Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (22): 5804-5813.doi: 10.12307/2026.201
Previous Articles Next Articles
Postbiotic targeting muscle aging: mechanistic insights and application prospects of urolithin A
Yang Zijiang1, Guo Chenggen2, Deng Ziao3, Xue Xinxuan2
- 1School of Physical Education, Nanchang Jiaotong Institute, Nanchang 330100, Jiangxi Province, China; 2School of Sports Training, Wuhan Sports University, Wuhan 430079, Hubei Province, China; 3School of Law and Business, Hubei University of Economics, Wuhan 430205, Hubei Province, China
-
Received:2025-07-22Accepted:2025-09-11Online:2026-08-08Published:2025-12-27 -
Contact:Xue Xinxuan, Professor, Master’s supervisor, School of Sports Training, Wuhan Sports University, Wuhan 430079, Hubei Province, China -
About author:Yang Zijiang, MS, School of Physical Education, Nanchang Jiaotong Institute, Nanchang 330100, Jiangxi Province, China -
Supported by:Youth Fund Project for Humanities and Social Sciences Research, Ministry of Education, No. 24YJC890016 (to GCG); Key Project for Philosophy and Social Sciences Research, Hubei Provincial Department of Education, No. 24D100 (to GCG); Youth Project for Science and Technology Research, Hubei Provincial Department of Education, No. Q20234103 (to GCG)
CLC Number:
Cite this article
Yang Zijiang, Guo Chenggen, Deng Ziao, Xue Xinxuan. Postbiotic targeting muscle aging: mechanistic insights and application prospects of urolithin A[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5804-5813.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
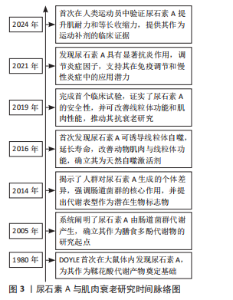
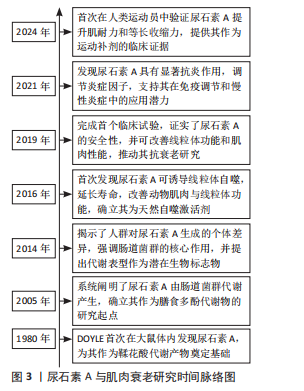
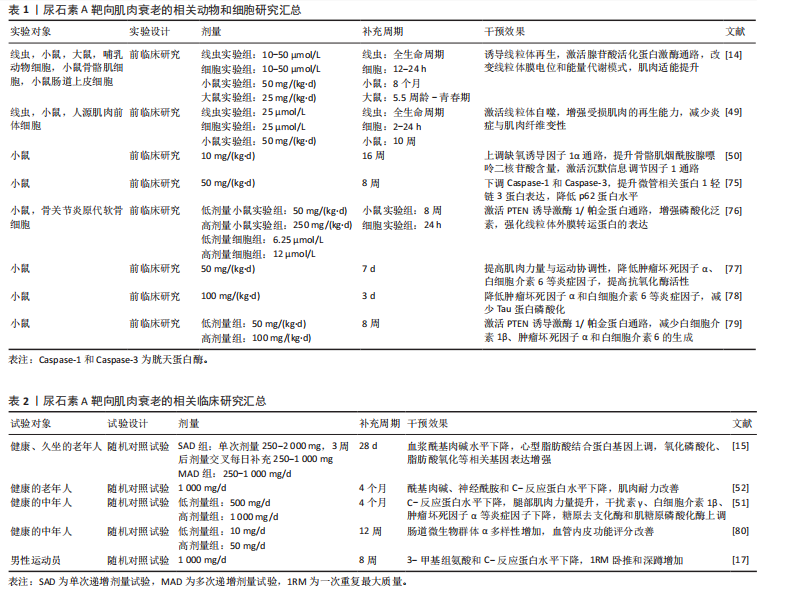
2.1 尿石素A与肌肉衰老的研究历程 1980年,DOYLE在大鼠体内首次发现尿石素A,该物质最初被定义为鞣花酸的代谢产物[11]。2005年,CERDá-VILLALBA等首次系统阐明了鞣花酸在人体内如何被肠道菌群代谢为尿石素A,这一发现奠定了尿石素A作为膳食多酚代谢物的基础,并开启了后续的生物活性研究[12]。2014年,TOMáS-BARBERáN等[13]研究揭示了人类对鞣花酸代谢的显著个体差异,强调了肠道微生物群在这一过程中的关键作用,并提出尿石素代谢表型可能成为评估个体肠道健康状况和疾病易感性的潜在生物标志物的观点。2016年,RYU等[14]首次发现尿石素A能够促进线粒体自噬,作为一种天然的线粒体自噬诱导剂,尿石素A不仅显著延长了秀丽隐杆线虫的寿命并增强其运动能力,还能够明显改善小鼠的肌肉功能并促进线粒体健康。2019年,ANDREUX等[15]开展了首个关于尿石素A的随机对照临床试验,研究证实尿石素A具有良好的安全性和生物利用度,可调控线粒体相关基因表达,从而促进肌肉健康并优化代谢功能,这项研究为尿石素A在抗衰老及线粒体健康领域提供临床证据。2021年,BOBOWSKA等[16]研究发现:尿石素A在抑制炎症因子表达方面效果尤为突出,可显著抑制肿瘤坏死因子α和白细胞介素6,从而有效降低炎症反应;同时,上调白细胞介素10,增强免疫调节能力。此外,尿石素A能够激活细胞外信号调节激酶1/2,这一发现为尿石素A在免疫调节和慢性炎症管理方面的潜在应用提供了重要理论支持。2024年,ZHAO等[17]首次在男性运动员中系统评估了尿石素A对多项肌肉功能指标的影响,重点揭示了尿石素A在提升肌肉耐力和最大自主等长肌收缩能力方面的显著优势,为尿石素A作为运动营养补剂在增强肌肉功能与提高机体恢复效率中的应用潜力提供了临床证据。见图3。"
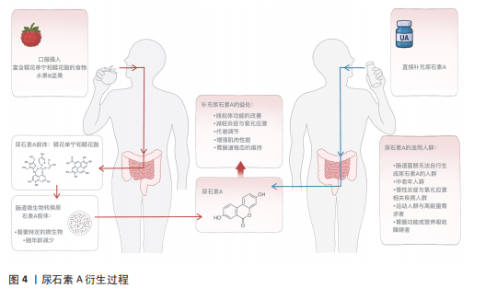
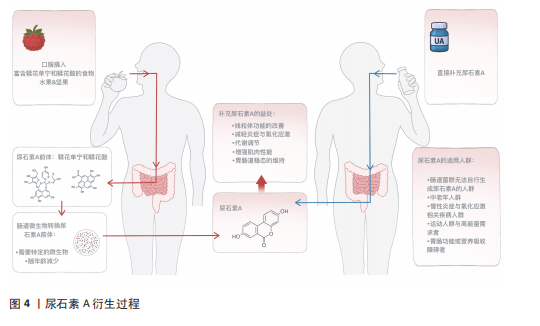
2.2 尿石素A概述 2.2.1 尿石素A的来源与结构特点 尿石素A是天然多酚类物质鞣花单宁和鞣花酸在结肠中经肠道微生物转化后产生的代谢产物,这些多酚类物质广泛存在于石榴、草莓、树莓和核桃等食物中[18],最早于1980年由DOYLE在大鼠体内发现[11],其化学结构的特征是含有α-苯并香豆素骨架,分子式为C13H8O4,分子质量228.20 g/mol (图4)。核心结构为三环稠合的芳香族化合物,含有一个内酯环,赋予其较强的稳定性和生物活性基础。尿石素A结构较为简单,具有较强的亲脂性,有助于其穿透生物膜,从而在体内被高效地吸收和利用[19]。 2.2.2 尿石素A的转化过程 天然多酚类化合物鞣花单宁和鞣花酸广泛存在于多种植物性食物中,是尿石素A的主要膳食前体[20]。由于鞣花单宁的生物利用度极低,它们通常被直接排出体外,或在体内转化为生物利用度更高的衍生物。在肠道细菌分泌的单宁酶作用下,鞣花单宁被水解为鞣花酸,随后经过一系列酶促反应,鞣花酸被进一步转化为尿石素A及其他尿石素(图4)。尿石素A和尿石素B是最常见的最终代谢产物,其中尿石素A是目前跨物种研究中最为广泛的尿石素[21]。 2.2.3 尿石素A的代谢类型 尿石素A的生成高度依赖于个体肠道微生物的组成,研究表明,并非所有人都具备自然合成尿石素A的能力。根据尿石素A的生成效率,可将个体划分为3种代谢类型:仅产生尿石素A (UM-A)、产生多种尿石素(UM-B)和不产生尿石素(UM-0)[22]。年轻人群以A型为主,而随着年龄的增长,B型和0型的比例显著上升,表明肠道菌群功能随衰老逐渐减弱。与尿石素A合成相关的关键菌属在老年人中显著减少,同时菌群多样性下降,导致多酚类营养素的代谢能力降低[23]。因此,尿石素A肠道菌群代谢类型不仅反映个体的营养代谢潜力,也可作为评估肠道健康状态和生理老化程度的潜在生物标志物[24]。"
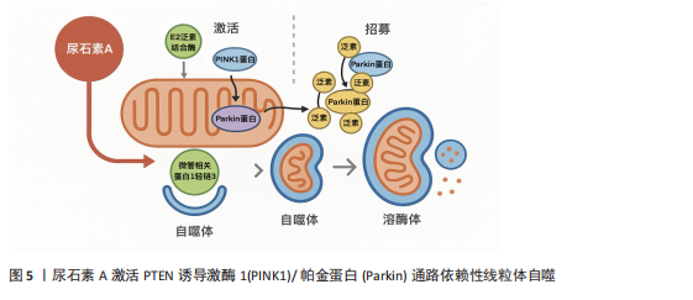
