Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (10): 2594-2607.doi: 10.12307/2026.626
Previous Articles Next Articles
Effects of high-intensity interval training and moderate-intensity continuous training on cognitive function in patients with neurodegenerative diseases: a meta-analysis
Tang Zhuolin1, Yin Mingyue2, Bai Mingyang3, Zheng Huakun1, 4, Yan Henghao1, 5, Xu Kai2, Liu Qian1
- Tang Zhuolin1, Yin Mingyue2, Bai Mingyang3, Zheng Huakun1, 4, Yan Henghao1, 5, Xu Kai2, Liu Qian1
1School of Physical Education, Sichuan Agricultural University, Yaan 625014, Sichuan Province, China; 2School of Athletic Performance, 4School of Sports and Health, Shanghai University of Sport, Shanghai 200438, China; 3School of Sports Human Science, Beijing Sport University, Beijing 100091, China; 5Institute of Sports Science, Institute of Physical Education, Southwest University, Chongqing 400715, China
-
Received:2025-04-09Accepted:2025-06-08Online:2026-04-08Published:2025-08-30 -
Contact:Liu Qian, MS, Professor, Master’s supervisor, School of Physical Education, Sichuan Agricultural University, Yaan 625014, Sichuan Province, China -
About author:Tang Zhuolin, School of Physical Education, Sichuan Agricultural University, Yaan 625014, Sichuan Province, China Yin Mingyue, MS candidate, School of Athletic Performance, Shanghai University of Sport, Shanghai 200438, China -
Supported by:Sichuan Innovation Training Program for Undergraduates, No. S202410626075 (to TZL)
CLC Number:
Cite this article
Tang Zhuolin, Yin Mingyue, Bai Mingyang, Zheng Huakun, Yan Henghao, Xu Kai, Liu Qian. Effects of high-intensity interval training and moderate-intensity continuous training on cognitive function in patients with neurodegenerative diseases: a meta-analysis[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2594-2607.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
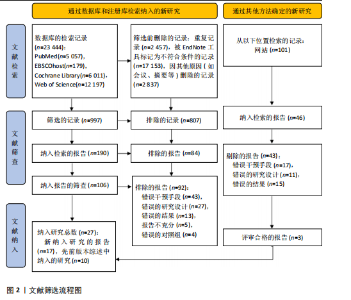
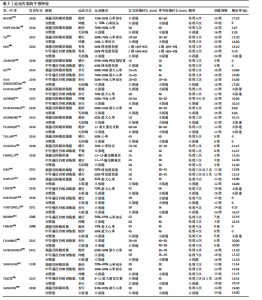
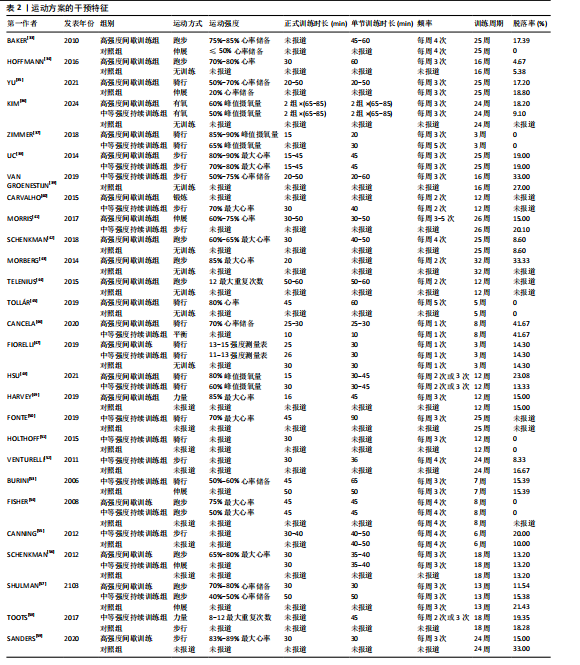
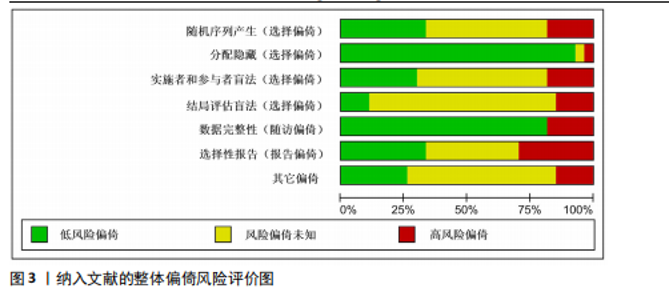
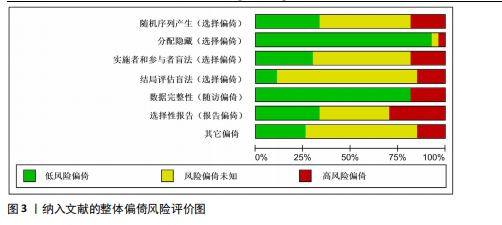
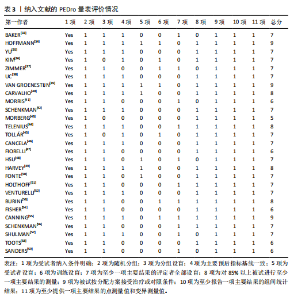
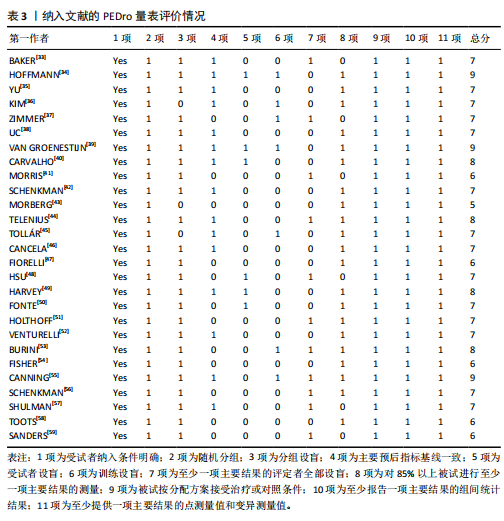
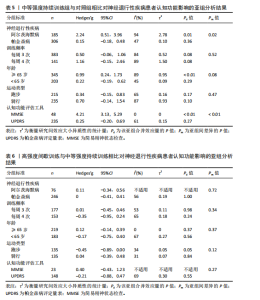
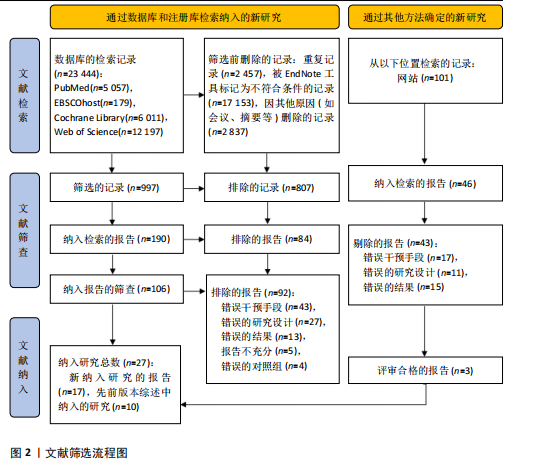
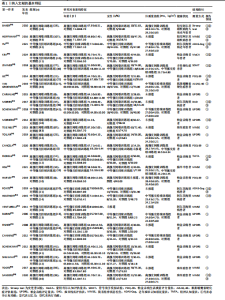
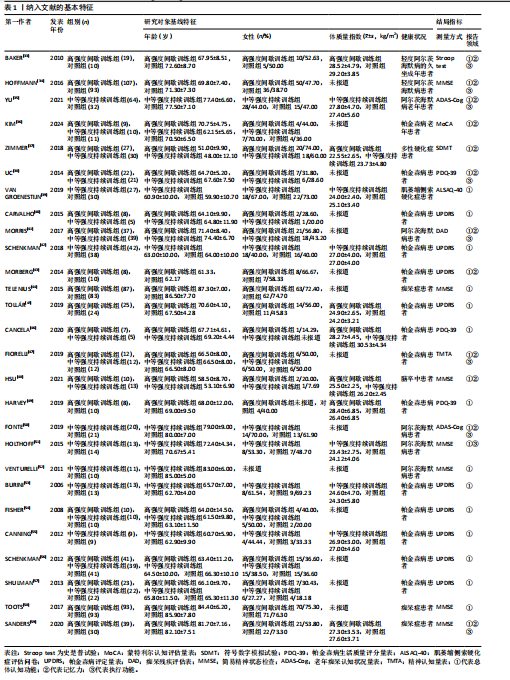
2.1 文献检索结果 共检索到23 444篇文献,通过检索先前版本综述中纳入的研究和Google学术补充了10项研究,按照标准筛选后共纳入27篇文献[33-59]。文献筛选流程见图2。 2.2 纳入文献的基本特征 共纳入1 613例神经退行性疾病患者,52%的研究对象为女性,总体平均年龄(72.06±12.89)岁,体质量指数(26.16±4.31) kg/m2。有1项研究未报告性别比例[52],有2项研究未报告完整性别比例[46,49]。在纳入的27篇文献中,研究对象多为帕金森病[36,38,40,42-43,45-47,49,53-57]、阿尔茨海默病患者[33–35,41,50-52]。在结局指标的报道中,16项研究使用MMSE和UPDRS[34,40,42–45,48,51-59];此外,有7项研究使用特定领域的量表评估干预后研究对象的认知功能变 化[35,38-39,41,46,49-50]。所有研究均报告了干预前后研究对象的总体认知功能变化。在27项研究中,仅有1项研究未在监督条件下进行[51],有3项研究未详细描述干预环境[36,40,46],5项研究在实验室环境下开展[34,42,47,50,54], 3项研究于家庭环境中实施[51,55-56],4项研究在社区环境下进行[33,35,41,49],9项研究在医院或疗养院中完成[37,44-45,48,52-53,57-59],1项研究是在健身房中进行[43],2项研究是在混合环境下进行(家庭和社区[38];家庭和医院[39])。纳入文献的基本特征见表1。 2.3 运动方案的干预特征 表2总结了所有运动方案的干预特征。13项研究比较了高强度间歇训练干预后与对照"
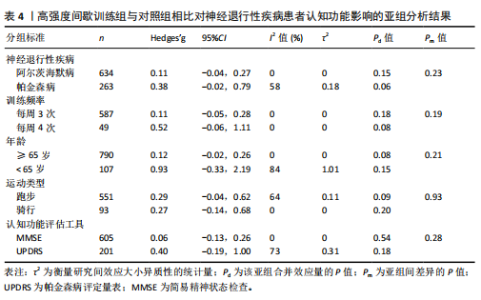
