Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (12): 3100-3108.doi: 10.12307/2026.736
Previous Articles Next Articles
Regulatory mechanism of leptin in bone metabolism
Sun Long1, Wu Haiyang2, Tong Linjian3, Liu Rui3, Yang Weiguang4, Xiao Jian5, Liu Lice6, Sun Zhiming7
- 1Class 2024 MS Candidate, 5Class 2022 MS Candidate, 6Class 2023 MS Candidate, Tianjin Medical University, Tianjin 300170, China; 2Department of Orthopedics, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450047, Henan Province, China; 3Tianjin Huanhu Hospital, Affiliated Hospital of Tianjin Medical University, Tianjin 300350, China; 4Tianjin Medical University General Hospital, Tianjin 300052, China; 7Tianjin Third Central Hospital/Tianjin University Central Hospital, Tianjin 300170, China
-
Received:2025-07-09Accepted:2025-08-30Online:2026-04-28Published:2025-09-30 -
Contact:Sun Zhiming, Chief physician, Professor, Doctoral supervisor, Tianjin Third Central Hospital/Tianjin University Central Hospital, Tianjin 300170, China -
About author:Sun Long, MS candidate, Class 2024 MS Candidate, Tianjin Medical University, Tianjin 300170, China -
Supported by:Tianjin Natural Science Foundation (General Program), No. 23JCYBJC01740 (to SZM)
CLC Number:
Cite this article
Sun Long, Wu Haiyang, Tong Linjian, Liu Rui, Yang Weiguang, Xiao Jian, Liu Lice, Sun Zhiming. Regulatory mechanism of leptin in bone metabolism[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(12): 3100-3108.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
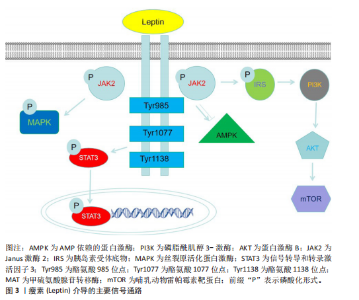
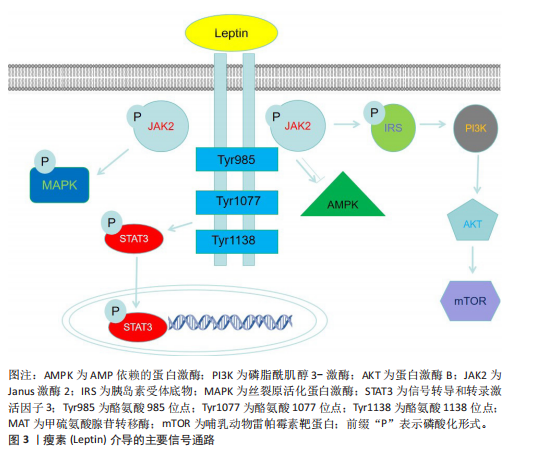
性的形态[16]。除白色脂肪组织外,瘦素还存在于其他多种组织中,如乳腺、卵巢、胎盘、垂体、骨骼肌、胃、淋巴组织、间充质干细胞及骨组织[17],但白色脂肪细胞是瘦素最主要的分泌来源。血清瘦素水平与体脂含量呈正相关关系,因此瘦素主要反映了机体脂肪组织中的能量储备状况[18]。瘦素表达和循环浓度不仅呈现昼夜节律性波动,还会随营养状态的改变而变化。瘦素分子的氨基末端含有一段由21个氨基酸残基组成的信号肽序列,负责引导前体分子进入分泌途径[19],分泌入血清的成熟瘦素分子则是该信号肽被切除后形成的由146个氨基酸残基构成的肽链[20]。 瘦素的合成与分泌受控于复杂的内分泌、神经内分泌及旁分泌信号网络。总体而言,瘦素的分泌水平与体质量及营养状态呈正相关,例如,饥饿状态下血清瘦素水平显著下降,这是机体对能量缺乏状态的适应性生理反应。此外,皮下脂肪组织相较于内脏脂肪组织具有更高的瘦素分泌能力[21]。瘦素的分泌受到多种生理因素的调节,这些因素包括体脂分布、性别、年龄、青春期阶段、禁食、进食状态以及运动等,同时还受多种激素的影响。胰岛素是瘦素生成的关键调节因子,长期高胰岛素血症可导致血浆瘦素浓度升高,而短期高胰岛素血症对血浆瘦素浓度则无明显影响。在人体研究中,胰岛素输注可增加血浆瘦素浓度;相反,儿茶酚胺则通过结合β2-肾上腺素能受体和β3-肾上腺素能受体来抑制瘦素合成;此外,皮质类固醇和肿瘤坏死因子α能刺激瘦素合成,而甲状腺激素对瘦素可能起抑制作用[21]。 在骨代谢领域,瘦素与脂联素形成了复杂的相互作用网络,二者通过协同与拮抗作用共同维持骨稳态。瘦素具有中枢抑制与外周促进的双重调控特性:瘦素通过下丘脑-交感神经轴间接抑制骨形成,同时又可激活成骨细胞内的Janus激酶2/信号传导和转录激活蛋白3信号通路直接促进骨矿化。脂联素通过其受体激活AMP依赖的蛋白激酶通路,进而刺激成骨分化、抑制炎症因子介导的骨破坏。在骨髓微环境中,这两种脂肪因子保持动态平衡:瘦素促进骨髓间充质干细胞向成骨细胞分化,脂联素抑制骨髓间充质干细胞向脂肪细胞分化,共同调控着成骨与成脂分化的相对比例[22]。 大量研究表明,瘦素在肥胖、免疫、生殖、循环、癌症发生发展、牙周炎、骨生成及阻塞性睡眠呼吸暂停等过程中发挥着重要的生理与病理作用[23-28]。 2.2.2 瘦素的受体及信号通路 瘦素受体是Ⅰ类细胞因子受体家族成员,广泛表达于中枢神经系统及外周组织。根据胞内结构域的长度和氨基酸序列差异,瘦素受体主要分为长型、短型和可溶3种亚型。目前已鉴定的瘦素受体异构体a、瘦素受体异构体b、瘦素受体异构体c、瘦素受体异构体d、瘦素受体异构体e和瘦素受体异构体f 6种异构体均由db基因通过转录后可变剪接产生[16]。所有异构体均含有配体结合的共有胞外结构域,但羧基末端的胞内结构域则显著不同,其中瘦素受体异构体e因独特的缺乏跨膜结构域(即仅含胞外域)成为可溶性异构体,能够结合循环中的瘦素并阻碍它向中枢转运,其余5种异构体具有完全一致的胞外域和跨膜域。 在具有完整结构的受体中,瘦素受体异构体b因胞内区包含全部功能性结构域而最为关键,瘦素与瘦素受体异构体b结合后可启动下游多重信号传导。瘦素受体自身无酶活性,但可结合并激活胞质酪氨酸激酶Janus激酶2。瘦素结合诱导受体构象改变,触发Janus激酶2分子间的反式自磷酸化,进而激活包括丝裂原活化蛋白激酶、Janus激酶/信号传导和转录激活蛋白、磷脂酰肌醇3-激酶-蛋白激酶B、AMP依赖的蛋白激酶和哺乳动物雷帕霉素靶蛋白在内的多条信号级联通路。最新研究发现,瘦素受体异构体b的乙酰化修饰能增强瘦素信号敏感性;药理学抑制组蛋白去乙酰酶6可显著逆转饮食诱导的肥胖,但具体机制尚未完全阐明,推测可能涉及脂肪源性的循环因子或中枢直接调控瘦素受体异构体b乙酰化状态[29]。 瘦素受体作为跨系统调控的核心分子,它的功能已超越经典的代谢调控,比如通过Janus激酶 2/信号传导和转录激活蛋白 3、磷脂酰肌醇3-激酶/蛋白激酶B/哺乳动物雷帕霉素靶蛋白通路调控能量平衡及乳腺脂质合成,现已拓展至骨骼重塑、神经免疫及病理适应等多个领域[30]。