Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (1): 153-162.doi: 10.12307/2025.572
Previous Articles Next Articles
Application and progress of dental pulp stem cells and their derivatives in dental pulp regeneration
Xu Haichao, Luo Lihua, Pan Yihuai
- School and Hospital of Stomatology, Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China
-
Received:2024-11-18Accepted:2025-01-17Online:2026-01-08Published:2025-07-02 -
Contact:Pan Yihuai, PhD, Professor, Doctoral supervisor, School and Hospital of Stomatology, Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China; Co-corresponding author: Luo Lihua, PhD, Associate researcher, Master's supervisor, School and Hospital of Stomatology, Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China -
About author:Xu Haichao, School and Hospital of Stomatology, Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China -
Supported by:Zhejiang Provincial Basic Public Welfare Research Project, No. LGF21H140007 (to LLH)
CLC Number:
Cite this article
Xu Haichao, Luo Lihua, Pan Yihuai. Application and progress of dental pulp stem cells and their derivatives in dental pulp regeneration[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 153-162.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
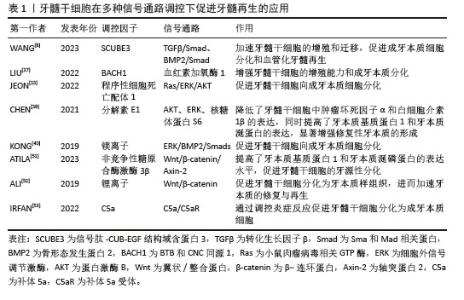
2.1 牙髓干细胞在牙髓组织工程中的应用 2.1.1 牙髓干细胞的定义及特征 牙髓干细胞是来源于牙髓组织的多能干细胞,主要通过酶消化法从正畸拔除的牙齿、埋伏牙和第三磨牙等牙髓组织中分离、提取,具有易于获取、无伦理问题以及免疫排斥反应低等独特优势。牙髓干细胞源于胚胎发育早期的神经嵴组织,具有多向分化能力和高度增殖潜力,可以作为牙髓再生的关键种子细胞来源。研究表明,牙髓干细胞可以分化为成牙本质细胞[13]、成骨细胞[14-16]、血管内皮细胞[4]、神经细胞等多种细胞类型[2,17]。此外,牙髓干细胞可通过旁分泌机制释放血管内皮生长因子、成纤维细胞生长因子和骨形态发生蛋白等多种生长因子,有助于促进血管生成和神经再生[8,18-20]。 2.1.2 牙髓干细胞在牙髓再生中的应用 在牙髓再生过程中,提高牙髓干细胞活性及促进其向成牙本质细胞、血管内皮细胞分化是恢复牙髓组织结构和功能的理想方法之一[18]。近年来,工程化干细胞作为干细胞研究领域的重要发展方向,已逐渐应用于牙髓再生治疗中。通过细胞因子调控、生物材料支持、物理条件调节和基因工程编辑等手段,工程化干细胞能够显著提升牙髓干细胞的活性,并促进其向成牙本质细胞及血管内皮细胞分化,从而加速牙髓组织的修复与再生。