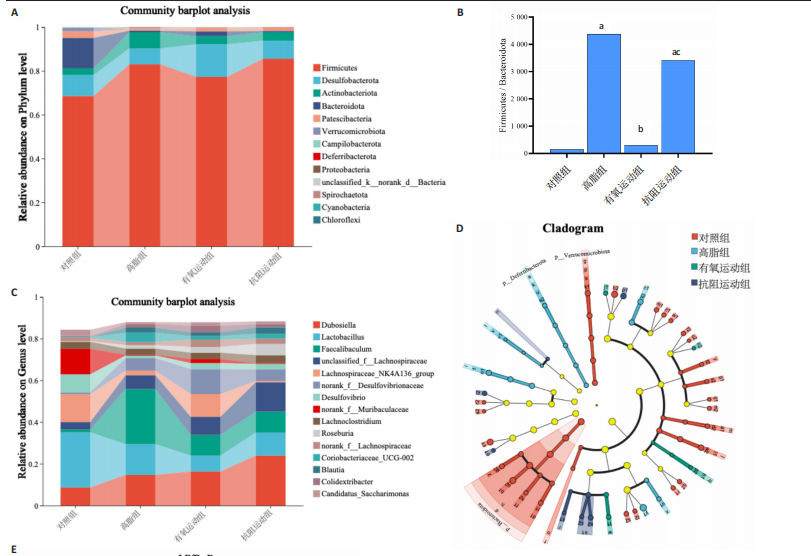
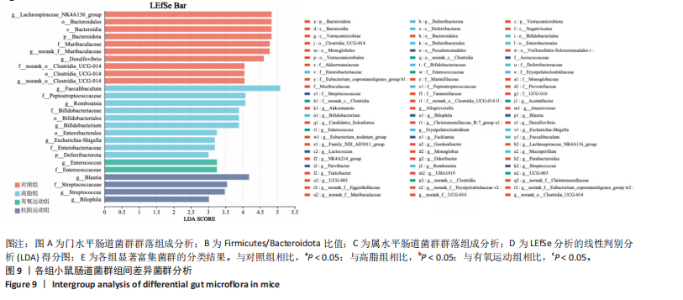
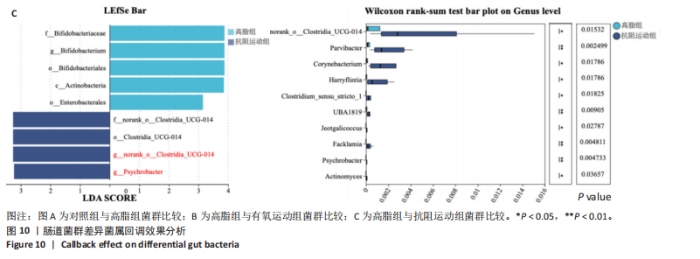
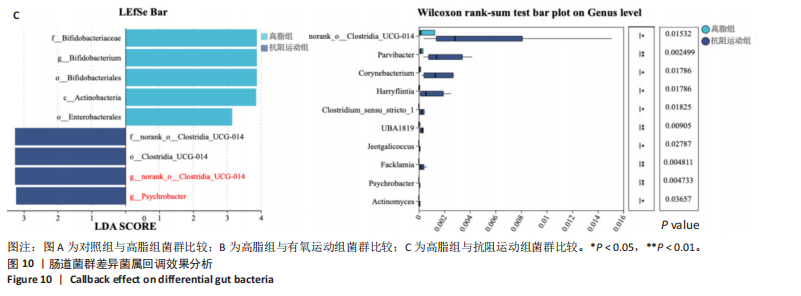
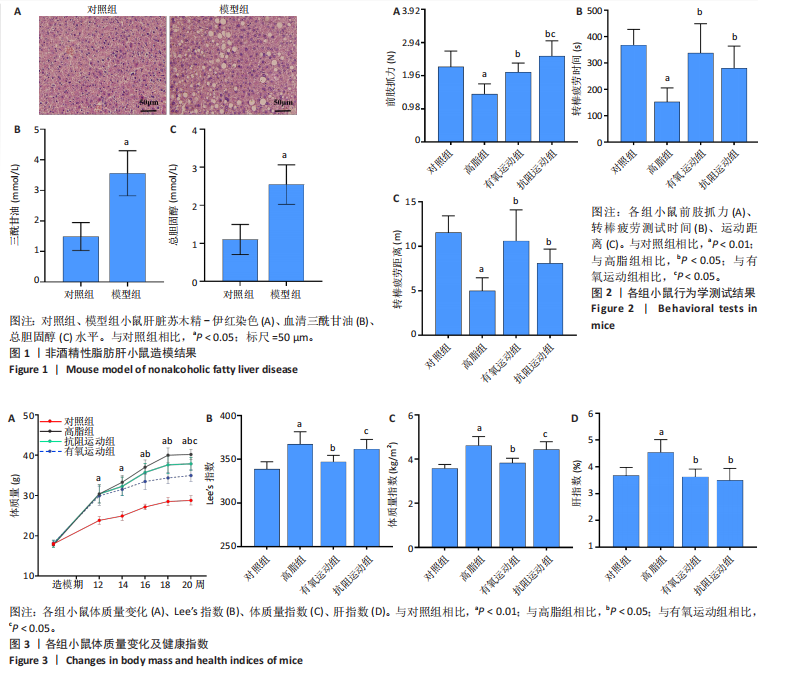
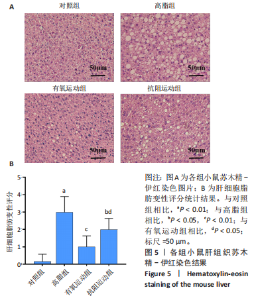
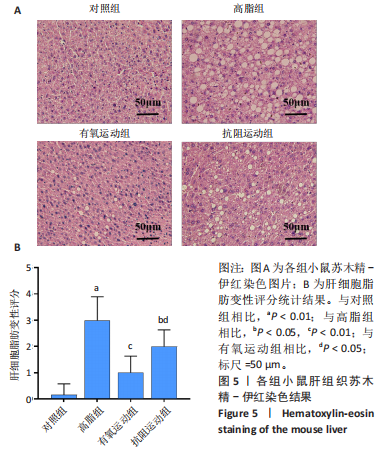
2.1 实验动物数量分析 初始时共购入48只小鼠,第12周造模后将对照组和造模组分别处死2只,运动过程中剔除3只无法完成训练小鼠,其中有氧运动组1只,抗阻运动组2只,余41只纳入结果分析,其中对照组10只、高脂组10只、有氧运动组11只、抗阻运动组10只。 2.2 非酒精性脂肪肝小鼠造模结果 造模期间各组小鼠饮食、活跃度等身体状态良好。如图1所示,高脂饲养12周后,苏木精-伊红染色显示模型组肝脏出现大量空泡、细胞排列紊乱;模型组小鼠血清三酰甘油(P < 0.05)、总胆固醇(P < 0.05)水平显著高于对照组,表明模型组小鼠出现明显肝脏脂肪变性,非酒精性脂肪肝模型构建成功。 2.3 行为学实验结果 如图2所示,与对照组相比,高脂组转棒疲劳时间、运动距离显著降低(P < 0.01);与高脂组相比,有氧运动组和抗阻运动组转棒疲劳时间、运动距离均显著增加(P < 0.05)。与对照组相比,高脂组前肢抓力显著降低(P < 0.01);与高脂组相比,有氧运动组和抗阻运动组前肢抓力均显著增加(P < 0.05);与有氧运动组相比,抗阻运动组前肢抓力显著增加(P < 0.05)。 2.4 小鼠体质量、Lee’s指数、体质量指数、肝指数 如图3A所示,与高脂组相比,有氧运动组第16周开始出现体质量显著降低(P < 0.05),抗阻运动组第20周体质量显著降低(P < 0.05),实验结果表明有氧运动比抗阻运动减脂效果更具优势。如图3B,C所示,与对照组相比,高脂组Lee’s指数、体质量指数显著升高(P < 0.01);与高脂组相比,有氧运动组Lee’s指数、体质量指数显著降低(P < 0.05),抗阻运动组Lee’s指数、体质量指数相对下调但无显著性差异(P > 0.05);与有氧运动组相比,抗阻运动组Lee’s指数、体质量指数显著升高(P < 0.05)。如图3D所示,与对照组相比,高脂组肝指数升高(P < 0.01);与高脂组相比,有氧运动组和抗阻运动组肝指数显著降低(P < 0.05)。 2.5 各组小鼠血清生化指标检测结果 如图4所示,与对照组相比,高脂组血清总胆固醇、三酰甘油、低密度脂白显著增高(P < 0.05),高密度脂蛋白显著降低(P < 0.01);与高脂组相比,有氧运动组和抗阻运动组血清总胆固醇、三酰甘油、低密度脂蛋白显著降低(P < 0.05),高密度脂蛋白显著升高(P < 0.05)。与对照组相比,高脂组血清丙氨酸氨基转氨酶、天门冬氨酸氨基转移酶活力显著升高(P < 0.01);与高脂组相比,有氧运动组和抗阻运动组血清丙氨酸氨基转氨酶、天门冬氨酸氨基转移酶活力显著降低 (P < 0.05)。与有氧运动组相比,抗阻运动组血清三酰甘油和低密度脂蛋白水平、天门冬氨酸氨基转移酶活力显著升高(P < 0.05),其余指标组间比较无显著差异。 2.6 苏木精-伊红染色结果 如图5所示,对照组肝脏细胞状态完好,形态规整,无空泡和炎症病变;与对照组相比,高脂组肝细胞脂肪变性评分极显著增加(P < 0.01),且出现大量空泡、细胞排列紊乱;与高脂组相比,有氧运动组(P < 0.01)、抗阻运动组 (P < 0.05)空泡数量显著减少,炎性浸润得到明显改善;有氧运动组肝脏改善显著优于抗阻运动组(P < 0.05)。 2.7 各组小鼠肝组织蛋白表达 如图6所示,与对照组相比,高脂组NLRP3、核因子κB、白细胞介素1β、肿瘤坏死因子α蛋白表达量显著上调(P < 0.05),鸢尾素蛋白表达量显著下调(P < 0.01);与高脂组相比,有氧运动组和抗阻运动组NLRP3、核因子κB、白细胞介素1β、肿瘤坏死因子α蛋白表达量显著下调(P < 0.05),鸢尾素蛋白表达量显著上调(P < 0.05)。与有氧运动组相比,抗阻运动组肿瘤坏死因子α蛋白表达量显著上调(P < 0.05),鸢尾素蛋白表达量显著下调(P < 0.05),NLRP3、核因子κB、白细胞介素1β蛋白表达量无显著差异(P > 0.05)。 2.8 肠道菌群分析 2.8.1 α多样性分析 通过对盲肠内容物进行16S rRNA测序,分析了不同运动方式干预对非酒精性脂肪肝小鼠肠道菌群结构和组成的影响。韦恩图结果显示(图7A),在所有样本中共同包含338个操作分类单元,其中对照组、高脂组、有氧运动组和抗阻运动组分别包含467,105,156,99个独特的操作分类单元。