近期研究揭示血管生成素样蛋白4可作为瘦素受体的第二配体,在应激状态下通过结合骨髓间充质细胞上的瘦素受体驱动异位骨化,这解释了瘦素受体突变小鼠与瘦素缺乏小鼠之间的表型差异[7]。在骨稳态维持中,瘦素受体通过信号转导和转录激活因子3非依赖性途径支持皮质骨矿化,瘦素功能缺失会加剧骨孔隙率及低密度骨比例[31]。同时,骨髓基质细胞表达的瘦素受体与交感神经形成双向交互,骨髓基质细胞分泌神经生长因子维持神经存活,而交感神经通过β-肾上腺素能信号反馈促进再生因子释放,共同加速骨髓修复[32]。在脂肪组织内,存在于交感神经周围的屏障细胞通过分泌白细胞介素33调控棕色脂肪组织的免疫微环境,对维持脂肪组织产热功能及代谢稳态至关重要;屏障功能失调可导致局部炎症加剧、瘦素抵抗及肥胖易感性增加[33]。此外,可溶性瘦素受体通过动态调节循环瘦素生物利用度参与代谢性疾病进程[34]。这些突破性进展不仅重塑了瘦素受体作为“多配体-多通路”信号整合中心的生物学意义,也为肥胖、异位骨化及代谢性骨病等疾病的跨学科治疗策略开辟了新途径。瘦素介导的主要信号通路见图3。 2.3 瘦素对骨相关细胞的直接作用 2.3.1 瘦素受体在骨相关细胞中的表达 作为连接能量代谢与骨重塑的关键信号分子,瘦素及其受体在骨组织微环境中的特异性表达与功能调控,构成了骨骼系统动态平衡的核心枢纽。近年研究证实,瘦素受体广泛表达于骨髓间充质干细胞、成骨细胞、破骨细胞及软骨细胞等骨相关细胞中[35-37],通过激活Janus激酶 2/信号传导和转录激活蛋白、丝裂原活化蛋白激酶、核因子κB受体活化因子配体/骨保护素等多条信号通路构成的调控网络,协调骨形成与骨吸收的动态平衡。在免疫调节方面,瘦素促进NK细胞、巨噬细胞的活化和促炎性细胞因子的分泌,同时通过抑制调节性T细胞的功能、促进辅助性T细胞1或辅助性T细胞17分化来增强适应性免疫反应。在关节疾病如类风湿关节炎中,瘦素水平升高与疾病活动度相关,它能刺激滑膜成纤维细胞产生白细胞介素6、白细胞介素8等炎症递质,并且瘦素缺陷小鼠的关节炎程度减轻[38]。在能量缺乏状态下,例如先天性瘦素缺乏或者脂肪营养不良,瘦素水平降低会导致骨代谢异常,而瘦素替代治疗可显著改善这种症状,例如,瘦素通过直接作用于成骨细胞和间接调节激素通路促进骨形成,在女性运动员中可恢复月经周期并提高骨密度。然而,在肥胖等高瘦素血症状态下,瘦素抵抗限制了其治疗效果[39]。下面将系统性阐述瘦素受体在骨相关细胞中的表达。 2.3.2 瘦素对骨髓间充质干细胞的作用 人类骨髓间充质干细胞中存在高亲和力的瘦素受体,瘦素可以促进骨髓间充质干细胞增殖并向成骨细胞谱系分化,同时抑制骨髓间充质干细胞向脂肪细胞分化[2]。瘦素受体阳性骨髓间充质干细胞是骨髓微环境中的关键组成成分,不仅是成骨细胞和骨髓脂肪细胞的主要来源,还通过分泌干细胞因子、基质细胞衍生因子1等"

细胞因子维持造血稳态。瘦素受体阳性骨髓间充质干细胞具有显著的异质性,部分亚群高表达干细胞标志物,而其他亚群则偏向成骨或成脂分化,比如在骨髓损伤时,AdipoQ+亚群能快速增殖并分化为脂肪细胞或成骨细胞参与组织修复[40]。瘦素通过抑制磷脂酰肌醇3-激酶/蛋白激酶B信号通路依赖的伴侣介导自噬,上调骨间充质干细胞中巨蛋白的表达,从而增强25-羟基维生素D3的摄取与代谢,协同促进成骨分化。瘦素以剂量依赖性方式显著提高骨髓间充质干细胞内巨蛋白的mRNA和蛋白水平,通过抑制分子伴侣介导自噬中的关键分子溶酶体相关膜蛋白2A与热休克同源蛋白70的表达减少巨蛋白的溶酶体降解,进而增加细胞对25-羟基维生素D3的内化效率,促进25-羟基维生素D3转化为活性形式1-α,25-二羟基维生素D3。瘦素与25-羟基维生素D3联合作用可协同提升成骨标志基因表达、碱性磷酸酶活性及钙沉积能力,并且该效应可被磷脂酰肌醇3-激酶/蛋白激酶B抑制剂部分阻断,证实该通路通过调控分子伴侣介导自噬高度参与成骨分化进程[5]。 2.3.3 瘦素对成骨细胞的作用 瘦素对成骨细胞谱系细胞具有直接的合成代谢作用。有研究证实,在1-100 ng/mL质量浓度范围内,瘦素可以呈剂量依赖性地增加原代成骨细胞培养中矿化骨结节的形成[37]。瘦素可通过诱导人骨髓基质细胞向成骨分化,表现为碱性磷酸酶、Ⅰ型胶原及骨钙素的mRNA和蛋白水平呈剂量-时间依赖性升高,同时抑制人骨髓基质细胞脂肪转化,以维持骨形成正向调控。体外实验表明,生理浓度(≥10-11 mol/L)的瘦素可显著促进胎儿大鼠原代成骨细胞的增殖和分化,通过胸苷掺入实验发现,瘦素的最大促增殖效果与胰岛素样生长因子1、转化生长因子β、表皮生长因子和胰岛素等其他主要成骨细胞促增殖因子相当。在分子机制上,瘦素通过激活Ap-1基因上调细胞周期蛋白D1,直接刺激成骨细胞增殖[40-42]。在分化调控层面,瘦素通过增强维生素D代谢基因的表达来促进成骨潜能;人髂嵴成骨细胞持续暴露于瘦素后,转化生长因子β、胰岛素样生长因子1、Ⅰ型胶原α链等成骨标志物基因表达上调,伴随胶原合成增加、矿化能力提升及细胞存活率升高。体内实验验证了瘦素的骨合成效应,皮下注射瘦素导致小鼠胫骨中骨和软骨基质蛋白的mRNA水平升高;在骨折修复模型中,瘦素缺陷小鼠的骨痂成熟延迟现象可被局部瘦素治疗逆转,表现为骨痂形成加速但伴随皮质骨变薄的特征[43-46]。通过细胞模型研究发现,瘦素对早期成骨细胞分化的关键基因表达无显著影响,表明瘦素在成骨细胞早期分化阶段的作用较弱,这种阶段特异性作用需要进一步验证[47]。 总之,瘦素对成骨细胞谱系的调控揭示了它在骨代谢中的复杂角色:一方面,通过激活多条信号通路协同上调成骨标志物,直接驱动成骨细胞增殖分化;另一方面,通过抑制脂肪细胞生成来维持骨髓微环境的成骨倾向。 2.3.4 瘦素对破骨细胞的作用 MISCH等[48]研究首次揭示了瘦素在骨吸收中的局部调控作用,证明瘦素能够抑制人外周血单核细胞培养体系中破骨细胞的生成;同时,瘦素能显著增加人外周血单核细胞中骨保护素的mRNA和蛋白表达,提示瘦素抑制破骨细胞生成的作用可能是通过核因子κB受体活化因子配体/核因子κB受体活化因子/骨保护素系统介导的;瘦素能够强烈刺激成骨细胞表达骨保护素。此外,瘦素还可通过嵌合抗原受体T细胞免疫疗法途径抑制破骨细胞的分化并提升骨保护素水平,骨保护素与核因子κB受体活化因子配体结合从而降低破骨细胞活性。瘦素能够明显提高人骨髓基质细胞的骨保护素mRNA稳态水平和蛋白质分泌,同时降低核因子κB受体活化因子配体mRNA水平[48]。在去卵巢诱导骨质疏松的啮齿动物模型中,瘦素可显著减少破骨细胞数量、下调骨源性碱性磷酸酶和抗酒石酸磷酸酶5b活性,显著上调骨形态发生蛋白2表达;瘦素能够减少小鼠骨髓培养中破骨细胞的形成,但对成熟破骨细胞的骨吸收活性无显著影响,这表明瘦素抑制破骨细胞生成,但不影响成熟破骨细胞的功能[49]。瘦素对成熟破骨细胞功能的“无效性”可能源于瘦素受体异构体b在成熟破骨细胞中的表达沉默现象,而此现象与破骨细胞终末分化过程中DNA甲基转移酶3a的特异性激活密切相关。瘦素在破骨细胞分化过程中还表现出显著的促炎作用,瘦素能显著上调破骨细胞中促炎基因肿瘤坏死因子和一氧化氮合酶2的表达水平,表明瘦素可能通过炎症途径调节破骨细胞的功能,然而,瘦素并未显著改变破骨细胞分化标志物的表达或细胞活性,说明瘦素对破骨细胞分化的直接影响有限;而一氧化氮合酶2的上调可能通过负反馈机制抑制骨吸收,从而间接调节骨代谢平衡[47]。 2.3.5 瘦素对软骨细胞的作用 软骨细胞表达瘦素受体,并且瘦素作为软骨代谢的核心调节因子,通过多重机制调控软骨稳态。