2.3 肌肉衰老的机制 肌肉衰老是一个复杂的、多方面的过程,涉及多种相互关联的分子机制和细胞系统。 (1)线粒体功能障碍与氧化应激:线粒体中的氧化磷酸化过程产生各细胞所需的三磷酸腺苷[25],烟酰胺腺嘌呤二核苷酸和黄素腺嘌呤二核苷酸通过此过程将电子转移至电子传递链[26]。电子传递链是一个四复合体酶系统,可依次氧化和转移电子,因此当电子从复合体Ⅰ移动到Ⅳ时,质子会通过复合体Ⅰ、Ⅲ和Ⅳ将自己主动运输到线粒体膜间隙[27]。这种离子运动在膜间隙和基质之间形成电化学梯度,其中基质带负电荷,而膜间隙带正电荷。当质子通过三磷酸腺苷合酶流入基质时,二磷酸腺苷会被磷酸化为三磷酸腺苷[28]。三磷酸腺苷的合成量取决于电子传递链通量以及氧气吸收量。静息骨骼肌中,细胞内二磷酸腺苷的浓度低于三磷酸腺苷,这抑制了线粒体中的氧化磷酸化活性,并增加了烟酰胺腺嘌呤二核苷酸氧化型的比例[29]。这种压力降低会增加电子传递链中未配对电子(由于过剩电子的流动)向泛醌(Q)复合物的泄漏。细胞内的氧气被电子还原形成超氧阴离子,然后由超氧化物歧化酶自发转化为过氧化氢[30]。 随着年龄增长,线粒体功能逐渐受损,呼吸链酶复合体活性下降,自由基产生显著增加,形成恶性循环。活性氧的积累会破坏线粒体脱氧核糖核酸、膜脂和蛋白,进一步降低线粒体合成三磷酸腺苷的能力[31]。老年小鼠对比年轻小鼠其过氧化氢生成比例高出50%–80%,伴随线粒体脱氧核糖核酸拷贝数减少,柠檬酸合酶、细胞色素氧化酶等关键酶活性下降,三磷酸腺苷产量亦减少约30%[32]。此外,活性氧在肌纤维类型中的产生存在差异,ⅡB型纤维中的活性氧水平是Ⅰ型纤维的两三倍,这使得ⅡB纤维在衰老中更易萎缩[33]。 综上,线粒体是生成活性氧的主要场所,同时也极易受到活性氧的攻击,两者相互作用,是导致肌肉衰老的关键因素之一。 (2)炎症:常常作为一条重要通路贯穿于肌肉衰老的整个过程。炎症广泛存在于衰老的肌肉组织细胞内,并诱发免疫反应,进而导致肌肉功能的衰退[34]。核因子κB通路是典型的促炎信号通路,以单二聚体和异二聚体蛋白的形式存在。通常,当促炎因子(肿瘤坏死因子α、白细胞介素1)与其受体结合时[35],会刺激核因子κB通路的激活,最终导致靶基因的转录,包括泛素-蛋白酶体系统相关分子、细胞因子、趋化因子、细胞黏附分子和生长因子[36]。这些靶基因共同作用于泛素-蛋白酶体系统,引起蛋白水解,从而导致肌肉萎缩[37]。与此同时,核因子κB的激活会间接影响哺乳动物雷帕霉素靶蛋白信号[38],从而影响肌肉卫星细胞的增殖与代谢,导致肌肉纤维分化功能受损,阻碍肌肉的修复与再生[39]。 (3)神经肌肉功能障碍:神经肌肉接头是运动神经元轴突与骨骼肌的连接点,由运动神经末梢、突触周围施万细胞和骨骼肌组成。该连接点的主要功能是将突触前运动神经元的动作电位转化为突触后肌纤维的收缩。随着年龄的增长,肌肉和神经元中的线粒体功能逐渐受损,三磷酸腺苷的生产效率降低,活性氧激增,这些内部变化导致神经肌肉接头功能受损,驱动了肌肉衰老的病理进程[40]。此外,蛋白质精氨酸甲基转移酶1型等关键基因的表达也会影响神经肌肉接头的稳定性[41]。终末施万细胞是神经肌肉接头维持功能的关键元素,也是神经肌肉突触中不可或缺的参与者,衰老相关的终末施万细胞退化可能是导致肌肉去神经化的关键因素[42]。一项关于大鼠的电生理测试证实,神经肌肉接头功能障碍导致了大鼠肌肉量的减少,驱动了肌肉衰老的进程[43]。综上,肌肉衰老与神经肌肉接头之间存在着密切而复杂的关系,神经肌肉接头的功能退化可能是驱动肌肉衰老的关键因素。 2.4 尿石素A改善肌肉衰老的药物动力学机制 2.4.1 线粒体和线粒体功能 当线粒体受损或暴露于外源性诱导剂时,尿石素A能够促使线粒体自噬的激活,帮助清除受损线粒体并维持线粒体稳态[44]。尿石素A可通过多种途径启动该过程,尤其是激活PTEN诱导激酶1/帕金蛋白依赖性线粒体自噬:PTEN诱导激酶1在受损线粒体膜上稳定积聚,招募并磷酸化帕金蛋白,进而促进线粒体蛋白的泛素化,形成锚点吸引微管相关蛋白1轻链3和吞噬体膜包裹线粒体[45],最终与溶酶体融合完成降解(图5)[46]。此外,尿石素A也可激活PTEN诱导激酶1/帕金蛋白非依赖性路径,通过Bcl-2/腺病毒E1B相互作用蛋白3和线粒体外膜功能性蛋白1等线粒体受体蛋白直接招募微"
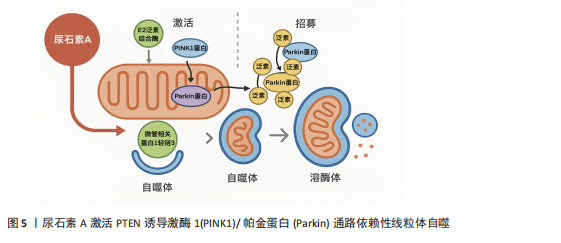
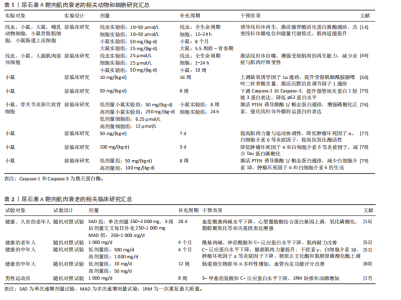
管相关蛋白1轻链3,促进自噬体形成并实现线粒体清除[47]。 尿石素A在细胞、线虫、小鼠和人类等多种生物中均表现出改善线粒体健康的显著作用,其核心机制是通过促进选择性清除功能异常线粒体的过程——线粒体自噬来实现[48]。在动物实验中,尿石素A显著上调了秀丽隐杆线虫中自噬相关基因1同源蛋白、PTEN诱导激酶1和帕金相关蛋白1的表达,这些基因分别对应哺乳动物的微管相关蛋白1轻链3B、PTEN诱导激酶1和帕金蛋白同源基因。值得注意的是,在缺失PTEN诱导激酶1或双顺反子载体转运蛋白1的突变线虫中,尿石素A对线粒体自噬和寿命延长的有益作用被显著削弱,提示上述基因在尿石素A诱导线粒体自噬及其延缓衰老作用中具有关键调控作用[14]。在杜氏肌营养不良症的小鼠模型中,补充尿石素A后,骨骼肌组织中线粒体自噬相关标志物水平显著升高,包括帕金蛋白、泛素化水平以及磷酸化泛素修饰的线粒体蛋白等[49]。另外一项针对中年小鼠的研究表明,经尿石素A持续干预16周后,骨骼肌中血管生成标志物表达增加,沉默信息调节因子1-过氧化物酶体增殖物激活受体γ辅激活因子1α通路被激活,三磷酸腺苷和烟酰胺腺嘌呤二核苷酸氧化型水平也显著提高[50]。 在人体临床研究中,SINGH等[51]在一项针对中年人群开展的为期4个月的随机双盲临床试验中发现,尿石素A低剂量组(500 mg)显著上调泛素结合酶E2 N型和泛素结合酶E2 R2型的表达,并提高帕金蛋白磷酸化水平,这是启动PTEN诱导激酶1/帕金蛋白介导线粒体自噬的关键步骤;而在高剂量组(1 000 mg)中,尿石素A激活三羧酸循环、脂肪酸氧化、电子传递链和氧化磷酸化等代谢通路,系统性增强了线粒体的能量代谢能力。值得注意的是,LIU等[52]在一项针对老年人随机双盲试验中发现,试验组 (1 000 mg尿石素A/d)对手部肌肉最大三磷酸腺苷产量的改善并不显著,这可能与干预时长以及受试对象有关。此外,DENK等[53]研究发现,在为期28 d、每日补充1 000 mg 尿石素A后,CD8+ T细胞的线粒体质量显著提升,并伴随细胞增殖活性增强。虽然该研究未直接测定三磷酸腺苷产量,但通过质谱分析和流式细胞术检测发现,其代谢通路中氧化磷酸化效率明显提高,从而进一步支持了尿石素A增强线粒体功能的潜在作用。 2.4.2 炎症 尿石素A的抗炎特性与早期前临床研究的发现一致[54]。尿石素A的抗炎机制主要通过抑制核因子κB和蛋白激酶B/丝裂原活化蛋白激酶信号通路,降低环氧合酶2的mRNA和蛋白表达水平[55]。在脂多糖刺激的巨噬细胞中,尿石素A可抑制肿瘤坏死因子α的产生,并促进抗炎因子白细胞介素10的合成和转化生长因子β1的表达[16]。尽管尿石素A对白细胞介素的调控作用在不同研究中存在一定差异,但已有研究表明,尿石素A可通过降低糖尿病小鼠胰腺中白细胞介素1β水平发挥保护作用[56],并减弱白细胞介素1β诱导的大鼠关节软骨细胞的炎症反应[57]。 尿石素A的抗炎作用首次在急性结肠炎大鼠模型中得到验证,该模型通过右旋糖酐硫酸钠诱导,研究发现尿石素A可显著降低结肠中炎症标志物环氧合酶2的mRNA和蛋白水平[58];后续研究在急性三硝基苯磺酸诱导和慢性葡聚糖硫酸钠诱导的以及小鼠结肠炎模型中均观察到血浆中促炎因子白细胞介素1β、白细胞介素6及肿瘤坏死因子α水平均显著下降,呈现一致性的抗炎效果。在链脲佐菌素诱导的糖尿病小鼠中,尿石素A同样降低了这些细胞因子的炎症水平,并显著提高了抗炎因子白细胞介素10的表达[56]。此外,尿石素A还可降低高脂饮食诱导的肥胖小鼠肝脏中白细胞介素1β水平[59]。在一项针对中年人群的随机对照试验中,持续4个月服用500 mg尿石素A可显著降低白细胞介素1β水平,但对C-反应蛋白、干扰素γ和肿瘤坏死因子α无显著影响;相比之下,更高剂量的尿石素A(1 000 mg/ d,持续4个月)则显著降低了C-反应蛋白、干扰素γ和肿瘤坏死因子α水平,但对白细胞介素1β无明显作用;两个剂量在干预4个月后,与安慰剂相对比白细胞介素13和白细胞介素6水平均未显示出显著差异[51]。