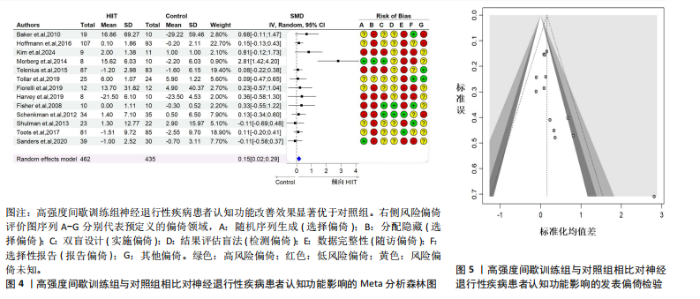
组的效果差异[33-34,36,43-45,47,49,54,56–59],13项研究比较了中等强度持续训练干预后与对照组的效果差异[35-36,39,42,47,50-57],11项研究比较了高强度间歇训练与中等强度持续训练的干预效果差异[36-38,40-41,46-48 ,54,56-57], 5项研究包含高强度间歇训练、中等强度持续训练组与对照组的对比[36,47,54,56-57]。训练频率从每周1次到5次不等,大多数研究采用每周3次的训练频率[34-39,41,48-51,53,56-59],12,24周的训练周期最为常见[36,38,40,44,48-49,51-52,59]。 共有17项研究报道了高强度间歇训练的依从性或脱落率,总体依从性较高(73%-100%),而由于研究间样本量差异较大,脱落率跨度较为宽泛(0%-41.67%)[33-34,36-38,41,43,45-49,54,56-59];17项研究报道了中等强度持续训练组的依从性或脱落率,总体依从性略高于高强度间歇训练(77.78%-100%),脱落率范围从0%到41.67%不等[35-39,41-42,46-48,51-57]。 2.4 文献风险偏倚评价 在纳入27项文献中,有10项在风险偏倚评估的2个或更多领域中存在高风险[38-40,43,45-46,52-54,56],其中最常见的风险偏倚与研究中的实施偏倚和报告偏倚有关[33,39-40,44-46,51-52,54,56-57],见图3。 2.5 文献质量评价 纳入的27篇文献中,受试者均按分配方案接受干预或对照条件,均报道了受试者的纳入条件和至少一项主要结果的组间统计结果以及点测量值和变异测量值[33-59],在受试者设盲、训练设盲和评估设盲方面报道较少,但整体研究的质量评分较高[33-42,44-59],详见表3。 2.6 Meta分析结果 2.6.1 高强度间歇训练与对照组相比对神经退行性疾病患者认知功能的改善效果 13项研究分析了高强度间歇训练与对照组相比对神经退行性疾病患者认知功能的改善效果[33-34,36,43-45,47,49,54,56-59],结果显示,与对照组相比,高强度间歇训练对神经退行性疾病患者的认知功能有显著改善作用[Hedges’g=0.15,95%CI(0.02,0.29),P=0.02],见图4,研究间存在中等异质性[I2=41.1%,P= 0.06,PI(0.02,0.29)]。Egger’s检验显示主效应的合并结果存在一定发表偏倚(P=0.03),详见图5。"
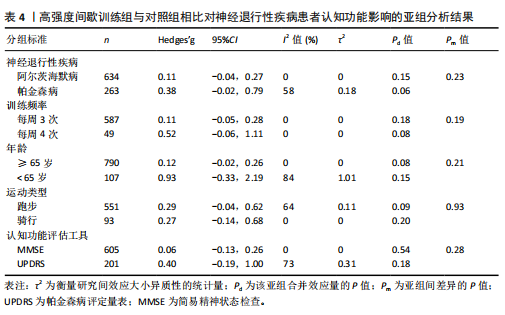
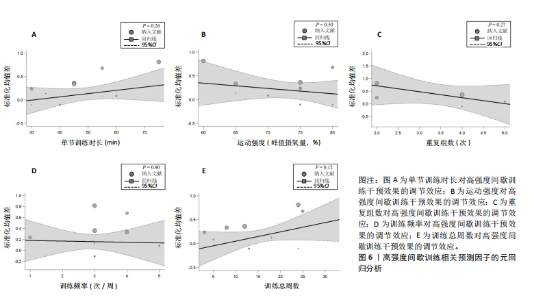
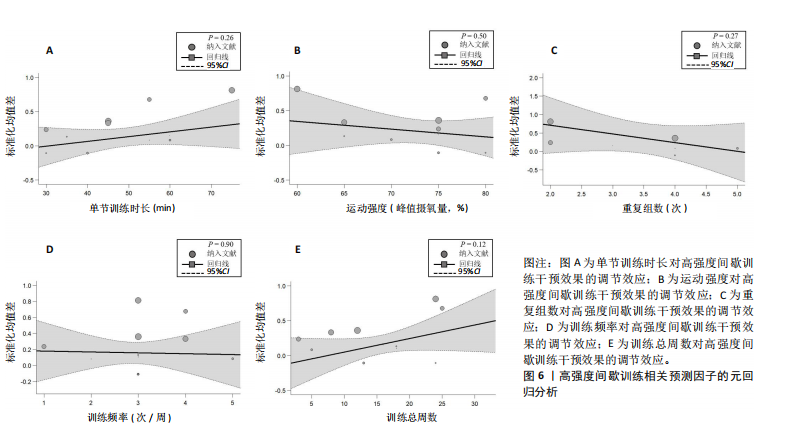
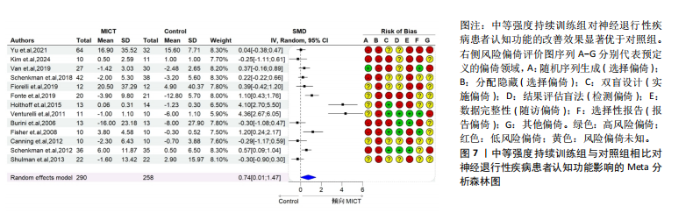
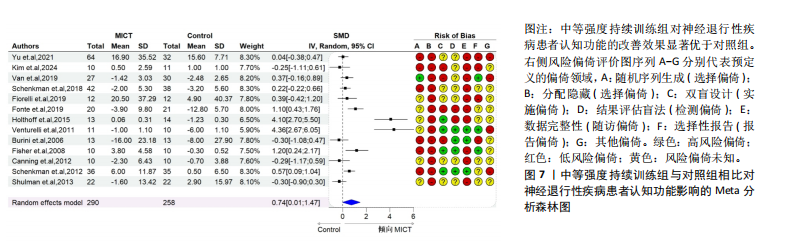
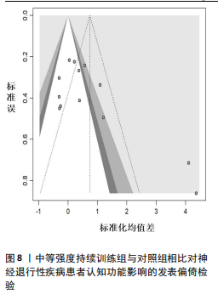
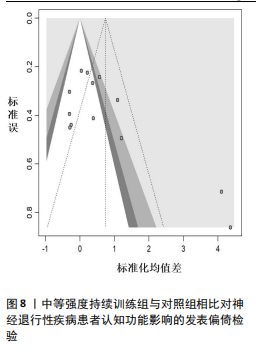
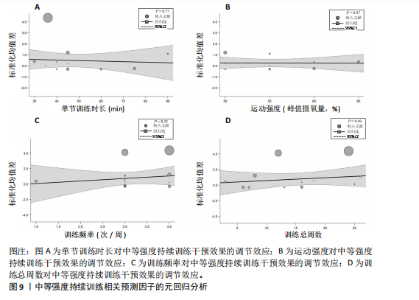
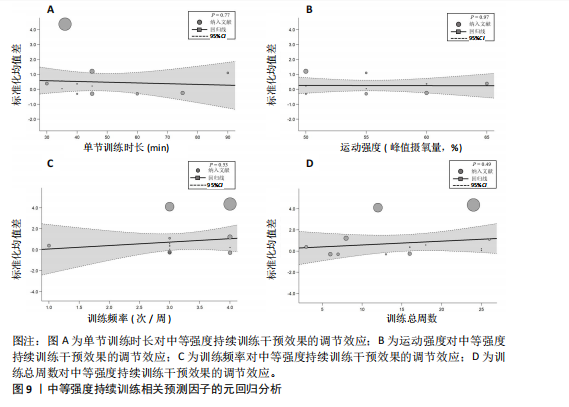
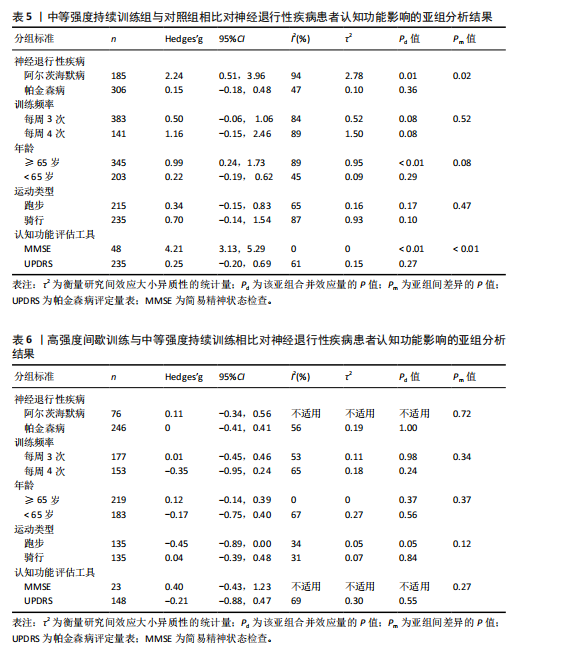
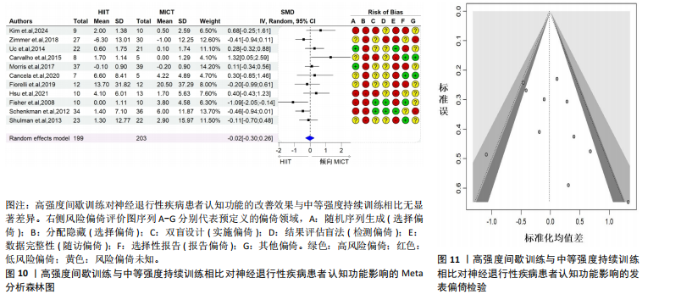
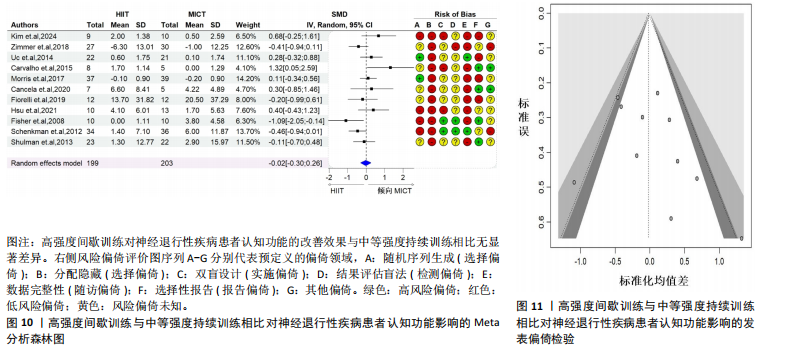
2.6.2 中等强度持续训练组与对照组相比对神经退行性疾病患者认知功能的改善效果 13项研究分析了中等强度持续训练组与对照组相比对神经退行性疾病患者认知功能的改善效果[35-36,39,42,47,50-57],结果显示,与对照组相比,中等强度持续训练对神经退行性疾病患者的认知功能有显著改善作用[Hedges’g=0.74,95%CI(0.01,1.47),P=0.04],见图7,但这些研究间的异质性极高[I2=83.2%,P < 0.01,PI(0.18,0.53)]。Egger’s检验显示主效应合并结果存在一定发表偏倚(P=0.04),详见图8。 亚组分析结果见表5。干预后使用不同认知功能评估工具会对中等强度持续训练干预效果产生显著影响,使用MMSE能比UPDRS捕捉到神经退行性疾病患者更细致的认知变化(Hedges’g=4.21 vs. 0.25,Pm < 0.01)。不同神经退行性疾病类型对中等强度持续训练干预效果存在显著影响(Pm=0.02),相比于帕金森病患者,中等强度持续训练对阿尔茨海默病患者认知功能具有更大的改善效果(Hedges’g=2.24 vs. 0.15)。年龄对中等强度持续训练干预效果存在显著影响趋势,≥65岁患者的认知改善效果可能优于< 65岁患者(Hedges’g =0.99 vs. 0.22,Pm=0.08)。其他亚组未发现存在显著影响(Pm > 0.05)。 中等强度持续训练的训练总周数从3周到27周不等,单次训练时长在30-45 min分布较为集中,训练频率在每周3次最多。运动强度则较为分散,从50%峰值摄氧量到65%峰值摄氧量不等。回归分析结果显示,没有因素对中等强度持续训练提升神经退行性疾病患者认知功能具有显著调节作用(P > 0.05),见图9。 2.6.3 高强度间歇训练与中等强度持续训练相比对神经退行性疾病患者认知功能改善的效果差异 11项研究分析了高强度间歇训练与中等强度持续训练相比对神经退行性疾病患者认知功能的改善效果[36-38,40-41,46-48,54,56-57],结果显示高强度间歇训练和中等强度持续训练对神经退行性疾病患者"
| [1] AMOR S, PUENTES F, BAKER D, et al. Inflammation in neurodegenerative diseases. Immunology. 2010;129(2):154-169. [2] GUO Q, LEHMER C, MARTÍNEZ-SÁNCHEZ A, et al. In situ structure of neuronal C9orf72 poly-GA aggregates reveals proteasome recruitment. Cell. 2018;172(4):696-705.e12. [3] GITLER AD, DHILLON P, SHORTER J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis Model Mech. 2017;10(5):499-502. [4] SELKOE DJ. Treatments for alzheimer’s disease emerge. Science. 