基于操作分类单元的Shannon指数和Simpson指数表明随着物种数量的增加,指数曲线逐渐趋于平缓(图7B,C),这表明样本的群落丰富度和多样性良好,且测序深度足以反映小鼠盲肠内容物中绝大多数肠道微生物信息。α多样性分析数据显示,sobs(P=0.04)、chao(P=0.038)和coverage(P=0.042)指数在4个组别间存在显著差异(图7D-F)。与对照组相比,高脂组的sobs和chao指数显著降低(P < 0.05),而coverage指数则显著增加(P < 0.05)。结果表明,非酒精性脂肪肝小鼠肠道菌群的多样性在各个组别中存在差异。 2.8.2 β多样性分析 通过主坐标分析和非度量多维尺度分析(non-metric multidimensional scaling,NMDS)评估了各组肠道菌群的β多样性,以分析其组成的相似性和差异性。主坐标分析结果表明(图8A-C),在操作分类单元水平(R=0.341 1,P=0.001)和属水平(R=0.295 5,P=0.002),对照组独立成簇,与其他组别显著分离,而其余3组的样本相互聚集。在门水平(R=0.096 5,P=0.077),所有组别均相互聚集,无法分离。NMDS分析结果(图8D-F)显示,在操作分类单元水平(R2=0.289 4,P=0.001)和属水平(R2=0.283 5,P=0.001),对照组同样独立成簇,与其他组别显著分离;而在门水平(R2=0.304 2,P=0.032),所有组别再次相互聚集,无法分离。结果表明,对照组的肠道菌群组成与其他组别存在显著差异,而高脂组、有氧运动组和抗阻运动组的肠道菌群组成多样性则较为相似。 2.8.3 组间差异菌群分析 群落分析(图9A-C)显示,在门水平上,Firmicutes、Desulfobacterota、Actinobacteriota、Bacteroidota和Proteobacteria是4个组别中的优势菌群。与对照组相比,高脂组和抗阻运动组中Firmicutes和Actinobacteriota的相对丰度增加,而Bacteroidota的相对丰度降低;有氧运动组则与高脂组和抗阻运动组相反。Firmicutes/Bacteroidota(F/B)比值是脂代谢紊乱的一个重要指标,结果显示,与对照组相比,高脂组和抗阻运动组的F/B比值显著增加(P < 0.05),而有氧运动组的F/B比值则显著降低(P < 0.05)。在属水平上,Dubosiella、Lactobacillus、Faecalibaculum、unclassified_f__Lachnospiraceae 和Lachnospiraceae_NK4A136_group在4个组别中为优势菌属。利用线性判别分析效应量(LEfSe)分析从门到属水平丰度显著变化的物种(图9D),设定LDA值> 3,在4个组别中筛选出各个组别中显著富集的菌群(图9E),结果显示:对照组显著富"
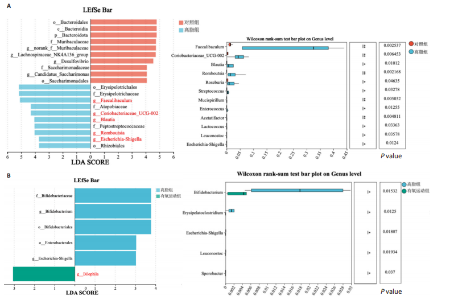
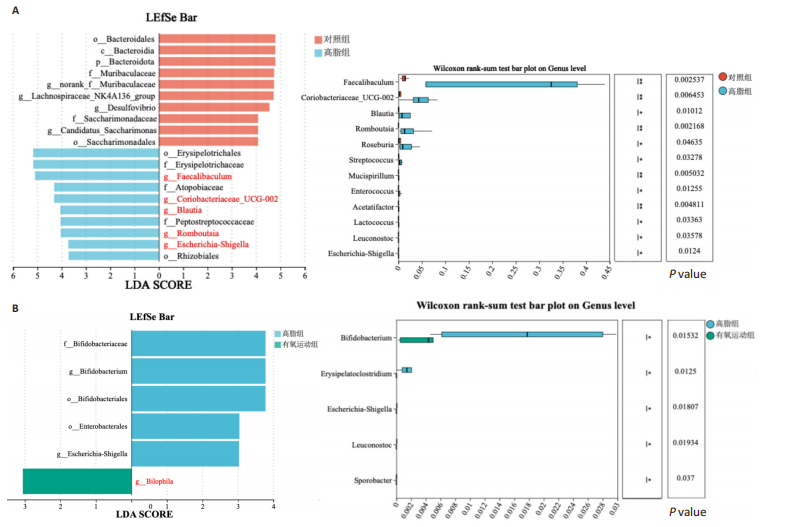
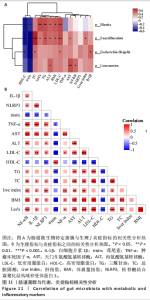
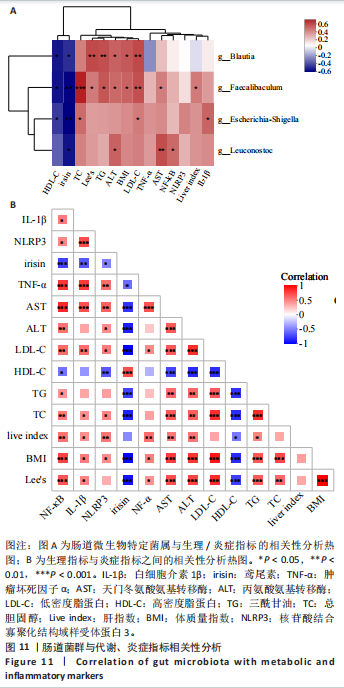
集g__Lachnospiraceae_NK4A136_group (LDA=4.83,P=0.023)、o__Bacteroidales(LDA=4.83,P=0.039)、c__Bacteroidia (LDA=4.83,P=0.039)、p__Bacteroidota(LDA=4.831,P=0.039)、f__Muribaculaceae (LDA=4.77,P=0.024)、g__norank_f__Muribaculaceae(LDA=4.77,P=0.024)等菌群。高脂组显著富集g__Faecalibaculum(LDA=5.08,P=0.024)、f__Peptostreptococcaceae(LDA=4.07,P=0.005)、g__Romboutsia(LDA=4.77,P=0.024)、f__Bifidobacteriaceae(LDA=3.88,P=0.031)、o__Enterobacterales(LDA=3.25,P=0.022)、g__Escherichia-Shigella(LDA=3.19,P=0.033)等菌群。有氧运动组显著富集g__Enterococcus(LDA=3.25,P=0.014)和f__Enterococcaceae(LDA=3.25,P=0.014)菌群。抗阻运动组显著富集g__Blautia(LDA=4.17,P=0.01)、f__Streptococcaceae(LDA=3.54,P=0.041)、g__Streptococcus(LDA=3.47,P=0.037)和g__Bilophila(LDA=3.01,P=0.025)菌群。 