例如,相关细胞因子的添加可以增强牙髓干细胞的增殖潜力和分化能力[18],实现细胞状态的定向调控;生物支架和纳米载体的优化设计为牙髓干细胞提供了更为理想的再生微环 境[21];通过调节培养环境中的pH值、氧气体积分数及电磁场等物理因素[22-23],可精确调控牙髓干细胞的增殖和分化;此外,基因转染技术通过调控特定转录因子的表达[24-26],促进牙髓干细胞向目标细胞类型分化,进一步提升牙髓干细胞在牙髓再生中的效果。 (1)牙髓干细胞活性:为了提高牙髓再生的效果,增强和维持牙髓干细胞的活性包括增殖、黏附、迁移、分化和免疫调节能力等方面,是实现有效修复的关键。研究者们提出了多种工程化干细胞方案来提高牙髓干细胞的活性,为后续血运重建以及形成牙髓-牙本质样结构奠定了坚实的基础[27-28]。YU等[18]发现大麻二酚通过下调促炎细胞因子如肿瘤坏死因子α和白细胞介素1β的表达,有效恢复炎症环境中牙髓干细胞的增殖与分化活性,进一步增强成牙分化潜力。AYADILORD等[29]将植物体姜黄素纳米颗粒作用于牙髓干细胞,发现通过调控多个miRNA(如miR-21、miR-23、miR-155等)和CD200的表达,抑制磷脂酰肌醇3激酶/蛋白激酶B/核因子κB信号通路,从而调节干细胞的免疫活性并降低促炎细胞因子的表达。WANG等[22]利用等离子体纳米材料的电磁场效应和光热效应的协同作用,靶向增加牙髓干细胞线粒体内羟脯氨酸的含量,从而促进牙髓干细胞向成牙本质细胞的定向分化。ZHANG等[23]构建了一种磁性纳米颗粒交联的超分子聚合物纳米纤维,并通过施加人工磁场成功实现对牙髓干细胞极性的调控,促进牙髓干细胞定向极化及延伸等细胞行为,这一机制策略为牙髓再生提供了新的研究方向。JEONG等[30]利用牙髓干细胞构建了一种牙髓牙本质样类器官,这些类器官能够对生物刺激器展现出适当的反应,具有干细胞和分化细胞(如成牙本质细胞)的特征,呈现了在牙髓再生领域作为新型研究工具的巨大潜力。MEZA等[31]首次将自体牙髓干细胞与白细胞富血小板纤维蛋白基质联合应用于不可逆牙髓炎患者的恒牙再生手术,结果表明,白细胞富血小板纤维蛋白基质不仅为牙髓干细胞提供了理想的黏附与迁移环境,还通过释放生长因子促进了细胞的增殖与分化,最终成功再生出具有血管结构的均匀致密牙髓样组织。LIU等[32]通过在牙本质切片表面修饰多巴胺-透明质酸涂层来增强牙髓干细胞与根管壁之间的黏附力,研究表明涂层处理后的牙本质组在细胞数量、细胞伸长率和黏附力上明显优于未处理的牙本质组,进而促进了牙髓干细胞在根管内的增殖和迁移。TERRANOVA等[33]将鞣酸修饰的聚己内酯微粒引入电纺聚乳酸纳米纤维支架中,得到的3D锥体结构支架具备优良的结构可控性,易于插入根管,同时具有高度多孔性,能够有效促进牙髓干细胞的浸润与定植,增强增殖、分化和迁移活性,为牙髓组织再生与修复提供有力支持。 (2)牙髓干细胞向成牙本质细胞分化:牙髓-牙本质复合体的重建是牙髓再生的关键因素之一,大量研究表明,牙髓干细胞能够在多种信号通路的调控下向成牙本质细胞分化[34-36],从而促进牙髓-牙本质复合体的再生。LIU等[37]研究发现,过表达BTB和CNC同源1能够影响血红素加氧酶1信号通路从而促进牙髓干细胞向成牙本质细胞的分化。JEON和LIU等[13,38]研究发现,通过阻断程序性细胞死亡配体1信号,可以激活Ras-细胞外信号调节激酶(extracellular signal-regulated kinase,ERK)和蛋白激酶B信号通路,进一步促进牙髓干细胞的成牙本质分化。CHEN等[39]研究表明,分解素E1通过调节蛋白激酶B、ERK以及核糖体蛋白S6的磷酸化水平,显著促进牙髓干细胞中牙本质磷蛋白(dentin sialoprotein,DSP)的表达。另一项研究证明了镁离子(Mg2+)通过激活ERK/骨形态发生蛋白2/Smads信号通路促进牙髓干细胞向成牙本质细胞分化[40]。 WANG等[24]通过反转录病毒转染技术使牙髓干细胞过表达分泌型Frizzled相关蛋白2,并将其移植到上下颌牙拔除的兔模型中,实验组拔牙槽被大量高密度的牙本质样结构填充,并且表达了牙本质特异性蛋白,如牙本质涎磷蛋白(dentin sialophosphoprotein,DSPP)和牙本质基质蛋白1(dentin matrix protein 1,DMP1)等。