LIU等[52]的研究进一步支持了这一发现,指出1 000 mg/d、持续4个月的尿石素A对C-反应蛋白水平无显著影响。此外,BROOME等[60]研究发现,尿石素A可通过降低炎症标志物,从而减弱对线粒体生物合成关键分子(如过氧化物酶体增殖物激活受体γ辅激活因子1α)的负面调控,进而促进新线粒体的生成。尿石素A不仅具备上调线粒体和代谢相关基因表达的作用,还能协调多种与运动相关的肌肉可塑性和功能适应,同时有效抑制广泛的炎症反应。 2.4.3 抗氧化活性 尿石素A通过多靶点机制对抗体内的活性氧。在鱼藤酮诱导的大鼠帕金森病模型中,通过摄入富含鞣花单宁的石榴汁后,观察到脑组织中过氧化氢酶、谷胱甘肽过氧化物酶和谷胱甘肽S-转移酶活性增强,以及谷胱甘肽水平下降[61]。在顺铂诱导的小鼠肾毒性模型中也获得了类似的抗氧化作用[62],尿石素A可防止顺铂诱导的肾脏谷胱甘肽储备耗竭,提高谷胱甘肽过氧化物酶和超氧化物歧化酶活性以及抑制烟酰胺腺嘌呤二核苷酸磷酸氧化酶2基因表达,而烟酰胺腺嘌呤二核苷酸磷酸氧化酶2正是氧化应激的主要来源。此外,多种慢性疾病小鼠模型亦证实,尿石素A能够强化病理小鼠铜锌超氧化物歧化酶和锰超氧化物歧化酶基因表达,并提高胰腺谷胱甘肽水平[63],从而预防脂质过氧化[56]。在体外细胞实验中,尿石素A显著增强了神经细胞的抗氧化活性,通过抑制氧化酶的形式降低活性氧产生,并表现出清除氧化受损神经细胞中自由基的潜力[64]。尿石素A还被证实能够有效减少晚期糖基化终产物的形成,这类物质通过结合神经元细胞表面受体从而诱发氧化应激[65]。 临床试验方面,尿石素A表现出良好的安全性与抗氧化功效。在一项面向健康、久坐的老年人试验中发现[15],尿石素A可显著改善线粒体功能,激活线粒体自噬,间接减少活性氧的产生。ZHAO等[17]研究也进一步证实了这个观点,经过8周的尿石素A干预,实验组的超氧化物歧化酶水平上升,显著改善由运动而引起的氧化应激。在一项针对心力衰竭的小样本临床试验中发现,试验组对比安慰剂组高密度脂蛋白胆固醇水平显著提高[66],尽管高密度脂蛋白胆固醇不是传统抗氧化标志物,但它可在一定程度上反映细胞抗氧化能力的增强。 2.5 尿石素A改善肌肉性能的机制 2.5.1 肌肉蛋白质代谢 尿石素A通过调控肌肉蛋白质的合成与降解改善肌肉性能。KONDO等[67]通过一项L6骨骼肌前体细胞实验发现,尿石素A通过激活磷脂酰肌醇3激酶/蛋白激酶B与腺苷酸活化蛋白激酶信号通路,促进葡萄糖转运蛋白4向细胞膜的转位,提高肌肉对葡萄糖的吸收效率,并可能间接激活哺乳动物雷帕霉素靶点/核糖体蛋白S6激酶通路,为肌肉蛋白质的合成提供能量。哺乳动物雷帕霉素靶点通路在调节肌肉蛋白质合成和细胞生长中起着关键作用[68],研究表明,尿石素A可以通过抑制哺乳动物雷帕霉素靶点信号通路中的特定调控因子(如磷脂酰肌醇3激酶和蛋白激酶B)来调节肌肉蛋白质的生成[69]。值得注意的是,RODRIGUEZ等[70]研究发现,6周石榴汁提取物喂养的小鼠在注射肿瘤坏死因子α后蛋白激酶B/哺乳动物雷帕霉素靶点通路活性仍被保留,提示尿石素A对肌肉蛋白质合成的积极作用。TOTIGER等[71]的研究则进一步支撑该观点,小鼠经过3-7周的尿石素A干预后,磷脂酰肌醇-3-激酶/蛋白激酶B/哺乳动物雷帕霉素靶点信号通路显著激活。此外,一项体外细胞实验发现,尿石素A通过抑制蛋白激酶B激活叉头框蛋白O1调节肌肉蛋白代谢[72],由于叉头框蛋白O1在肌肉中是肌萎缩相关基因1与肌肉特异性RING指蛋白1等E3泛素连接酶的关键转录因子,这提示尿石素A可能通过激活叉头框蛋白O1增强泛素蛋白酶连接系统介导的蛋白质降解,然而这一假设仍需后期实验佐证。综上,大量动物和细胞实验证明了尿石素A通过多条信号通路调节肌肉蛋白质的代谢。但应注意的是,未来在开展临床试验时应充分考虑不同疾病模型与个体生理状态的差异。例如,蛋白激酶B–叉头框蛋白O1通路在健康状态下可以起到促进蛋白质合成、抑制肌肉分解的作用,但在病理状态下(糖尿病、慢性炎症以及癌症恶液质)该通路可能会加速蛋白质降解,导致更严重的肌肉萎缩。 2.5.2 肌肉力量 目前,尽管针对尿石素A改善肌肉力量的临床试验十分有限,但现有证据表明尿石素A可能在改善肌肉力量方面发挥积极作用[73]。GHOSH等[50]通过对成年小鼠进行16周的尿石素A干预后发现,尿石素A可通过沉默信息调节因子1-过氧化物酶体增殖物激活受体γ辅激活因子1α通路增强骨骼肌中的三磷酸腺苷和烟酰胺腺嘌呤二核苷酸水平,从而改善肌肉代谢能力和血液供给。LUAN等[49]的研究进一步支撑这一观点,在一项针对杜氏肌营养不良模型小鼠实验中发现,尿石素A能够显著改善肌肉干细胞的再生能力,同时激活沉默信息调节因子1,增强肌细胞中线粒体的生物合成功能。ZHAO等[17]则通过一项针对男性运动员的试验发现,试验组1次重复最大质量卧推和深蹲成绩显著提升,但这一差异对比安慰剂组并不具备显著性。与此同时,针对体能中等且最大三磷酸腺苷合成速率较低的老年人群,每日补充1 000 mg尿石素A,持续4个月后,肌肉耐力显著提升,尤其在重复肌肉收缩测试中,其表现显著优于安慰剂组;此外,受试者右手第一背侧骨间肌及小腿胫前肌的肌肉耐力亦有明显增强,并在统计学上具有显著性[52]。另一项针对超重中年人群的随机对照临床试验进一步证实,尿石素A的补充(500或1 000 mg/d,持续4个月)可显著提高腿后肌群(股二头肌)的平均峰值扭矩以及膝关节屈曲时的最大扭矩,均显著优于安慰剂组[51]。 2.5.3 运动表现 尿石素A在多种动物模型中被证实具有提升运动表现的潜力。动物实验显示,尿石素A可延缓肌肉衰老并提升多项运动能力。在秀丽隐杆线虫中,尿石素A可预防与年龄相关的肌肉退化,表现为运动能力提高及咽部泵动频率上升。在哺乳动物中,这一效果同样显著。中龄小鼠在接受34周尿石素A膳食干预后,有氧运动表现改善;老龄小鼠在6周尿石素A干预后也表现出相似的运动表现提升[14]。此外,尿石素A显著增强了杜氏肌营养不良症小鼠的耐力、握力和肌肉强直收缩力[74]。在人类临床研究中,ZHAO等[17]在一项为期8周、每日补充1 g尿石素A的试验中发现,尿石素A可显著改善最大自愿等长收缩、重复次数至力竭等肌肉耐力指标。然而,还研究表明,在每日补充500 mg尿石素A持续4个月干预后,握力、步行速度、峰值功率输出、峰值摄氧量、最大摄氧量、骑行总距离、6 min步行距离及疲劳出现时间等运动表现指标并未表现出显著优于安慰剂的效果。尽管每日1 000 mg尿石素A补充组在步行速度、峰值摄氧量、最大摄氧量及骑行距离等方面显示出一定的改善趋势,且整体优于安慰剂组,但相关差异尚未达到统计学显著水平[51]。综上,尿石素A在提升人体运动表现方面具有潜在作用,但其机制效应仍需通过长期干预及更大样本量的随机对照试验进一步验证。"