2021;373(6555): 624-626. [5] ARMSTRONG MJ, OKUN MS. Diagnosis and treatment of parkinson disease: A review. JAMA. 2020;323(6):548-560. [6] TABRIZI SJ, SCHOBEL S, GANTMAN EC, et al. A biological classification of huntington’s disease: The integrated staging syste. Lancet Neurol. 2022;21(7):632-644. [7] ZHANG S, ZHEN K, SU Q, et al. The effect of aerobic exercise on cognitive function in people with alz[heimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Int J Environ Res Public Health. 2022;19(23):15700. [8] SACHS GA, CARTER R, HOLTZ LR, et al. Cognitive impairment: An independent predictor of excess mortality: a cohort study. Ann Intern Med. 2011;155(5):300-308. [9] TAHAMI MONFARED AA, BYRNES MJ, WHITE LA, et al. The humanistic and economic burden of alzheimer’s disease. Neurol Ther. 2022;11(2):525-551. [10] GUBERT C, HANNAN AJ. Exercise mimetics: Harnessing the therapeutic effects of physical activity. Nat Rev Drug Discov. 2021; 20(11):862-879. [11] LIU J, MIN L, LIU R, et al. The effect of exercise on cerebral blood flow and executive function among young adults: A double-blinded randomized controlled trial. Sci Rep. 2023;13(1):8269. [12] DOUGHERTY RJ, BOOTS EA, LINDHEIMER JB, et al. Fitness, independent of physical activity is associated with cerebral blood flow in adults at risk for alzheimer’s disease. Brain Imaging Behav. 2020;14(4):1154-1163. [13] ISO-MARKKU P, KUJALA UM, KNITTLE K, et al. Physical activity as a protective factor for dementia and alzheimer’s disease: Systematic review, meta-analysis and quality assessment of cohort and case-control studies. Brit J Sport Med. 2022;56(12):701-709. [14] YU F, SALISBURY D, MATHIASON MA. Inter-individual differences in the responses to aerobic exercise in alzheimer’s disease: Findings from the FIT-AD trial. J Sport Health Sci. 2021;10(1):65-72. [15] ENETTE L, VOGEL T, MERLE S, et al. Effect of 9 weeks continuous vs. interval aerobic training on plasma BDNF levels, aerobic fitness, cognitive capacity and quality of life among seniors with mild to moderate alzheimer’s disease: a randomized controlled trial. Eur Rev Aging Phys Act. 2020;17:1-16. [16] OLNEY N, WERTZ T, LAPORTA Z, et al. Comparison of acute physiological and psychological responses between moderate-intensity continuous exercise and three regimes of high-intensity interval training. J Strength Cond Res. 2018;32(8):2130-2138. [17] 殷明越,陈志力,李汉森,等.碎片化运动: 兼具应用可行性与健康促进效果的新策略[J].西安体育学院学报,2023,40(5):615-627. [18] RAMOS JS, DALLECK LC, TJONNA AE, et al. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: A systematic review and meta-analysis. Sports Med. 2015;45(5):679-692. [19] KATHIA MM, DUPLEA SG, BOMMARITO JC, et al. High-intensity interval versus moderate-intensity continuous cycling training in parkinson’s disease: A randomized trial. J Appl Physiol. 