XUAN等[41]使用自体乳牙牙髓干细胞治疗因创伤导致的年轻恒牙牙髓坏死,成功再生出含有正常成牙本质细胞层的牙髓组织,经过24个月的随访,发现再生牙髓仍然保持活性。另外,牙髓干细胞与生物材料的联合应用已被广泛探索,被认为是牙髓再生的有效策略之一。HAN等[42]采用双网络羧乙基几丁质水凝胶携载牙髓干细胞,在体外促进成牙本质分化,并在体内实验中展现出与天然牙髓组织生成相匹配的支架降解速率。JUN等[43]将氧化铈纳米颗粒引入到三氧化二矿物骨料并作用于牙髓干细胞,发现细胞内活性氧水平下降约70%,而牙髓干细胞成牙本质分化能力得到明显提升。其他支架材料如光交联丝素蛋白甲基丙烯酸酯复合水凝胶、富含氯化钾的卡拉胶/壳聚糖/明胶复合支架等为牙髓干细胞提供了优异的仿生微环境,进一步提升了牙髓干细胞的成牙本质分化能力[7,44-53]。相关文献见表1。 "
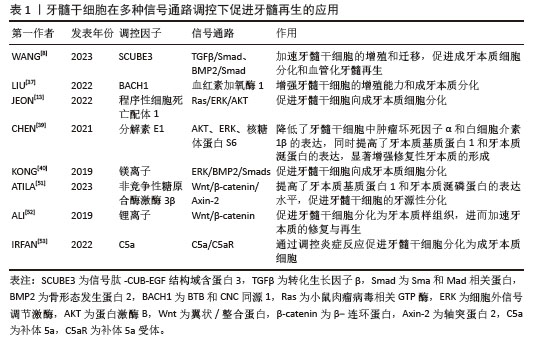
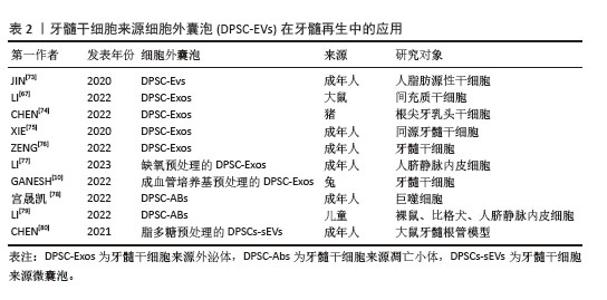
(3)牙髓干细胞向血管内皮细胞分化:在牙髓重建过程中,新生血管为周围组织提供必要的营养支持[54],有效促进牙髓干细胞向血管内皮细胞分化,并分泌相关血管生成活性因子。为了克服牙髓干细胞在单细胞培养模型中增殖缓慢和微环境模拟不足等问题,研究者们通过在单细胞培养模型中引入其他类型的细胞[55]、各种生长因子和活性材料建立细胞共培养体系,显著提高了牙髓干细胞的血管生成能力[56-58]。吕继忠[58]将小鼠成纤维细胞来源的诱导多能干细胞诱导分化为血管内皮样细胞后,与牙髓干细胞共培养,发现共培养体系中牙髓干细胞的血管内皮生长因子mRNA表达水平显著上升。KATATA等[4]以及ITOH等[59]的研究表明,使用内皮分化培养基对牙髓干细胞进行预处理,显著增强了牙髓干细胞的血管生成能力。BINDAL等[60]研究发现,人血小板裂解液可以显著提升牙髓干细胞中血管生成相关基因的表达。LIANG等[61]在比格犬和裸鼠体内模型中的研究表明,骨形态发生蛋白7能够诱导牙髓干细胞迁移,并促进新生血管向内生长,同时促进丰富的牙髓样组织形成。WU等[62]通过铜离子(Cu2+)激活晚期牙髓干细胞中染色盒蛋白同源物7 (chromobox protein homolog 7,CBX7)的表达,并将干细胞注射到裸鼠皮下移植模型中,发现CD31表达上调,提示有血管生成。NAKASHIMA等[63]在犬牙髓切除的临床前模型中,通过自体牙髓干细胞与粒细胞集落刺激因子联合移植到根管中,成功促进了血管和神经丰富的牙髓样组织形成,且治疗后的犬临床体征恢复正常。在验证干细胞疗法用于牙髓再生的临床前安全性和有效性后,NAKASHIMA等[64]首次将自体牙髓干细胞应用于人体多根磨牙的牙髓再生治疗,治疗后4周,患牙电活力测试呈阳性反应,患者未见全身毒性;24周时,核磁共振成像结果显示再生牙髓与对照牙髓几乎无差异,并形成血管-神经支配的牙本质样矿化组织;48周时,锥形束计算机断层扫描未见根尖周射线可透性,表明治疗后的牙髓组织稳定无渗漏,证明该疗法在人体牙髓再生中的临床安全性与疗效性。同时,研究者们构建封装牙髓干细胞与血管内皮生长因子的可注射海藻酸钠/锂皂石水凝胶微球,不但可以注射到常规细胞片层或刚性支架难以覆盖的区域,同时能够持续释放血管内皮生长因子长达28 d[65]。ATILA等[51,66]在水凝胶核/壳结构上分别负载了褪黑素和非竞争性糖原合酶激酶3β,成功实现了双重可控释放,其中非竞争性糖原合酶激酶3β作为首要释放成分,用于刺激血管生成,褪黑素起到延迟释放的作用,有效调控牙髓干细胞的早期增殖速率。此外,LI等[26]提出了一种新型非病毒基因纳米载体,通过携载血管内皮生长因子基因显著提升了转染后的牙髓干细胞血管生成潜力,有效促进了牙根血运重建与牙髓组织再生。 2.2 牙髓干细胞来源活性因子在牙髓组织工程中的应用 2.2.1 细胞外囊泡 (1)细胞外囊泡的定义及特征:牙髓干细胞来源细胞外囊泡是由牙髓干细胞分泌的一类被细胞膜包裹的纳米级囊泡,具有高度稳定性和生物相容性。根据国际细胞外囊泡学会的建议,通常将细胞外囊泡根据大小和形成机制分为3类:小的内体衍生的外泌体(直径30-150 nm)、大的质膜衍生的微囊泡(直径100-1 000 nm)和凋亡过程中产生的凋亡小体(直径50-1 000 nm)。由于牙髓干细胞基因工程的可行性和便利性,可作为细胞外囊泡的合适来源[67]。