| [1] LÓPEZ-OTÍN C, BLASCO MA, PARTRIDGE L, et al. Hallmarks of aging: An expanding universe. Cell. 2023;186(2):243-278. [2] LI Y, TIAN X, LUO J, et al. Molecular mechanisms of aging and anti-aging strategies. Cell Commun Signal. 2024; 22(1):285. [3] MARCANGELI V, YOUSSEF L, DULAC M, et al. Impact of high-intensity interval training with or without l-citrulline on physical performance, skeletal muscle, and adipose tissue in obese older adults. J Cachexia Sarcopenia Muscle. 2022;13(3):1526-1540. [4] HOU Y, CHU X, PARK JH, et al. Urolithin A improves Alzheimer’s disease cognition and restores mitophagy and lysosomal functions. Alzheimers Dement. 2024;20(6):4212-4233. [5] HUANG JR, ZHANG MH, CHEN YJ, et al. Urolithin A ameliorates obesity-induced metabolic cardiomyopathy in mice via mitophagy activation. Acta Pharmacol Sin. 2023;44(2):321-331. [6] KARIM S, MADANI B, BURZANGI AS, et al. Urolithin A’s Antioxidative, Anti-Inflammatory, and Antiapoptotic Activities Mitigate Doxorubicin-Induced Liver Injury in Wistar Rats. Biomedicines. 2023;11(4):1125. [7] 乌里盼·托乎达阿里,孙媛,丁宛婷,等.基于miRNA155-5p介导的MAPK/NF-κB通路探讨尿石素A的抗炎机制[J].中国药理学通报,2024,40(6):1066-1074. [8] ZHAO H, SONG G, ZHU H, et al. Pharmacological Effects of Urolithin A and Its Role in Muscle Health and Performance: Current Knowledge and Prospects. Nutrients. 2023;15(20):4441. [9] RIBEIRO M, ALVARENGA L, CARDOZO LFMF, et al. Urolithin as a Metabolite of Ellagitannins and Ellagic Acid from Fruits and Nuts Produced by the Gut Microbiota: Its Role on Non-Communicable Diseases. Curr Nutr Rep. 2025;14(1):55. [10] PIDGEON R, MITCHELL S, SHAMASH M, et al. Diet-derived urolithin A is produced by a dehydroxylase encoded by human gut Enterocloster species. Nat Commun. 2025;16(1):999. [11] D’AMICO D, ANDREUX PA, VALDÉS P, et al. Impact of the Natural Compound Urolithin A on Health, Disease, and Aging. Trends Mol Med. 2021;27(7):687-699. [12] GARCÍA-VILLALBA R, GIMÉNEZ-BASTIDA JA, CORTÉS-MARTÍN A, et al. Urolithins: a Comprehensive Update on their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol Nutr Food Res. 2022; 66(21):e2101019. [13] TOMÁS-BARBERÁN FA, GARCÍA-VILLALBA R, GONZÁLEZ-SARRÍAS A, et al. Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J Agric Food Chem. 2014; 62(28):6535-6538. [14] RYU D, MOUCHIROUD L, ANDREUX PA, et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016; 22(8):879-888. [15] ANDREUX PA, BLANCO-BOSE W, RYU D, et al. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat Metab. 2019;1(6):595-603. [16] BOBOWSKA A, GRANICA S, FILIPEK A, et al. Comparative studies of urolithins and their phase II metabolites on macrophage and neutrophil functions. Eur J Nutr. 2021;60(4):1957-1972. [17] ZHAO H, ZHU H, YUN H, et al. Assessment of Urolithin A effects on muscle endurance, strength, inflammation, oxidative stress, and protein metabolism in male athletes with resistance training: an 8-week randomized, double-blind, placebo-controlled study. J Int Soc Sports Nutr. 2024;21(1):2419388. [18] 邓宇,杨诗颖,冼文妍,等.鞣花酸在UM-A型人肠道菌群中体外转化及对肠道菌群的影响[J].食品与发酵工业,2023, 49(2):34-40. [19] HASHEMINEZHAD SH, BOOZARI M, IRANSHAHI M, et al. A mechanistic insight into the biological activities of urolithins as gut microbial metabolites of ellagitannins. Phytother Res. 2022;36(1):112-146. [20] ZHANG M, CUI S, MAO B, et al. Ellagic acid and intestinal microflora metabolite urolithin A: A review on its sources, metabolic distribution, health benefits, and biotransformation. Crit Rev Food Sci Nutr. 2023;63(24):6900-6922. [21] TOMÁS-BARBERÁN FA, GONZÁLEZ-SARRÍAS A, GARCÍA-VILLALBA R, et al. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol Nutr Food Res. 2017;61(1):1-36. [22] ROMO-VAQUERO M, FERNÁNDEZ-VILLALBA E, GIL-MARTINEZ AL, et al. Urolithins: potential biomarkers of gut dysbiosis and disease stage in Parkinson’s patients. Food Funct. 2022;13(11):6306-6316. [23] HU Y, ZHANG L, WEI LF, et al. Liposomes encapsulation by pH driven improves the stability, bioaccessibility and bioavailability of urolithin A: A comparative study. Int J Biol Macromol. 