2024; 137(3):603-615. [20] O’CALLAGHAN A, HARVEY M, HOUGHTON D, et al. Comparing the influence of exercise intensity on brain-derived neurotrophic factor serum levels in people with parkinson’s disease: a pilot study. Aging Clin Exp Res. 2020;32(9):1731-1738. [21] HUGUES N, PELLEGRINO C, RIVERA C, et al. Is high-intensity interval training suitable to promote neuroplasticity and cognitive functions after stroke? Int J Mol Sci. 2021; 22(6):3003. [22] AHMADI S, BÉLANGER M, O’BRIEN MW, et al. Acute effects of high-intensity interval training and moderate-intensity continuous training on executive functions in healthy older adults. Sci Rep. 2025;15(1):6749. [23] JUnG BK, KIM K. Effects of 12 weeks of moderate-intensity continuous exercise and high-intensity interval exercise on cognitive function in elderly subjects. Asian J Kinesiol. 2024;26(2):48-58. [24] TAYLOR JL, BARNES JN, JOHNSON BD. The utility of high intensity interval training to improve cognitive aging in heart disease patients. Int J Environ Res Public Health. 2022;19(24):16926. [25] TSAI CL, PAN CY, TSENG YT, et al. Acute effects of high-intensity interval training and moderate-intensity continuous exercise on BDNF and irisin levels and neurocognitive performance in late middle-aged and older adults. Behav Brain Res. 2021;413:113472. [26] COATES AM, JOYNER MJ, LITTLE JP, et al. A perspective on high-intensity interval training for performance and health. Sports Med. 2023;53(Suppl 1):85-96. [27] CUMPSTON M, LI T, PAGE MJ, et al. Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10(10):ED000142. [28] MAHER CG, SHERRINGTON C, HERBERT RD, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713-721. [29] NAKAGAWA S, NOBLE DWA, SENIOR AM, et al. Meta-evaluation of meta-analysis: Ten appraisal questions for biologists. BMC Biol. 2017;15(1):18. [30] EGGER M, DAVEY SMITH G, SCHNEIDER M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315(7109):629-634. [31] PETERS JL, SUTTON AJ, JONES DR, et al. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991-996. [32] EKERDT DJ. Frontiers of research on work and retirement. J Gerontol B-Psychol. 2010; 65(1):69-80. [33] BAKER LD, FRANK LL, FOSTER-SCHUBERT K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. JAMA Neurol. 2010;67(1):71-79. [34] HOFFMANN K, SOBOL NA, FREDERIKSEN KS, et al. Moderate-to-high intensity physical exercise in patients with alzheimer’s disease: A randomized controlled trial. J Alzheimers Dis. 2016;50(2):443-453. [35] YU F, VOCK D M, ZHANG L, et al. Cognitive effects of aerobic exercise in alzheimer’s disease: A pilot randomized controlled trial. J Alzheimers Dis. 2021;80(1):233-244. [36] KIM R, CHOI S, KANG N, et al. Effects of high-intensity interval training and moderate-intensity continuous training on non-motor symptoms in patients with parkinson’s disease: a randomised pilot trial. J Neurol Neurosurg Psychiatry. 2024;95(9):886-888. [37] ZIMMER P, BLOCH W, SCHENK A, et al. High-intensity interval exercise improves cognitive performance and reduces matrix metalloproteinases-2 serum levels in persons with multiple sclerosis: A randomized controlled trial. Mult Scler. 2018;24(12):1635-1644. [38] UC EY, DOERSCHUG KC, MAGNOTTA V, et al. Phase I/II randomized trial of aerobic exercise in parkinson disease in a community setting. Neurology. 2014; 83(5):413-425. [39] VAN GROENESTIJN AC, SCHRÖDER CD, VAN EIJK RPA, et al. Aerobic exercise therapy in ambulatory patients with ALS: a randomized controlled trial. Neurorehabil Neural Repair. 