通过收集牙髓干细胞上清液,离心即可获得富含蛋白质、脂质和核酸如信使RNA(messenger RNA,mRNA)、微小RNA(microRNA,miRNA)、长链非编码RNA(long non-coding RNA,lncRNA)等活性因子的牙髓干细胞来源细胞外囊泡[68-70],牙髓干细胞来源细胞外囊泡可以停留在原始细胞附近或进入生物体液[71],通过细胞膜融合或内吞作用进入靶细胞,发挥信号通路传递作用。因较低的免疫排斥风险和较高的基因工程改造可行性[67,72],牙髓干细胞来源细胞外囊泡被认为是牙髓组织工程中一种极具潜力的无细胞治疗手段[73-80],相关文献见表2。 "
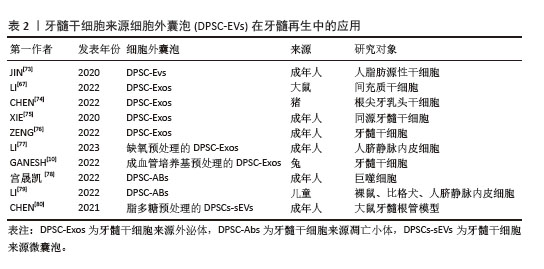
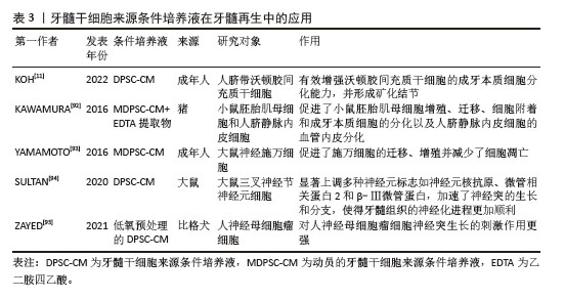
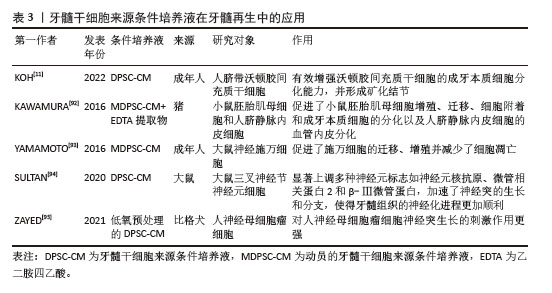
(2)细胞外囊泡在牙髓再生方面的应用 促进成牙本质分化:在牙髓组织修复过程中,牙髓干细胞来源细胞外囊泡内部的活性因子能够通过多种机制有效调控干细胞向成牙本质细胞的分化。研究表明,牙髓干细胞来源细胞外囊泡通过激活多条信号通路如ERK、C-Jun氨基末端激酶(C-Jun N-terminal kinase,JNK)和丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)等[73,81],促进人脂肪来源干细胞的成骨分化,效果与美国食品和药物管理局批准的骨形态发生蛋白2相当。此外,SWANSON等[82]实验表明,牙髓干细胞来源外泌体能够通过内吞作用刺激人源性间充质干细胞激活DSPP的转录,促进其向成牙本质细胞分化。CHEN等[74]研究发现,牙髓干细胞来源外泌体通过细胞归巢机制,成功招募了根尖牙乳头干细胞,并在体内形成牙髓样结缔组织。值得注意的是,牙髓干细胞来源细胞外囊泡中的miRNA在促进成牙本质分化过程中也发挥了重要作用。XIE等[75]研究发现,在牙髓干细胞来源外泌体被同型牙髓干细胞吞噬后,上调的环状溶血磷脂酸受体1与miR-31结合,消除了对牙髓干细胞成骨分化的抑制作用。LI等[67]研究表明,牙髓干细胞来源外泌体携载的长链非编码RNA锚定蛋白重复结构域26能够通过调控miR-150/Toll样受体4信号通路促进成骨细胞分化。 促进成血管和成神经分化:牙髓干细胞来源细胞外囊泡在牙髓再生中的另一个关键作用在于其强大的促进血管和神经再生能力。牙髓神经的再生依赖于健康血管提供的营养支持,牙髓干细胞来源细胞外囊泡通常包含可溶性血管生成相关蛋白(如血管内皮生长因子和成纤维细胞生长因子)及相关miRNA[83-85],在血管再生方面发挥着尤为重要的作用。ZHANG等[9]通过将含有牙髓干细胞来源细胞外囊泡的纤维蛋白凝胶与牙髓干细胞共培养,发现牙髓干细胞来源细胞外囊泡中的miRNA促进了血管内皮生长因子的分泌,并且伴随多种胶原蛋白的沉积,后者为新生血管的成熟和稳定提供了支持。GANESH等[10]发现,血管分化条件下的牙髓干细胞来源外泌体在促进血管生成方面有显著优势。蓝彬园等[86]研究发现,脂多糖预处理的牙髓干细胞分泌的外泌体能够在根管内形成更多新生血管密度的牙髓样组织。LI等[87]进一步表明,脂多糖预处理的牙髓干细胞分泌的外泌体还能够增强施万细胞的增殖、迁移及成牙本质分化的能力。CHEN等[80]将脂多糖预处理的牙髓干细胞产生的微囊泡植入到大鼠牙髓根管模型中,发现血管内皮生长因子、神经成束蛋白和牙本质涎磷蛋白等标志物表达显著上升,显示出脂多糖预处理的牙髓干细胞分泌的微囊泡在血管、神经和牙本质再生中的潜力。此外,不同年龄供者来源的牙髓干细胞衍生细胞外囊泡在血管和神经再生能力方面存在差异[88],年轻组的牙髓干细胞来源外泌体相较于年老组在促进血管内皮生长因子和肝细胞生长因子分泌上具有显著优势,显示出更高的无细胞治疗潜力。MAS-BARGUES等[89]研究发现,衰老牙髓干细胞来源细胞外囊泡能够诱导年轻牙髓干细胞中抗氧化基因过表达,进而增强抗氧化能力,并促进年轻牙髓干细胞的增殖、迁移与存活,从而调节年轻牙髓干细胞的微环境。 2.2.2 条件培养液 (1)条件培养液的定义及特征:牙髓干细胞来源条件培养液是通过收集在体外培养牙髓干细胞的基液,经过过滤、离心和浓缩等处理步骤,从而获得高生物活性的条件培养液。牙髓干细胞来源条件培养液富含细胞因子、生长因子、细胞外基质的分解产物和代谢产物等,能够通过旁分泌机制调节周围细胞的行为。由于含有丰富的生物活性成分和低免疫排斥风险,牙髓干细胞来源条件培养液被认为在组织修复领域具有重要的应用潜力[90]。与牙髓干细胞来源细胞外囊泡相比,牙髓干细胞来源条件培养液含有更多的分解产物和代谢因子,能够在微环境中提供更有利的修复条件,促进成牙本质分化,诱导新生血管形成并减少内皮细胞凋亡,在牙髓再生领域相关文献如表3所示,受到了广泛关注[91-95]。 "