2023;253(Pt 7):127554. [24] XIAN W, YANG S, DENG Y, et al. Distribution of Urolithins Metabotypes in Healthy Chinese Youth: Difference in Gut Microbiota and Predicted Metabolic Pathways. J Agric Food Chem. 2021;69(44):13055-13065. [25] MEMBREZ M, MIGLIAVACCA E, CHRISTEN S, et al. Trigonelline is an NAD+ precursor that improves muscle function during ageing and is reduced in human sarcopenia. Nat Metab. 2024;6(3):433-447. [26] AL AMIR DACHE Z, THIERRY AR. Mitochondria-derived cell-to-cell communication. Cell Rep. 2023;42(7):112728. [27] CAI X, NG CP, JONES O, et al. Lactate activates the mitochondrial electron transport chain independently of its metabolism. Mol Cell. 2023;83(21):3904-3920.e7. [28] LIANG R, ZHU L, HUANG Y, et al. Mitochondria: fundamental characteristics, challenges, and impact on aging. Biogerontology. 2024;25(6):923-941. [29] CESARE MM, FELICE F, SANTINI V, et al. Antioxidants in Sport Sarcopenia. Nutrients. 2020;12(9):2869. [30] OKOYE CN, KOREN SA, WOJTOVICH AP. Mitochondrial complex I ROS production and redox signaling in hypoxia. Redox Biol. 2023;67:102926. [31] 肖玉欣,王楠,王婧,等.鞣花酸和尿石素类代谢产物的生物活性及其对肠道健康的作用研究进展[J].食品科学,2022, 43(9):275-284. [32] HARPER C, GOPALAN V, GOH J. Exercise rescues mitochondrial coupling in aged skeletal muscle: a comparison of different modalities in preventing sarcopenia. J Transl Med. 2021;19(1):71. [33] PARRY HA, ROBERTS MD, KAVAZIS AN. Human Skeletal Muscle Mitochondrial Adaptations Following Resistance Exercise Training. Int J Sports Med. 2020;41(6):349-359. [34] LI X, LI C, ZHANG W, et al. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct Target Ther. 2023;8(1):239. [35] TYLUTKA A, WALAS Ł, ZEMBRON-LACNY A. Level of IL-6, TNF, and IL-1β and age-related diseases: a systematic review and meta-analysis. Front Immunol. 2024;15:1330386. [36] AGNIHOTRI P, DEKA H, CHAKRABORTY D, et al. Anti-inflammatory potential of selective small compounds by targeting TNF-α & NF-kB signaling: a comprehensive molecular docking and simulation study. J Biomol Struct Dyn. 2023;41(23):13815-13828. [37] GUO Q, JIN Y, CHEN X, et al. NF-κB in biology and targeted therapy: new insights and translational implications. Signal Transduct Target Ther. 2024;9(1):53. [38] FU W, WU G. Targeting mTOR for Anti-Aging and Anti-Cancer Therapy. Molecules. 2023;28(7):3157. [39] KUNZ HE, LANZA IR. Age-associated inflammation and implications for skeletal muscle responses to exercise. Exp Gerontol. 2023;177:112177. [40] ARNOLD WD, CLARK BC. Neuromuscular junction transmission failure in aging and sarcopenia: The nexus of the neurological and muscular systems. Ageing Res Rev. 2023;89:101966. [41] SO HK, KIM H, LEE J, et al. Protein Arginine Methyltransferase 1 Ablation in Motor Neurons Causes Mitochondrial Dysfunction Leading to Age-related Motor Neuron Degeneration with Muscle Loss. Research (Wash D C). 2023;6:0158. [42] HASTINGS RL, AVILA MF, SUNEBY E, et al. Cellular and molecular evidence that synaptic Schwann cells contribute to aging of mouse neuromuscular junctions. Aging Cell. 2023;22(11):e13981. [43] PADILLA CJ, HARRIGAN ME, HARRIS H, et al. Profiling age-related muscle weakness and wasting: neuromuscular junction transmission as a driver of age-related physical decline. Geroscience. 2021;43(3):1265-1281. [44] 周友,朱小艳,黄建荣,等.尿石素A通过促进线粒体自噬保护糖尿病小鼠心脏损伤的作用研究[J].