以LDA值> 3进行两组比较分析,筛选出相较于对照组而言高脂组显著富集的菌属,相较于高脂组而言有氧运动组和抗阻运动组中显著富集的菌属,并采用Wilcoxon秩和检验对上述筛选出的富集菌属进行组间差异分析,进一步探究不同方式的运动干预对高脂组vs.对照组中显著差异的菌属是否具有回调效果。 图10结果显示,与对照组相比,高脂组g__Faecalibaculun(P=0.002 5)、Coriobacteriaceae_UCG-002(P=0.006)、g__Blautia (P=0.01)、g__Romboutsia(P=0.002)和g__Escherichia- Shigella(P=0.012)的相对丰度均显著增加。与高脂组相比,有氧运动组中g__Bilophila显著富集,且g__Bifidobacterium(P=0.015)、g__Erysipelatoclostridium (P=0.012)、g__Escherichia-Shigella (P=0.018)、g__Leuconostoc(P=0.019)和g__Sporobacter(P=0.037)的相对丰度显著降低,其中g__Escherichia-Shigella和g__Leuconostoc为回调的差异菌属(高脂组vs.对照组)。与高脂组相比,抗阻运动组显著富集的菌属g__norank_o__Clostridia_UCG-014(P=0.015)和g__Psychrobacter(P=0.002)的相对丰度均显著降低,抗阻运动组中未发现回调的差异菌属。 2.9 肠道菌群与生理、炎症指标相关性分析 将上述显著富集和回调的核心菌属与生理、炎症相关的指标进行相关性分析(图11A),结果发现:g__Escherichia-Shigella与总胆固醇 (r=0.458 8,P=0.024 1)、低密度脂蛋白(r=0.413 6,P=0.044 6)及白细胞介素1β(r=0.476 8,P=0.018 5)呈正相关,与高密度脂蛋白(r=-0.406 0,P=0.049 0)及鸢尾素(r= -0.541 5,P=0.006 3)呈负相关;g__Faecalibaculum与总胆固醇(r=0.655 8,P=0.000 5)、三酰甘油(r= 0.514 6,P=0.010 1)、低密度脂蛋白(r=0.531 3,P=0.007 5)及丙氨酸氨基转氨酶(r=0.462 6,P=0.022 8)、天门冬氨酸氨基转移酶(r=0.407 0,P=0.048 4)呈正相关,与高密度脂蛋白(r=-0.500 9,P=0.012 7)及鸢尾素(r=-0.533 9,P=0.007 2)呈负相关;g__Leuconostoc 与总胆固醇(r=0.397 9,P=0.054 1)、低密度脂蛋白(r=0.403 1,P=0.050 8)及丙氨酸氨基转氨酶(r=0.484 2,P=0.016 5)、天门冬氨酸氨基转移酶(r=0.538 3,P=0.006 7)、核因子κB(r=0.462 7,P=0.022 8 )呈正相关,与鸢尾素(r=-0.498 7,P=0.013 1)呈负相关;g__Blautia 与 Lee’s 指数(r=0.549 6,P=0.005 4)、体质量指数(r=0.508 7,P=0.011 1)、三酰甘油(r=0.532 8,P=0.007 3)、低密度脂蛋白 (r=0.548 7,P=0.005 5)及丙氨酸氨基转氨酶(r=0.486 1,P=0.016 0)呈正相关,与高密度脂蛋白(r=-0.490 4,P=0.015 0)及鸢尾素(r=-0.436 5,P= 0.033 0)呈负相关。 此外,代谢指标与炎症指标存在显著关联,相关性分析结果显示(图11B),肿瘤坏死因子α与体质量指数(r=0.503,P=0.013)、低密度脂蛋白(r=0.465,P=0.023)及天门冬氨酸氨基转移酶(r=0.663,P < 0.001)呈正相关;白细胞介素1β与体质量指数(r=0.516,P=0.011)、低密度脂蛋白(r=0.576,P=0.004)、天门冬氨酸氨基转移酶(r=0.670,P < 0.001)及肿瘤坏死因子α(r=0.754,P < 0.001)均呈强正相关;NLRP3炎症小体则与体质量指数(r=0.450,P=0.029)、总胆固醇(r=0.477,P=0.019)及天门冬氨酸氨基转移酶(r=0.608,P=0.002)呈显著正相关,核因子κB不仅与肿瘤坏死因子α(r=0.774,P < 0.001)和白细胞介素1β(r=0.516,P=0.011)呈正相关,还与肝损伤指标天门冬氨酸氨基转移酶(r=0.813,P < 0.001)、体质量指数(r=0.721,P < 0.001)及低密度脂蛋白(r=0.573,P=0.004)呈强正相关。鸢尾素与血脂、肥胖指标及肝损伤指标呈极显著负相关(P < 0.001),与核因子κB(r=-0.660,P < 0.001)、肿瘤坏死因子α (r=-0.506,P=0.013)等促炎因子呈显著负相关。"