当瘦素受体异构体b过表达的人软骨细胞暴露于瘦素时,瘦素会激活哺乳动物雷帕霉素靶蛋白通路并加速细胞衰老,而瘦素通路的长期激活可能会降低细胞活力并诱发软骨退变,而瘦素通路的短期激活可能发挥保护作用[50]。瘦素通过刺激赖氨酰氧化酶样蛋白3表达、激活哺乳动物雷帕霉素靶蛋白通路以及抑制软骨细胞自噬来诱导软骨细胞凋亡。值得注意的是,Janus激酶2/信号传导和转录激活蛋白3通路具有拮抗凋亡的作用,提示该通路在瘦素介导的软骨细胞死亡中扮演关键角色。瘦素不仅影响软骨细胞存活,还调控其分泌功能,瘦素通过诱导基质金属蛋白酶表达促进胶原蛋白、弹性蛋白及聚集蛋白聚糖的降解。此外,软骨前体细胞作为软骨修复的关键细胞群,它的功能亦受瘦素显著抑制,瘦素能够降低软骨前体细胞迁移能力及软骨分化潜能,同时增强成骨转化倾向,从而重塑软骨前体细胞的分化命运轨迹[51]。 2.4 瘦素通过中枢神经系统途径调节骨代谢 下丘脑为瘦素调控骨骼代谢的核心中枢区域[1],脑室内注射瘦素可显著影响骨代谢进程[48]。在瘦素缺陷的小鼠模型中,脑室内输注瘦素不仅使小鼠体质量恢复正常,还诱导小鼠骨骼表型向野生型小鼠趋近,具体表现为股骨和椎骨松质骨体积减少、股骨长度和总骨量同步增加,表明瘦素对松质骨具有抑制作用,而对皮质骨呈现合成代谢效应[52]。近来有研究通过对比瘦素缺乏肥胖小鼠与正常小鼠的股骨特征发现,瘦素缺乏导致骨骼呈现“外弱内强”的特殊改变,即皮质骨质量下降的同时松质骨代偿性增生,证实了瘦素通过直接作用于成骨细胞和间接通过中枢神经调控双重途径影响骨代谢,提示瘦素信号紊乱可能导致骨折风险增加[6]。值得注意的是,虽脑室内注射瘦素可恢复骨微结构,但同时伴随脊柱成骨细胞活性显著增强,提示瘦素对骨骼的作用机制复杂。脑室内注射瘦素后可提高椎骨和胫骨的矿物质沉积率,与皮下注射瘦素的效果相当,并且股骨骨髓基质细胞中与成骨相关的基因表达增加,而与破骨细胞生成、脂肪生成和脂肪细胞脂质储存相关的基因表达减少[53],表明脑室内注射瘦素促进骨髓中促成骨因子的表达,从而增强骨形成[54]。但在成年母羊模型中,长期低剂量脑室输注瘦素导致全身骨形成指标如骨碱性磷酸酶和骨钙素降低,腰椎与股骨远端松质骨体积减少,表明瘦素的中枢效应具有物种与剂量依赖性差异[55]。联体共生实验表明,瘦素对骨骼的影响依赖于神经元信号而非循环因子,进一步支持中枢神经系统直接调控骨骼的假说[56]。瘦素还通过下丘脑-垂体-性腺轴间接影响骨代谢,例如恢复运动相关能量缺乏综合征患者的下丘脑-垂体-性腺轴功能后雌激素水平上升,促进骨形成,并可能通过抑制皮质醇分泌来减少骨吸收[57]。 2.5 瘦素通过交感神经系统调节骨代谢 下丘脑通过交感神经系统介导瘦素对骨骼的双向调控[58]。研究表明,中枢瘦素可激活腹内侧下丘脑,促进儿茶酚胺分泌,进而通过β2-肾上腺素能受体影响骨代谢[56]。系统性使用β-受体激动剂会减少骨量和骨形成率,而β-受体阻滞剂(例如普萘洛尔)则能逆转这一效应。例如,在野生型小鼠中,普萘洛尔可完全阻断卵巢切除后的骨丢失,而脑室内注射瘦素虽减少脂肪质量但对β-受体阻断小鼠的骨量无影响,证实交感神经系统是瘦素调控骨骼的关键通路[59]。瘦素通过交感神经系统激活成骨细胞中的核因子κB受体活化因子配体表达,促进破骨分化,而β2-肾上腺素受体缺陷小鼠的成骨细胞核因子κB受体活化因子配体水平较低,破骨活性减弱[60]。因此,来自大脑的瘦素信号通过交感神经系统作用于成骨细胞,不仅影响骨形成,还影响核因子κB受体活化因子配体表达,随后影响破骨细胞分化和松质骨吸收。神经元中瘦素受体的细胞特异性缺失导致骨形成增加、椎骨中骨吸收增加以及脊柱和胫骨远端松质骨体积增加[61]。交感神经系统还介导瘦素对脂质代谢的调节,中枢瘦素通过下丘脑-交感神经轴抑制脂肪生成,并促进脂肪组织和肝脏中的脂肪分解和脂肪酸氧化,这些效应也发生在骨髓脂肪细胞中,因此,瘦素的中枢作用可能由于骨髓脂肪细胞中脂肪因子和脂肪酸产生的变化而直接影响局部骨细胞活性[62-65]。瘦素通过中枢-交感神经轴实现多维度调控:一方面,通过下丘脑直接抑制松质骨形成;另一方面,通过交感神经系统激活β2-肾上腺素受体信号,协调成骨与破骨活动,同时重塑骨髓微环境。瘦素受体在产生5-羟色胺的中缝核脑干神经元中表达[66]。瘦素与瘦素受体结合会抑制Tph2基因的表达,从而减少5-羟色胺的合成和释放[67]。研究发现,与弓状核和腹侧下丘脑中删除瘦素受体的小鼠相比,5-羟色胺能神经元中删除瘦素受体的小鼠表现为高骨量[68]。此外,5-羟色胺2C受体在下丘脑核中表达,这些神经元中5-羟色胺2C受体的缺失会导致严重的骨质流失,这种现象是由于骨形成下调、骨吸收上调以及交感神经活动增加引起的[68]。大脑中的5-羟色胺通过5-羟色胺2C受体在下丘脑神经元中发挥重要作用,减少交感神经活动,从而对骨量产生积极影响。瘦素依赖的中枢骨量调节机制依赖于通过大脑5-羟色胺对交感神经系统的调控[69]。细胞黏附分子1在大脑多个区域表达,参与调节体质量与能量稳态,兴奋性神经元中细胞黏附分子1表达缺失会增强瘦素敏感性,导致骨量减少。在缺乏细胞黏附分子1的动物中,股骨长度、骨矿物质含量、骨干横截面积和骨强度均显著降低;相反,在兴奋性神经元中诱导细胞黏附分子1表达可降低瘦素敏感性,增加股骨长度、骨矿物质含量和骨干横截面积[70],表明细胞黏附分子1通过调节瘦素敏感性进而影响骨代谢和骨结构。 2.6 瘦素在“肠-骨轴”中的作用 瘦素在“肠-骨轴”中通过多途径调控骨代谢。肠道菌群通过调节宿主代谢、免疫功能和激素分泌来调节骨骼稳态,影响骨代谢平衡[70]。骨质疏松症是一种代谢性骨病,它的特征是骨矿物质密度降低、骨微结构的改变,从而致骨折发生率增加[71]。骨量在胎儿出生后逐渐增加,到成年时达到峰值,并受到生活方式、年龄和生物性别等多方面的影响[72]。激素环境、免疫系统、代谢途径以及肠道微生物群都可以影响骨代谢[73],特别是肠道微生物群的改变(即所谓的菌群失调)可能诱导骨质疏松效应[74]。有研究表明,移植年轻大鼠的肠道微生物群可减少老年骨质疏松症大鼠的骨丢失,逆转老年大鼠骨质疏松症,并降低骨转换标志物水平;此外,移植的肠道微生物群还改善了老年骨质疏松症大鼠的肠道微生物组成和屏障功能[8]。肠道微生物产生的短链脂肪酸(特别是丁酸盐)能够促进调节性T细胞的分化,进而激活Wnt信号通路刺激成骨细胞活性,同时抑制破骨细胞分化。菌群失衡会导致肠道通透性增加,引发慢性炎症并促进Th17细胞增殖,诱导破骨细胞增殖,从而加速骨吸收过程。肠道微生物还通过影响血清素和瘦素等激素的分泌来间接调控骨代谢,其中肠道来源的5-羟色胺抑制骨形成[75]。基于这些发现,通过益生菌如乳酸杆菌和益生元等干预手段可以优化肠道菌群组成,增加有益代谢产物的产生,改善矿物质吸收和骨密度。这些研究成果为开发针对骨质疏松等骨骼疾病的新型微生物靶向治疗策略提供了重要理论基础。微生物群与瘦素水平具有相关性,使用万古霉素会导致大鼠瘦素水平急剧下降[76]。大量细菌种类,如乳球菌属、黏螺旋菌属、乳酸菌属和双歧杆菌属与外周瘦素浓度呈正相关,而其他细菌种类,如梭菌属、普雷沃菌属、拟杆菌属和异杆菌属,与瘦素水平呈负相关[77]。植物乳杆菌通过减少白色脂肪组织中脂肪细胞的大小来抑制瘦素的分泌[78]。在吸烟人群中使用益生菌干预也显著降低了受试者的瘦素水平[79],这为通过调节肠道菌群来干预瘦素相关代谢性疾病提供了新的思路。瘦素作为连接能量代谢与骨稳态的关键激素,通过中枢抑制、直接调控骨细胞、调节肠道菌群及介导炎症与内分泌信号等多重机制影响“肠-骨轴”,它的作用既包括直接促进骨形成,也涉及通过肠道微环境间接调节骨代谢平衡。"