| [1] LIANG Q, LIANG C, LIU X, et al. Vascularized dental pulp regeneration using cell-laden microfiber aggregates. J Mater Chem B. 2022; 10(48):10097-10111. [2] SUI B, CHEN C, KOU X, et al. Pulp Stem Cell–Mediated Functional Pulp Regeneration. J Dent Res. 2019;98(1):27-35. [3] ROTHERMUND K, CALABRESE TC, SYED-PICARD FN. Differential Effects of Escherichia coli- Versus Porphyromonas gingivalis-derived Lipopolysaccharides on Dental Pulp Stem Cell Differentiation in Scaffold-free Engineered Tissues. J Endod. 2022;48(11):1378-1386.e2. [4] KATATA C, SASAKI JI, LI A, et al. Fabrication of Vascularized DPSC Constructs for Efficient Pulp Regeneration. J Dent Res. 2021;100(12): 1351-1358. [5] XIE Z, SHEN Z, ZHAN P, et al. Functional Dental Pulp Regeneration: Basic Research and Clinical Translation. Int J Mol Sci. 2021;22(16):8991. [6] LAI CF, SHEN J, BALIC A, et al. Nogo-A Regulates the Fate of Human Dental Pulp Stem Cells toward Osteogenic, Adipogenic, and Neurogenic Differentiation. Cells. 2022; 11(21):3415. [7] LOUKELIS K, MACHLA F, BAKOPOULOU A, et al. Kappa-Carrageenan/Chitosan/Gelatin Scaffolds Provide a Biomimetic Microenvironment for Dentin-Pulp Regeneration. Int J Mol Sci. 2023;24(7):6465. [8] WANG Z, CHEN C, ZHANG J, et al. Epithelium-derived SCUBE3 promotes polarized odontoblastic differentiation of dental mesenchymal stem cells and pulp regeneration. Stem Cell Res Ther. 2023;14(1):130. [9] ZHANG S, THIEBES AL, KREIMENDAHL F, et al. Extracellular Vesicles-Loaded Fibrin Gel Supports Rapid Neovascularization for Dental Pulp Regeneration. Int J Mol Sci. 2020;21(12): 4226. [10] GANESH V, SEOL