| [1] WANG S, YIN J, LIU Z, et al. Metabolic disorders, inter-organ crosstalk, and inflammation in the progression of metabolic dysfunction-associated steatotic liver disease. Life Sci. 2024;359:123211. [2] GU Y, GUO C, LIU Z, et al. The trend in incidence of non-alcoholic fatty liver disease and its impact on cirrhosis and liver cancer: An analysis from Global Burden of Disease 2021. Public Health. 2025;242:79-86. [3] KAN C, ZHANG K, WANG Y, et al. Global burden and future trends of metabolic dysfunction-associated Steatotic liver disease: 1990-2021 to 2045. Ann Hepatol. 2025;30(2):101898. [4] WAYAL V, WANG SD, HSIEH CC. Novel bioactive peptides alleviate Western diet-induced MAFLD in C57BL/6J mice by inhibiting NLRP3 inflammasome activation and pyroptosis via TLR4/NF-κB and Keap1/Nrf2/HO-1 signaling pathways. Int Immunopharmacol. 2025;148: 114177. [5] ZHOU M, LV J, CHEN X, et al. From gut to liver: Exploring the crosstalk between gut-liver axis and oxidative stress in metabolic dysfunction-associated steatotic liver disease. Ann Hepatol. 2025;30(1):101777. [6] GERBER L, OTGONSUREN M, MISHRA A, et al. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population-based study. Aliment Pharmacol Ther. 2012;36(8):772-781. [7] CHEN M, ZHU JY, MU WJ, et al. Cdo1-Camkk2-AMPK axis confers the protective effects of exercise against NAFLD in mice. Nat Commun. 2023;14(1):8391. [8] 雷森林,李先辉,伍方佳.脂噬在非酒精性脂肪性肝病中的作用及运动调节机制的研究进展[J].中国病理生理杂志,2025,41(1):165-172. [9] FARZANEGI P, DANA A, EBRAHIMPOOR Z, et al. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur J Sport Sci. 2019;19(7):994-1003. [10] YE X, SUN P, LAO S, et al. Fgf21-Dubosiella axis mediates the protective effects of exercise against NAFLD development. Life Sci. 2023;334: 122231. [11] GU X, YUAN L, GAN L, et al. Understanding the Role of Exercise and Probiotic Interventions on Non-Alcoholic Fatty Liver Disease Alleviation in Zebrafish: Dialogue Between the Gut and Liver. Int J Mol Sci. 2025; 26(3):1360. [12] KOVYNEV A, YING Z, ZHANG S, et al. Timing Matters: Late, but Not Early, Exercise Training