2019;33(2):153-164. [40] CARVALHO A, BARBIRATO D, ARAUJO N, et al. Comparison of strength training, aerobic training, and additional physical therapy as supplementary treatments for parkinson’s disease: Pilot study. Clin Interv Aging. 2015;10:183-191. [41] MORRIS JK, VIDONI ED, JOHNSON DK, et al. Aerobic exercise for alzheimer’s disease: A randomized controlled pilot trial. PLoS One. 2017;12(2):e0170547. [42] SCHENKMAN M, MOORE CG, KOHRT WM, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol. 2018;75(2):219-226. [43] MORBERG BM, JENSEN J, BODE M, et al. The impact of high intensity physical training on motor and non-motor symptoms in patients with parkinson’s disease (PIP): a preliminary study. Neurorehabilitation. 2014;35(2):291-298. [44] TELENIUS EW, ENGEDAL K, BERGLAND A. Long-term effects of a 12 weeks high-intensity functional exercise program on physical function and mental health in nursing home residents with dementia: A single blinded randomized controlled trial. BMC Geriatr. 2015;15:158. [45] TOLLÁR J, NAGY F, HORTOBÁGYI T. Vastly different exercise programs similarly improve parkinsonian symptoms: A randomized clinical trial. Gerontology. 2019;65(2):120-127. [46] CANCELA JM, MOLLINEDO I, MONTALVO S, et al. Effects of a high-intensity progressive-cycle program on quality of life and motor symptomatology in a parkinson’s disease population: A pilot randomized controlled trial. Rejuvenation Res. 2020;23(6):508-515. [47] FIORELLI CM, CIOLAC EG, SIMIELI L, et al. Differential acute effect of high-intensity interval or continuous moderate exercise on cognition in individuals with parkinson’s disease. J Phys Act Health. 2019;16(2):157-164. [48] HSU CC, FU TC, HUANG SC, et al. Increased serum brain-derived neurotrophic factor with high-intensity interval training in stroke patients: A randomized controlled trial. Ann Phys Rehabil Med. 2021;64(4):101385. [49] HARVEY M, WESTON KL, GRAY WK, et al. High-intensity interval training in people with parkinson’s disease: A randomized, controlled feasibility trial. Clin Rehabil. 2019;33(3): 428-438. [50] FONTE C, SMANIA N, PEDRINOLLA A, et al. Comparison between physical and cognitive treatment in patients with MCI and alzheimer’s disease. Aging. 2019; 11(10):3138-3155. [51] HOLTHOFF VA, MARSCHNER K, SCHARF M, et al. Effects of physical activity training in patients with alzheimer’s dementia: Results of a pilot RCT study. PLoS One. 2015;10(4):e0121478. [52] VENTURELLI M, SCARSINI R, SCHENA F. Six-month walking program changes cognitive and ADL performance in patients with alzheimer. Am J Alzheimers Dis Other Demen. 2011;26(5):381-388. [53] BURINI D, FARABOLLINI B, IACUCCI S, et al. A randomised controlled cross-over trial of aerobic training versus qigong in advanced parkinson’s disease. Eur J Phys Rehabil Med. 2006;42(3):231-238. [54] FISHER BE, WU AD, SALEM GJ, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early parkinson’s disease. Arch Phys Med Rehabil. 