中国药理学通报, 2020,36(10):1385-1390. [45] MORADI N, CHAMPSI S, HOOD DA. Sulforaphane, Urolithin A, and ZLN005 induce time-dependent alterations in antioxidant capacity, mitophagy, and mitochondrial biogenesis in muscle cells. Sports Med Health Sci. 2024;7(1):16-27. [46] LU Y, LI Z, ZHANG S, et al. Cellular mitophagy: Mechanism, roles in diseases and small molecule pharmacological regulation. Theranostics. 2023;13(2):736-766. [47] DONG Y, ZHANG X. Targeting cellular mitophagy as a strategy for human cancers. Front Cell Dev Biol. 2024;12:1431968. [48] WANG S, LONG H, HOU L, et al. The mitophagy pathway and its implications in human diseases. Signal Transduct Target Ther. 2023;8(1):304. [49] LUAN P, D’AMICO D, ANDREUX PA, et al. Urolithin A improves muscle function by inducing mitophagy in muscular dystrophy. Sci Transl Med. 2021;13(588):eabb0319. [50] GHOSH N, DAS A, BISWAS N, et al. Urolithin A augments angiogenic pathways in skeletal muscle by bolstering NAD+ and SIRT1. Sci Rep. 2020;10(1):20184. [51] SINGH A, D’AMICO D, ANDREUX PA, et al. Urolithin A improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Rep Med. 2022;3(5):100633. [52] LIU S, D’AMICO D, SHANKLAND E, et al. Effect of Urolithin A Supplementation on Muscle Endurance and Mitochondrial Health in Older Adults: A Randomized Clinical Trial. JAMA Netw Open. 2022;5(1):e2144279. [53] DENK D, SINGH A, KASLER H, et al. Impact of urolithin A supplementation, a mitophagy activator on mitochondrial health of immune cells (MitoIMMUNE): A randomized, double-blind, placebo-controlled trial in healthy adults. J Clin Oncol. 2024;42(16):1-10. [54] TONEY AM, FOX D, CHAIDEZ V, et al. Immunomodulatory Role of Urolithin A on Metabolic Diseases. Biomedicines. 2021;9(2):192. [55] KUEREC AH, LIM XK, KHOO AL, et al. Targeting aging with urolithin A in humans: A systematic review. Ageing Res Rev. 2024; 100:102406. [56] TUOHETAERBAIKE B, ZHANG Y, TIAN Y, et al. Pancreas protective effects of Urolithin A on type 2 diabetic mice induced by high fat and streptozotocin via regulating autophagy and AKT/mTOR signaling pathway. J Ethnopharmacol. 2020;250:112479. [57] DING SL, PANG ZY, CHEN XM, et al. Urolithin a attenuates IL-1β-induced inflammatory responses and cartilage degradation via inhibiting the MAPK/NF-κB signaling pathways in rat articular chondrocytes. J Inflamm (Lond). 2020;17:13. [58] LARROSA M, GONZÁLEZ-SARRÍAS A, YÁÑEZ-GASCÓN MJ, et al. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem. 2010;21(8):717-725. [59] GUADA M, GANUGULA R, VADHANAM M, et al. Urolithin A Mitigates Cisplatin-Induced Nephrotoxicity by Inhibiting Renal Inflammation and Apoptosis in an Experimental Rat Model. J Pharmacol Exp Ther. 2017;363(1):58-65. [60] BROOME SC, WHITFIELD J, KARAGOUNIS LG, et al. Mitochondria as Nutritional Targets to Maintain Muscle Health and Physical Function During Ageing. Sports Med. 2024; 54(9):2291-2309. [61] KUJAWSKA M, JOURDES M, KURPIK M, et al. Neuroprotective Effects of Pomegranate Juice against Parkinson’s Disease and Presence of Ellagitannins-Derived Metabolite-Urolithin A-In the Brain. Int J Mol Sci. 2019;21(1):202. [62] JING T, LIAO J, SHEN K, et al. Protective effect of urolithin a on cisplatin-induced nephrotoxicity in mice via modulation of inflammation and oxidative stress. Food Chem Toxicol. 2019;129:108-114. [63] TONEY AM, FAN R, XIAN Y, et al. Urolithin A, a Gut Metabolite, Improves Insulin Sensitivity Through Augmentation of Mitochondrial Function and Biogenesis. Obesity (Silver Spring). 