Ameliorates MASLD in Part by Modulating the Gut-Liver Axis in Mice. J Pineal Res. 2024;76(8):e70003. [13] DE CÓL JP, DE LIMA EP, POMPEU FM, et al. Underlying Mechanisms behind the Brain-Gut-Liver Axis and Metabolic-Associated Fatty Liver Disease (MAFLD): An Update. Int J Mol Sci. 2024;25(7):3694. [14] ZHU W, SAHAR NE, JAVAID HMA, et al. Exercise-Induced Irisin Decreases Inflammation and Improves NAFLD by Competitive Binding with MD2. Cells. 2021;10(12):3306. [15] HUANGFU LX, CAI XT, YANG JN, et al. Irisin attenuates inflammation in a mouse model of ulcerative colitis by altering the intestinal microbiota. Exp Ther Med. 2021;22(6):1433. [16] 王佳倩,蒋昌君,彭毅,等.有氧运动对CNPY2基因调控AKT/GSK3β通路改善非酒精性脂肪肝的作用研究[J].中国组织工程研究,2025,29(30):6441-6448. [17] REYNOLDS JC, LEE C. Mouse Fitness as Determined Through Treadmill Running and Walking. Methods Mol Biol. 2020;2144:57-65. [18] HORNBERGER TA JR, FARRAR RP. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol. 2004;29(1):16-31. [19] ZHAO Q, JING Y, JIANG X, et al. SIRT5 safeguards against primate skeletal muscle ageing via desuccinylation of TBK1. Nat Metab. 2025; 7(3):556-573. [20] 颜惠敏,吕欣晨,宋雯燕,等.姜黄素调控肠道菌群及其代谢物提高小鼠运动表现的研究[J].食品与发酵工业,2025,51(8):37-44. [21] 张树玲,李军汉,王佳倩,等.有氧运动干预非酒精性脂肪肝小鼠肝脏JAK2/STAT5信号通路的变化[J].中国组织工程研究,2022, 26(17):2690-2695. [22] WEI S, WANG L, EVANS PC, et al. NAFLD and NASH: etiology, targets and emerging therapies. Drug Discov Today. 2024;29(3):103910. [23] LIN X, QU J, YIN L, et al. Aerobic exercise-induced decrease of chemerin improved glucose and lipid metabolism and fatty liver of diabetes mice through key metabolism enzymes and proteins. Biochim Biophys Acta Mol Cell Biol Lipids. 2023;1868(12):159409. [24] DINIZ TA, DE LIMA JUNIOR EA, TEIXEIRA AA, et al. Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-α signaling in obese mice. Life Sci. 2021;266:118868. [25] FANG C, LIU S, YANG W, et al. Exercise ameliorates lipid droplet metabolism disorder by the PLIN2-LIPA axis-mediated lipophagy in mouse model of non-alcoholic fatty liver disease. Biochim Biophys Acta Mol Basis Dis. 2024;1870(3):167045. [26] SANTOS JDM, SILVA JFT, ALVES