| [1] ARJUNAN D, PRASAD TN, DAS L, et al. Osteoporosis and Obesity. Indian J Orthop. 2023;57(Suppl 1):218-224. [2] KIRK B, FEEHAN J, LOMBARDI G, et al. Muscle, Bone, and Fat Crosstalk: the Biological Role of Myokines, Osteokines, and Adipokines. Curr Osteoporos Rep. 2020; 18(4):388-400. [3] DUCY P, AMLING M, TAKEDA S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197-207. [4] TAKEDA S, ELEFTERIOU F, LEVASSEUR R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305-317. [5] HE Q, QIN R, GLOWACKI J, et al. Synergistic stimulation of osteoblast differentiation of rat mesenchymal stem cells by leptin and 25(OH)D(3) is mediated by inhibition of chaperone-mediated autophagy. Stem Cell Res Ther. 2021;12(1):557. [6] GRAEF F, WEI Y, GARBE A, et al. Increased cancellous bone mass accompanies decreased cortical bone mineral density and higher axial deformation in femurs of leptin-deficient obese mice. J Mech Behav Biomed Mater. 2024;160:106745. [7] HU H, LUO S, LAI P, et al. ANGPTL4 binds to the leptin receptor to regulate ectopic bone formation. Proc Natl Acad Sci U S A. 2024;121(1):e2310685120. [8] MA S, WANG N, ZHANG P, et al. Fecal microbiota transplantation mitigates bone loss by improving gut microbiome composition and gut barrier function in aged rats. PeerJ. 2021;9:e12293. [9] MOHAMMADI SM, SANIEE N, BORZOO T, et al. Osteoporosis and Leptin: A Systematic Review. Iran J Public Health. 2024;53(1):93-103. [10] HANSEN MS, FROST M. Alliances of the gut and bone axis. Semin Cell Dev Biol. 2022;123:74-81. [11] ZHANG Y, PROENCA R, MAFFEI M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425-432. [12] ELEFTERIOU F, AHN JD, TAKEDA S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514-520. [13] 王寅,马丽焱.瘦素对骨重建的神经调节[J].生理科学进展,2010,41(3):229-231. [14] ABELLA V, SCOTECE M, CONDE J, et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol. 2017;13(2):100-109. [15] GUAN D, MEN Y, BARTLETT A, et al. Central inhibition of HDAC6 re-sensitizes leptin signaling during obesity to induce profound weight loss. Cell Metab. 2024;36(4):857-876. [16] OBRADOVIC M, SUDAR-MILOVANOVIC E, SOSKIC S, et al. Leptin and Obesity: Role and Clinical Implication. Front Endocrinol (Lausanne). 2021;12:585887. [17] SOMMER C, VANGBERG KG, MOEN G, et al. Insulin and Body Mass Index Decrease Serum Soluble Leptin Receptor Levels in Humans. J Clin Endocrinol Metab. 2023; 108(5):1110-1119. [18] GRASSO P. Harnessing the Power of Leptin: The Biochemical Link Connecting Obesity, Diabetes, and Cognitive Decline. Front Aging Neurosci. 2022;14:861350. [19] FAN X, YUAN W, HUANG W, et al. Recent progress in leptin signaling from a structural perspective and its implications for diseases. Biochimie. 2023;212:60-75. [20] FAN X, QIN R, YUAN W, et al. The solution structure of human leptin reveals a conformational plasticity important for receptor recognition. Structure. 2024;32(1): 18-23. [21] HANKIR MK, BRUNEAU M. Periphery-Brain Interactions and Leptin in the Regulation of Whole-Body Energy Metabolism. Nutrients. 2022;14(8):1594. [22] MANGION D, PACE NP, FORMOSA MM. The relationship between adipokine levels and bone mass-A systematic review. Endocrinol Diabetes Metab. 2023;6(3):e408. [23] AYED K, NABI L, AKROUT R, et al. Obesity and cancer: focus on leptin. Mol Biol Rep. 2023;50(7):6177-6189. [24] CHILDS GV, ODLE AK, MACNICOL MC, et al. The Importance of Leptin to Reproduction. Endocrinology (Philadelphia). 