2008;89(7): 1221-1229. [55] CANNING CG, ALLEN NE, DEAN CM, et al. Home-based treadmill training for individuals with parkinson’s disease: A randomized controlled pilot trial. Clin Rehabil. 2012;26(9):817-826. [56] SCHENKMAN M, HALL DA, BARÓN AE, et al. Exercise for people in early- or mid-stage parkinson disease: A 16-month randomized controlled trial. Phys Ther. 2012;92(11):1395-1410. [57] Shulman LM, Katzel LI, Ivey FM, et al. Randomized clinical trial of 3 types of physical exercise for patients with parkinson disease. JAMA Neurol. 2013;70(2):183-190. [58] TOOTS A, LITTBRAND H, BOSTRÖM G, et al. Effects of exercise on cognitive function in older people with dementia: A randomized controlled trial. J Alzheimers Dis. 2017;60(1):323-332. [59] SANDERS LMJ, HORTOBÁGYI T, KARSSEMEIJER EGA, et al. Effects of low- and high-intensity physical exercise on physical and cognitive function in older persons with dementia: A randomized controlled trial. Alzheimers Res Ther. 2020;12(1):28. [60] SENA IG, COSTA AVD, SANTOS IKD, et al. Feasibility and effect of high-intensity training on the progression of motor symptoms in adult individuals with parkinson’s disease: A systematic review and meta-analysis. PLoS One. 2023;18(11):e0293357. [61] 胡静芸,蔡明,商庆慧,等.高强度间歇训练改善认知功能及其机制研究进展[J].生理学报,2021,73(1):126-136. [62] VAYNMAN SS, YING Z, YIN D, et al. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006;1070(1):124-130. [63] INOUE K, OKAMOTO M, SHIBATO J, et al. Long-term mild, rather than intense, exercise enhances adult hippocampal neurogenesis and greatly changes the transcriptomic profile of the hippocampus. PLoS One. 2015;10(6):e0128720. [64] HAN YMY, CHAN MMY, CHOI CXT, et al. The neurobiological effects of mind-body exercise: A systematic review and meta-analysis of neuroimaging studies. Sci Rep. 2023;13(1):10948. [65] BAILEY DM, EVANS KA, MCENENY J, et al. Exercise-induced oxidative-nitrosative stress is associated with impaired dynamic cerebral autoregulation and blood-brain barrier leakage. Exp Physiol. 2011;96(11):1196-1207. [66] YIN M, LI H, BAI M, et al. Is low-volume high-intensity interval training a time-efficient strategy to improve cardiometabolic health and body composition? A meta-analysis. Appl Physiol Nutr Metab. 2023;49(3):273-292. [67] DANIELA M, CATALINA L, ILIE O, et al. Effects of exercise training on the autonomic nervous system with a focus on anti-inflammatory and antioxidants effects. Antioxidants (Basel). 2022;11(2):350. [68] ANDERSON T, WIDEMAN L. Exercise and the cortisol awakening response: A systematic review. Sports Med-Open. 2017;3(1):37. [69] STULTS-KOLEHMAINEN MA, SINHA R. The effects of stress on physical activity and exercise. Sports Med. 2014;44(1):81-121. [70] YU H, ZHAO X, WU X, et al. High-intensity interval training versus moderate-intensity continuous training on patient quality of life in cardiovascular disease: A systematic review and meta-analysis. Sci Rep. 2023; 13(1):13915. [71] HUGUES N, PIN-BARRE C, PELLEGRINO C, et al. Time-dependent cortical plasticity during moderate-intensity continuous training versus high-intensity interval training in rats. Cereb Cortex. 