EDS, et al. Strength Training Protects High-Fat-Fed Ovariectomized Mice against Insulin Resistance and Hepatic Steatosis. Int J Mol Sci. 2024;25(10):5066. [27] ARRESE M, CABRERA D, KALERGIS AM, et al. Innate Immunity and Inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61(5):1294-1303. [28] FREDRICKSON G, BARROW F, DIETSCHE K, et al. Exercise of high intensity ameliorates hepatic inflammation and the progression of NASH. Mol Metab. 2021;53:101270. [29] YANG S, XU B, HAN Y, et al. TAF15 exacerbates nonalcoholic steatohepatitis progression by regulating lipid metabolism and inflammation via FASN and p65 NF-κB. Liver Int. 2023;43(9):1920-1936. [30] HE M, CHIANG HH, LUO H, et al. An Acetylation Switch of the NLRP3 Inflammasome Regulates Aging-Associated Chronic Inflammation and Insulin Resistance. Cell Metab. 2020;31(3):580-591.e5. [31] LI H, SHEN J, WU T, et al. Irisin Is Controlled by Farnesoid X Receptor and Regulates Cholesterol Homeostasis. Front Pharmacol. 2019;10:548. [32] XIN C, LIU J, ZHANG J, et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int J Obes (Lond). 2016;40(3):443-451. [33] LIU Y, JIN Y, LI J, et al. Small Bowel Transit and Altered Gut Microbiota in Patients With Liver Cirrhosis. Front Physiol. 2018;9:470. [34] XU H, FANG F, WU K, et al. Gut microbiota-bile acid crosstalk regulates murine lipid metabolism via the intestinal FXR-FGF19 axis in diet-induced humanized dyslipidemia. Microbiome. 2023;11(1):262. [35] HAN H, ZHANG S, WANG M, et al. Retinol metabolism signaling participates in microbiota-regulated fat deposition in obese mice. J Nutr Biochem. 2025;136:109787. [36] ZHAO J, BAI M, NING X, et al. Expansion of Escherichia-Shigella in Gut Is Associated with the Onset and Response to Immunosuppressive Therapy of IgA Nephropathy. J Am Soc Nephrol. 2022;33(12):2276-2292. [37] SHANG X, CHE X, MA K, et al. Chronic Cr(VI) exposure-induced biotoxicity involved in liver microbiota-gut axis disruption in Phoxinus lagowskii Dybowski based on multi-omics technologies. Environ Pollut. 2025;368:125759. [38] LIU X, MAO B, GU J, et al. Blautia-a new functional genus with potential probiotic properties? Gut Microbes. 