2021;162(2):1. [25] GUO Z, PENG Y, HU Q, et al. The relationship between leptin and periodontitis: a literature review. PeerJ. 2023;11:e16633. [26] KIERNAN K, MACIVER NJ. The Role of the Adipokine Leptin in Immune Cell Function in Health and Disease. Front Immunol. 2020;11:622468. [27] PATIAL K, MISHRA HP, PAL G, et al. Assessment of Leptin Levels and Their Correlation With the Severity of Obstructive Sleep Apnea Syndrome: A Case-Control Study. Cureus. 2023;15(7):e42028. [28] VILARIÑO-GARCÍA T, POLONIO-GONZÁLEZ M, PÉREZ-PÉREZ A, et al. Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes. Int J Mol Sci. 2024;25(4):2338. [29] MUNZBERG H, HEYMSFIELD SB, BERTHOUD H, et al. History and future of leptin: Discovery, regulation and signaling. Metabolism. 2024;161:156026. [30] YANG Y, WANG Z, GE H, et al. Leptin signaling promotes milk fat synthesis via PI3K/AKT/mTOR/SREBP1 in mammary gland of dairy cow. J Dairy Res. 2024;91(4):433-444. [31] WEE NKY, DE LIMA TFC, MCGREGOR NE, et al. Leptin receptor in osteocytes promotes cortical bone consolidation in female mice. J Endocrinol. 2022;255(1):25-37. [32] GAO X, MURPHY MM, PEYER JG, et al. Leptin receptor(+) cells promote bone marrow innervation and regeneration by synthesizing nerve growth factor. Nat Cell Biol. 2023;25(12):1746-1757. [33] HABERMAN ER, SARKER G, ARÚS BA, et al.Immunomodulatory leptin receptor+ sympathetic perineurial barrier cells protect against obesity by facilitating brown adipose tissue thermogenesis. Immunity (Cambridge, Mass.). 2024;57(1):141-152. [34] BAKSHI A, SINGH R, RAI U. Trajectory of leptin and leptin receptor in vertebrates: Structure, function and their regulation. Comp Biochem Physiol B Biochem Mol Biol. 2022;257:110652. [35] XIAO H, LI W, QIN Y, et al. Crosstalk between Lipid Metabolism and Bone Homeostasis: Exploring Intricate Signaling Relationships. Research (Wash D C). 2024;7:447. [36] YAMAZAKI S, MABUCHI Y, KIMURA T, et al. Activated mesenchymal stem/stromal cells promote myeloid cell differentiation via CCL2/CCR2 signaling. Stem Cell Reports. 2024;19(3):414-425. [37] ZHAO Y, PENG X, WANG Q, et al. Crosstalk Between the Neuroendocrine System and Bone Homeostasis. Endocr Rev. 2024;45(1): 95-124. [38] TSUCHIYA H, FUJIO K. Emerging role of leptin in joint inflammation and destruction. Immunol Med. 2022;45(1):27-34. [39] STEFANAKIS K, UPADHYAY J, RAMIREZ-CISNEROS A, et al. Leptin physiology and pathophysiology in energy homeostasis, immune function, neuroendocrine regulation and bone health. Metabolism. 2024;161:156056. [40] PRASAD P, CANCELAS JA. From Marrow to Bone and Fat: Exploring the Multifaceted Roles of Leptin Receptor Positive Bone Marrow Mesenchymal Stromal Cells. Cells. 2024;13(11):910. [41] ANSARIN A, MAHDAVI A M, JAVADIVALA Z, et al. The cross-talk between leptin and circadian rhythm signaling proteins in physiological processes: a systematic review. Mol Biol Rep. 2023;50(12):10427-10443. [42] DEEPIKA F, BATHINA S, ARMAMENTO-VILLAREAL R. Novel Adipokines and Their Role in Bone Metabolism: A Narrative Review. Biomedicines. 2023;11(2):644. [43] APPELT J, TSITSILONIS S, OTTO E, et al. Mice Lacking the Calcitonin Receptor Do Not Display Improved Bone Healing. Cells (Basel, Switzerland). 