2022;32(17):3829-3847. [72] LIU W, LIN X, CHEN X, et al. Vision-based estimation of MDS-UPDRS scores for quantifying parkinson’s disease tremor severity. Med Image Anal. 2023;85:102754. [73] FOLSTEIN M, FOLSTEIN S, MCHUGH P. 5.2 mini-mental state examination (mmse). Manual of Screeners for Dementia, 2020:51. [74] REGNAULT A, BOROOJERDI B, MEUNIER J, et al. Does the MDS-UPDRS provide the precision to assess progression in early parkinson’s disease? Learnings from the parkinson’s progression marker initiative cohort. J Neurol. 2019;266(8):1927-1936. [75] ERKKINEN MG, KIM MO, GESCHWIND MD. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2018;10(4): a033118. [76] ABYADEH M, GUPTA V, PAULO JA, et al. Amyloid-beta and tau protein beyond Alzheimer’s disease. Neural Regen Res. 2024;19(6):1262-1276. [77] SHUR NF, CREEDON L, SKIRROW S, et al. Age-related changes in muscle architecture and metabolism in humans: The likely contribution of physical inactivity to age-related functional decline. Ageing Res Rev. 2021;68:101344. [78] PYKE W, IFRAM F, COVENTRY L, et al. The effects of different protocols of physical exercise and rest on long-term memory. Neurobiol Learn Mem. 2020;167:107128. [79] DE LIMA NS, DE SOUSA RAL, AMORIM FT, et al. Moderate-intensity continuous training and high-intensity interval training improve cognition, and BDNF levels of middle-aged overweight men. Metab Brain Dis. 2022;37(2):463-471. [80] ELBOIM-GABYZON M, BUXBAUM R, KLEIN R. The effects of high-intensity interval training (HIIT) on fall risk factors in healthy older adults: A systematic review. Int J Environ Res Public Health. 2021;18(22):11809. [81] EKKEKAKIS P, BIDDLE SJ. Extraordinary claims in the literature on high-intensity interval training (HIIT): IV. Is HIIT associated with higher long-term exercise adherence? Psychol Sport Exerc. 2023;64:102295. [82] 郑华坤,殷明越,刘骞.间歇与持续训练对体力活动不足成人生活质量影响的 Meta分析[J].中国组织工程研究, 2025,29(8):1727-1740. [83] LI Q, WANG F, LIU X, et al. Center-based vs home-based geriatric rehabilitation on sarcopenia components: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2022;103(8):1663-1675.e3. [84] ASHWORTH NL, CHAD KE, HARRISON EL, et al. Home versus center based physical activity programs in older adults. Cochrane Database Syst Rev. 2005;2005(1):CD004017. [85] 周香莲,周媛媛,王丽娜,等.老年性轻度认知功能障碍患者运动干预策略的研究进展[J].中国全科医学,2018,21(12): 1408-1412. [86] SANTOS A, BRAATEN K, MACPHERSON M, et al. Rates of compliance and adherence to high-intensity interval training: A systematic review and meta-analyses. Int J Behav Nutr Phys Act. 2023;20(1):134. |
| [1] | Sun Yaotian, Xu Kai, Wang Peiyun. Potential mechanisms by which exercise regulates iron metabolism in immune inflammatory diseases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1486-1498. |
| [2] | Chen Qiang, Wu Wenjuan, Jiang Shuhua, Huang Da. Physical exercise improves physical function in burn patients: a systematic review and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1269-1281. |