2021;13(1):1-21. [39] SHIBATA M, OZATO N, TSUDA H, et al. Mouse Model of Anti-Obesity Effects of Blautia hansenii on Diet-Induced Obesity. Curr Issues Mol Biol. 2023;45(9):7147-7160. [40] ZHAO T, ZHAN L, ZHOU W, et al. The Effects of Erchen Decoction on Gut Microbiota and Lipid Metabolism Disorders in Zucker Diabetic Fatty Rats. Front Pharmacol. 2021;12:647529. |
| [1] | Lyu Guoqing, Aizimaitijiang·Rouzi, Xiong Daohai. Irisin inhibits ferroptosis in human articular chondrocytes: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1359-1367. |
| [2] | Liu Yu, Lei Senlin, Zhou Jintao, Liu Hui, Li Xianhui. Mechanisms by which aerobic and resistance exercises improve obesity-related cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1171-1183. |
| [3] | Chen Qiang, Wu Wenjuan, Jiang Shuhua, Huang Da. Physical exercise improves physical function in burn patients: a systematic review and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1269-1281. |
| [4] | Gu Fucheng, Yang Meixin, Wu Weixin, Cai Weijun, Qin Yangyi, Sun Mingyi, Sun Jian, Geng Qiudong, Li Nan. Effects of Guilu Erxian Glue on gut microbiota in rats with knee osteoarthritis: machine learning and 16S rDNA analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1058-1072. |
| [5] | Sun Long, Wu Haiyang, Tong Linjian, Liu Rui, Yang Weiguang, Xiao Jian, Liu Lice, Sun Zhiming. Regulatory mechanism of leptin in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(12): 3100-3108. |
| [6] | Hu Yujie, Xie Ping, , Lu Weijie, Yang Kang, Deng Yaoting, Liu Mengyang. Meta-analysis of the clinical efficacy of high-intensity interval exercise and middle-intensity continuous training in patients with coronary heart disease [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2584-2593. |
| [7] | Qiu Xueli, Cui Hao, Wu Chenyang, Tao Lide, Yao Yuqian, Tian Bo, Bai Jinyu, Zhang Yingzi. Gut microbiota tryptophan metabolite indole-3-propionic acid alleviates inflammatory bowel disease-related osteoporosis in a mouse model [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2413-2421. |