2021;10(9):2304. [44] GARBE A, GRAEF F, APPELT J, et al. Leptin Mediated Pathways Stabilize Posttraumatic Insulin and Osteocalcin Patterns after Long Bone Fracture and Concomitant Traumatic Brain Injury and Thus Influence Fracture Healing in a Combined Murine Trauma Model. Int J Mol Sci. 2020;21(23):9144. [45] LI J, GAO Y, YU T, et al. Obesity and leptin influence vitamin D metabolism and action in human marrow stromal cells. J Steroid Biochem Mol Biol. 2020;198:105564. [46] NIWCZYK O, GRYMOWICZ M, SZCZESNOWICZ A, et al. Bones and Hormones: Interaction between Hormones of the Hypothalamus, Pituitary, Adipose Tissue and Bone. Int J Mol Sci. 2023;24(7):6840. [47] HSU C, KAO C, YANG C, et al. Leptin Promotes the Expression of Pro-inflammatory Mediator Genes but Does Not Alter Osteoclastogenesis and Early Stage Differentiation of Osteoblasts. J Physiol Investig. 2024;67(6):355-363. [48] MISCH M, PUTHANVEETIL P. The Head-to-Toe Hormone: Leptin as an Extensive Modulator of Physiologic Systems. Int J Mol Sci. 2022;23(10):5439. [49] SHI H, CHEN M. The brain-bone axis: unraveling the complex interplay between the central nervous system and skeletal metabolism. Eur J Med Res. 2024;29(1):317. [50] 汪青,林华.瘦素在骨性关节炎病理机制中的作用[J].中华骨质疏松和骨矿盐疾病杂志,2022,15(4):435-439. [51] CORDERO-BARREAL A, GONZALEZ-RODRIGUEZ M, RUIZ-FERNANDEZ C, et al. An Update on the Role of Leptin in the Immuno-Metabolism of Cartilage. Int J Mol Sci. 2021;22(5):2411. [52] BEGHINI M, BRANDT S, KORBER I, et al. Serum IGF1 and linear growth in children with congenital leptin deficiency before and after leptin substitution. Int J Obes (Lond). 2021;45(7):1448-1456. [53] XIAO Y, HAN C, WANG Y, et al. Interoceptive regulation of skeletal tissue homeostasis and repair. Bone Res. 2023;11(1):48. [54] BARRIOS V, FRAGO LM, CANELLES S, et al. Leptin Modulates the Response of Brown Adipose Tissue to Negative Energy Balance: Implication of the GH/IGF-I Axis. Int J Mol Sci. 2021;22(6):2827. [55] SALAMANNA F, CONTARTESE D, VERONESI F, et al. Osteoporosis Preclinical Research: A Systematic Review on Comparative Studies Using Ovariectomized Sheep. Int J Mol Sci. 2022;23(16):8904. [56] ZHU S, CHEN W, MASSON A, et al. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024;10(1):71. [57] PERAKAKIS N, MANTZOROS CS. Evidence from clinical studies of leptin: current and future clinical applications in humans. Metabolism. 2024;161(6):156053. [58] KARSENTY G, KHOSLA S. The crosstalk between bone remodeling and energy metabolism: A translational perspective. Cell Metab. 2022;34(6):805-817. [59] CAO L, CHOI EY, LIU X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14(3): 324-338. [60] LV X, GAO F, CAO X. Skeletal interoception in bone homeostasis and pain. Cell Metab. 2022;34(12):1914-1931. [61] ZENG W, YANG F, SHEN WL, et al. Interactions between central nervous system and peripheral metabolic organs. Sci China Life Sci. 2022;65(10):1929-1958. [62] LIU H, LIU L, ROSEN CJ. Bone Marrow Adipocytes as Novel Regulators of Metabolic Homeostasis: Clinical Consequences of Bone Marrow Adiposity. Curr Obes Rep. 2025;14(1):9. [63] PERAKAKIS NM, FARR OMP, MANTZOROS CSM. Leptin in Leanness and Obesity. J Am Coll Cardiol. 