2019;27(4):612-620. [64] CÁSEDAS G, LES F, CHOYA-FOCES C, et al. The Metabolite Urolithin-A Ameliorates Oxidative Stress in Neuro-2a Cells, Becoming a Potential Neuroprotective Agent. Antioxidants (Basel). 2020;9(2):177. [65] WOJCIECHOWSKA O, KUJAWSKA M. Urolithin A in Health and Diseases: Prospects for Parkinson’s Disease Management. Antioxidants (Basel). 2023;12(7):1479. [66] JAMIALAHMADI T, HASANPOUR M, VAKILIAN F, et al. Evaluation of Urolithin A Efficacy in Heart Failure Patients with Reduced Ejection Fraction: A Randomized, Double-blind, Crossover, Placebo-controlled Clinical Trial. Rev Recent Clin Trials. 2024;19(3):221-228. [67] KONDO S, ADACHI SI, KOMATSU W, et al. Antidiabetic Effect of Urolithin A in Cultured L6 Myotubes and Type 2 Diabetic Model KK-Ay/Ta Mice with Glucose Intolerance. Curr Issues Mol Biol. 2024;46(2):1078-1090. [68] LIU X, GUO B, LI Q, et al. mTOR in metabolic homeostasis and disease. Exp Cell Res. 2024;441(2):114173. [69] CHEN P, CHEN F, LEI J, et al. Activation of the miR-34a-Mediated SIRT1/mTOR Signaling Pathway by Urolithin A Attenuates D-Galactose-Induced Brain Aging in Mice. Neurotherapeutics. 2019;16(4):1269-1282. [70] RODRIGUEZ J, CAILLE O, FERREIRA D, et al. Pomegranate extract prevents skeletal muscle of mice against wasting induced by acute TNF-α injection. Mol Nutr Food Res. 2017;61(4):1-12. [71] TOTIGER TM, SRINIVASAN S, JALA VR, et al. Urolithin A, a Novel Natural Compound to Target PI3K/AKT/mTOR Pathway in Pancreatic Cancer. Mol Cancer Ther. 2019; 18(2):301-311. [72] BI J, SONG L, GUO Q, et al. Effect of urolithin A on intracellular survival of Mycobacterium tuberculosis by regulating AKT-FOXO1-mediated autophagy. mSphere. 2025;10(5): e0006125. [73] FAITG J, D’AMICO D, RINSCH C, et al. Mitophagy Activation by Urolithin A to Target Muscle Aging. Calcif Tissue Int. 2024; 114(1):53-59. [74] XIA B, SHI XC, XIE BC, et al. Urolithin A exerts antiobesity effects through enhancing adipose tissue thermogenesis in mice. PLoS Biol. 2020;18(3):e3000688. [75] NISHIMOTO Y, FUJISAWA K, UKAWA Y, et al. Effect of urolithin A on the improvement of vascular endothelial function depends on the gut microbiota. Front Nutr. 2023;9: 1077534. [76] ZHANG Y, ZHANG Y, HALEMAHEBAI G, et al. Urolithin A, a pomegranate metabolite, protects pancreatic β cells from apoptosis by activating autophagy. J Ethnopharmacol. 2021;272:113628. [77] D’AMICO D, OLMER M, FOUASSIER AM, et al. Urolithin A improves mitochondrial health, reduces cartilage degeneration, and alleviates pain in osteoarthritis. Aging Cell. 2022;21(8):e13662. [78] ZHU H, ZHAO H, QIAN H, et al. Urolithin A Ameliorates Athletic Ability and Intestinal Microbiota in Sleep Deprivation from the Perspective of the Gut-Muscle Axis. Mol Nutr Food Res. 2024;68(7):e2300599. [79] TU HJ, SU CJ, PENG CS, et al. Urolithin A exhibits a neuroprotective effect against Alzheimer’s disease by inhibiting DYRK1A activity. J Food Drug Anal. 2023;31(2):358-370. [80] ZHANG C, SONG Y, CHEN L, et al. Urolithin A Attenuates Hyperuricemic Nephropathy in Fructose-Fed Mice by Impairing STING-NLRP3 Axis-Mediated Inflammatory Response via Restoration of Parkin-Dependent Mitophagy. Front Pharmacol. 2022;13:907209. |
| [1] |
Dong Chunyang, Zhou Tianen, Mo Mengxue, Lyu Wenquan, Gao Ming, Zhu Ruikai, Gao Zhiwei.
Action mechanism of metformin combined with Eomecon chionantha Hance dressing in treatment of deep second-degree burn wounds#br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2001-2013.