| [8] | Zhao Xiaoxuan, Liu Shuaiyi, Li Qi, Xing Zheng, Li Qingwen, Chu Xiaolei. Different exercise modalities promote functional recovery after peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1248-1256. |
| [9] |
Zhao Wensheng, Li Xiaolin, Peng Changhua, Deng Jia, Sheng Hao, Chen Hongwei, Zhang Chaoju, He Chuan.
Gut microbiota and osteoporotic fractures #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1296-1304.
|
| [10] |
Sun Guanghan, Xie Zhencong, Sun Mi, Xu Yang, Guo Dong.
Therapeutic effect and mechanism by which Trichosanthis Fructus-Allii Macrostemonis Bulbus regulates gut microbiota in a rat model of coronary heart disease #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 917-927.
|
| [11] | Ji Long, Chen Ziyang, , Jin Pan, Kong Xiangkui, Pu Rui, . Lipophagy, exercise intervention and prevention and treatment of nonalcoholic fatty liver disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7611-7619. |
| [12] | Wang Tao, Wang Shunpu, Min Youjiang, Wang Min, Li Le, Zhang Chen, Xiao Weiping. Causal relationship between gut microbiota and rheumatoid arthritis: data analysis in European populations based on GWAS data [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7663-7668. |
| [13] | Wang Jiaqian, , Jiang Changjun, Peng Yi, Ma Mi, Li Junhan. Study on the role of aerobic exercise in regulating the CNPY2-mediated AKT/GSK3β pathway for improving non-alcoholic fatty liver [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6441-6448. |
| [14] | Cui Yuena, Chen Xiaoyu, Liang Meiting, Chen Wujin, He Yi, Dilinur·Ekpa, Du Manxi, Zhu Yuqiu, Abuduwupuer·Haibier, Sun Yuping. Differences of calorie restriction and time-restricted feeding on metabolic indices and gut microbiota of mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6449-6456. |
| [15] | Zeng Hao, Zou Shunyi, Li Zhengpeng, Chai Yuan, Huang Yourong, Zhang Xiaoyun. Intestinal flora regulates bone metabolism: a visual analysis of literature from the Web of Science Core Collection [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5652-5661. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||