2021;77(6):745-760. [64] PEREIRA S, CLINE DL, GLAVAS MM, et al. Tissue-Specific Effects of Leptin on Glucose and Lipid Metabolism. Endocr Rev. 2021; 42(1):1-28. [65] PICÓ C, PALOU M, POMAR CA, et al. Leptin as a key regulator of the adipose organ. Rev Endocr Metab Disord. 2022;23(1):13-30. [66] ASGARI R, CACERES-VALDIVIEZO M, WU S, et al. Regulation of energy balance by leptin as an adiposity signal and modulator of the reward system. Mol Metab. 2025; 91:102078. [67] CHOI W, KIM J, KANG H, et al. Interactive Effects of Serum Leptin Levels and Physical Comorbidity on the Pharmacotherapeutic Response of Depressive Disorders. Clin Psychopharmacol Neurosci. 2022;20(4): 662-674. [68] LI C, PI G, LI F. The Role of Intestinal Flora in the Regulation of Bone Homeostasis. Front Cell Infect Microbiol. 2021;11:579323. [69] ZHANG B, CUI J, ZHANG X, et al. Autophagy: regulating the seesaw of bone-fat balance. Front Cell Dev Biol. 2025;13:1465092. [70] LIN Z, XIONG S, LIN Y, et al. Impact of leptin or melatonin on Sema4D overexpression-related bone metabolism. J Orthop Surg Res. 2023;18(1):285. [71] WANG N, MA S, FU L. Gut Microbiota Dysbiosis as One Cause of Osteoporosis by Impairing Intestinal Barrier Function. Calcif Tissue Int. 2022;110(2):225-235. [72] DIMAI HP, MUSCHITZ C, AMREIN K, et al. [Osteoporosis-Definition, risk assessment, diagnosis, prevention and treatment (update 2024): Guidelines of the Austrian Society for Bone and Mineral Research]. Wien Klin Wochenschr. 2024;136(Suppl 16): 599-668. [73] ZHANG Y, LI Y, LU P, et al. The modulatory effect and implication of gut microbiota on osteoporosis: from the perspective of “brain-gut-bone” axis. Food Funct. 2021; 12(13):5703-5718. [74] OZAKI D, KUBOTA R, MAENO T, et al. Association between gut microbiota, bone metabolism, and fracture risk in postmenopausal Japanese women. Osteoporos Int. 2021;32(1):145-156. [75] INDRIO F, SALATTO A. Gut Microbiota-Bone Axis. Ann Nutr Metab. 2025;81(Suppl 1): 47-56. [76] LIU Q, ZHU Y, LI G, et al. Irisin ameliorates myocardial ischemia-reperfusion injury by modulating gut microbiota and intestinal permeability in rats. PLoS One. 2023;18(9): e0291022. [77] VAN SON J, KOEKKOEK LL, LA FLEUR SE, et al. The Role of the Gut Microbiota in the Gut-Brain Axis in Obesity: Mechanisms and Future Implications. Int J Mol Sci. 2021; 22(6):2993. [78] HOSSEINIFARD E, BAVAFA-VALENLIA K, SAGHAFI-ASL M, et al. Antioxidative and Metabolic Effects of Lactobacillus plantarum, Inulin, and Their Synbiotic on the Hypothalamus and Serum of Healthy Rats. Nutr Metab Insights. 2020;13: 1178638820925092. [79] YUAN L, LI Y, CHEN M, et al. Antihypertensive Activity of Milk Fermented by Lactiplantibacillus plantarum SR37-3 and SR61-2 in L-NAME-Induced Hypertensive Rats. Foods. 2022;11(15):2332. [80] GEWITZ A, MENDELL J, WANG Y, et al. Pharmacokinetics and pharmacodynamics of mibavademab (a leptin receptor agonist): Results from a first-in-human phase I study. Clin Transl Sci. 2024;17(4):e13762. |
| [1] | Zeng Hao, Sun Pengcheng, Chai Yuan, Huang Yourong, Zhang Chi, Zhang Xiaoyun. Association between thyroid function and osteoporosis: genome-wide data analysis of European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1019-1027. |