| [3] | Jiang Yang, Peng Hao, Song Yanping, Yao Na, Song Yueyu, Yin Xingxiao, Li Yanqi, Chen Qigang. Isometric exercise reduces resting blood pressure: a meta-analysis of moderating factors and dose effects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 975-986. |
| [4] | Sun Jiahe, Shi Jipeng, Zhu Tianrui, Quan Helong, Xu Hongqi. Effect of exercise intervention in elderly individuals with sarcopenia and its comorbidities: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 997-1007. |
| [5] | Hu Yujie, Xie Ping, , Lu Weijie, Yang Kang, Deng Yaoting, Liu Mengyang. Meta-analysis of the clinical efficacy of high-intensity interval exercise and middle-intensity continuous training in patients with coronary heart disease [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2584-2593. |
| [6] | Jiao Jingya, Zhang Yeting. Analysis of thematic evolution pathways in the field of physical activity and neurogenesis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2653-2661. |
| [7] | Wang Yida, Liu Jun, Wang Xiaoling, Wang Liyan, Yang Chengru, Zhang Xuexiao. Effects of wearable electronic device-based interventions on physical activity and sedentary behavior in healthy adolescents: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1693-1704. |
| [8] | Zhang Zixian, Xu Youliang, Wu Shaokui, Wang Xiangying. Effects of blood flow restriction training combined with resistance training on muscle indicators in college athletes: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1705-1713. |
| [9] | Wang Juan, Wang Guanglan, Zuo Huiwu. Efficacy of exercise therapy in the treatment of anterior cruciate ligament reconstruction patients: #br# a network meta-analysis #br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1714-1726. |
| [10] | Zheng Huakun, Yin Mingyue, Liu Qian. Effects of interval and continuous training on the quality of life in physically inactive adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1727-1740. |
| [11] | Tian Jinxin, Zhao Yuxin, Hu Tong, Cui Tiantian, Ma Lihong. Effects of different transcranial magnetic stimulation modes on refractory depression in adults: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7639-7648. |
| [12] | Deng Qing, Wang Qingjun, Zhang Yeting. Visual analysis of dynamic evolution of research topics in the field of physical activity and hippocampal tissue [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6997-7003. |
| [13] | Wang He, Yu Shaohong, . Meta-analysis of transcranial direct current stimulation in improving lower limb motor dysfunction in stroke patients [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6556-6565. |
| [14] | Zhu Tianrui, Shi Jipeng, Sun Jiahe, Wang Luyi, Zhang Chen, Xu Hongqi, Quan Helong. Effectiveness of different exercise regimens to reduce fall risks in older adults: a Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5662-5672. |
| [15] | Qiao Zhengji, Chai Niubing, Zheng Luyao, Gao Yunna, Wang Yang. Effect of whole‑body vibration training on bone mineral density in postmenopausal women: a meta‑analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5195-5202. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||