|
| [2] | Yang Xuetao, Zhu Menghan, Zhang Chenxi, Sun Yimin, Ye Ling. Applications and limitations of antioxidant nanomaterials in oral cavity [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2044-2053. |
| [3] | Cai Ziming, Yu Qinghe, Ma Pengfei, Zhang Xin, Zhou Longqian, Zhang Chongyang, Lin Wenping. Heme oxygenase-1 alleviates lipopolysaccharide-induced inflammatory response in nucleus pulposus mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1624-1631. |
| [4] | He Jiale, Huang Xi, Dong Hongfei, Chen Lang, Zhong Fangyu, Li Xianhui. Acellular dermal matrix combined with adipose-derived stem cell exosomes promotes burn wound healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1699-1710. |
| [5] | Xia Linfeng, Wang Lu, Long Qianfa, Tang Rongwu, Luo Haodong, Tang Yi, Zhong Jun, Liu Yang. Human umbilical cord mesenchymal stem cell-derived exosomes alleviate blood-brain barrier damage in mice with septic encephalopathy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1711-1719. |
| [6] | Cui Lianxu, Li Haomin, Xu Junrong, Tan Baodong, Lu Dahong, Peng Siwei, Wang Jinhui. Effect of umbilical cord mesenchymal stem cell conditioned medium on tissue repair after traumatic craniocerebral injury in miniature pigs [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1730-1735. |
| [7] | Liu Anting, Lu Jiangtao, Zhang Wenjie, He Ling, Tang Zongsheng, Chen Xiaoling. Regulation of AMP-activated protein kinase by platelet lysate inhibits cadmium-induced neuronal apoptosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1800-1807. |
| [8] | Cao Yong, Teng Hongliang, Tai Pengfei, Li Junda, Zhu Tengqi, Li Zhaojin. Interactions between cytokines and satellite cells in muscle regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1808-1817. |
| [9] | You Huijuan, Wu Shuzhen, Rong Rong, Chen Liyuan, Zhao Yuqing, Wang Qinglu, Ou Xiaowei, Yang Fengying. Macrophage autophagy in lung diseases: two-sided effects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1516-1526. |
| [10] | Hou Chaowen, Li Zhaojin, Kong Jianda, Zhang Shuli. Main physiological changes in skeletal muscle aging and the multimechanism regulatory role of exercise [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1464-1475. |
| [11] | Yin Yongcheng, Zhao Xiangrui, Yang Zhijie, Li Zheng, Li Fang, Ning Bin. Effect and mechanism of peroxiredoxin 1 in microglial inflammation after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1106-1113. |
| [12] | Zhang Di, Zhao Jun, Ma Guangyue, Sun Hui, Jiang Rong. Mechanism of depression-like behavior in chronic social defeat stress mice based on high-throughput sequencing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1139-1146. |
| [13] | Li Haojing, Wang Xin, Song Chenglin, Zhang Shengnan, Chen Yunxin. Therapeutic efficacy of extracorporeal shock wave therapy in the upper trapezius muscle area combined with exercise control training in patients with chronic non-specific neck pain [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1162-1170. |
| [14] | Liu Yu, Lei Senlin, Zhou Jintao, Liu Hui, Li Xianhui. Mechanisms by which aerobic and resistance exercises improve obesity-related cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1171-1183. |
| [15] | Yu Huifen, Mo Licun, Cheng Leping. The position and role of 5-hydroxytryptamine in the repair of tissue injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1196-1206. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||