| [2] | Gu Fucheng, Yang Meixin, Wu Weixin, Cai Weijun, Qin Yangyi, Sun Mingyi, Sun Jian, Geng Qiudong, Li Nan. Effects of Guilu Erxian Glue on gut microbiota in rats with knee osteoarthritis: machine learning and 16S rDNA analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1058-1072. |
| [3] | Shi Tengbo, Tang Yanfeng, Zhang Mengyu, Wang Xingfei, Li Chenyang, Shi Jinyu, Guo Chaowei, Li Yanzhou, He Zike, Wang Shangzeng. Effect of high-dose low molecular weight heparin on the healing of femoral shaft fractures [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(12): 2957-2964. |
| [4] | Wu Fangjia, Lei Senlin, Li Xianhui, Yang Yang. Aerobic and resistance exercise interventions in a mouse model of nonalcoholic fatty liver disease: correlation between gut microbiota and irisin [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(12): 3029-3043. |
| [5] | Qiu Xueli, Cui Hao, Wu Chenyang, Tao Lide, Yao Yuqian, Tian Bo, Bai Jinyu, Zhang Yingzi. Gut microbiota tryptophan metabolite indole-3-propionic acid alleviates inflammatory bowel disease-related osteoporosis in a mouse model [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2413-2421. |
| [6] | Li Xinying, Zhang Wenhua, Li Xun, Zhang Shihua, Wang Xiaoqiang. Regulation of bone metabolism by myokines under resistance exercise intervention [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2475-2483. |
| [7] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [8] | Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683. |
| [9] |
Zhao Wensheng, Li Xiaolin, Peng Changhua, Deng Jia, Sheng Hao, Chen Hongwei, Zhang Chaoju, He Chuan.
Gut microbiota and osteoporotic fractures #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1296-1304.
|
| [10] |
Sun Guanghan, Xie Zhencong, Sun Mi, Xu Yang, Guo Dong.
Therapeutic effect and mechanism by which Trichosanthis Fructus-Allii Macrostemonis Bulbus regulates gut microbiota in a rat model of coronary heart disease #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 917-927.
|
| [11] | Wang Dongyang, Yang Qiaohui, Lin Xinchao. Relationship between vitamin D levels and reproductive characteristics and exercise dietary situation in postmenopausal women [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1021-1025. |
| [12] |
Li Zhipeng, Xing Rongxin, Hu Lianghong.
Roles of SOX5 in bone metabolism and prevention of bone diseases and the relationship with exercise#br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7589-7600.
|
| [13] | Wang Tao, Wang Shunpu, Min Youjiang, Wang Min, Li Le, Zhang Chen, Xiao Weiping. Causal relationship between gut microbiota and rheumatoid arthritis: data analysis in European populations based on GWAS data [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7663-7668. |
| [14] | Lu Xiuli, Xu Huazhen, Chen Yuxing, Yao Nan, Hu Zixuan, Huang Dane. Mechanism of Jiangu Formula in treating osteoporosis based on osteoclast-osteoblast coupling [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6828-6835. |
| [15] | Cui Yuena, Chen Xiaoyu, Liang Meiting, Chen Wujin, He Yi, Dilinur·Ekpa, Du Manxi, Zhu Yuqiu, Abuduwupuer·Haibier, Sun Yuping. Differences of calorie restriction and time-restricted feeding on metabolic indices and gut microbiota of mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6449-6456. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||