D, GOMEZ-CONTRERAS PC, et al. Exosome-Based Cell Homing and Angiogenic Differentiation for Dental Pulp Regeneration. Int J Mol Sci. 2022;24(1):466. [11] KOH B, AB RAHMAN FH, MATLAN NA, et al. Potential role of dental pulp stem cells conditioned medium for odontoblastic differentiation. Biol Res. 2022;55(1):11. [12] LI J, RAO Z, ZHAO Y, et al. A Decellularized Matrix Hydrogel Derived from Human Dental Pulp Promotes Dental Pulp Stem Cell Proliferation, Migration, and Induced Multidirectional Differentiation In Vitro. J Endod. 2020;46(10):1438-1447.e5. [13] JEON SM, LIM JS, PARK SH, et al. Blockade of PD-L1/PD-1 signaling promotes osteo-/odontogenic differentiation through Ras activation. Int J Oral Sci. 2022;14(1):18. [14] KIM H, OH N, KWON M, et al. Exopolysaccharide of Enterococcus faecium L15 promotes the osteogenic differentiation of human dental pulp stem cells via p38 MAPK pathway. Stem Cell Res Ther. 2022;13(1):446. [15] ZHAI S, LIU C, VIMALRAJ S, et al. Glucagon-like peptide-1 receptor promotes osteoblast differentiation of dental pulp stem cells and bone formation in a zebrafish scale regeneration model. Peptides. 2023;163: 170974. [16] MANIMARAN K, SHARMA R, SANKARANARAYANAN S, et al. Regeneration of mandibular ameloblastoma defect with the help of autologous dental pulp stem cells and buccal pad of fat stromal vascular fraction. Ann Maxillofac Surg. 2016;6(1):97-100. [17] TAKAOKA S, UCHIDA F, ISHIKAWA H, et al. Transplanted neural lineage cells derived from dental pulp stem cells promote peripheral nerve regeneration. Hum Cell. 2022;35(2): 462-471. [18] YU L, ZENG L, ZHANG Z, et al. Cannabidiol Rescues TNF-α-Inhibited Proliferation, Migration, and Osteogenic/Odontogenic Differentiation of Dental Pulp Stem Cells. Biomolecules. 2023;13(1):118. [19] ALGHUTAIMEL H, YANG X, DRUMMOND B, et al. Investigating the vascularization capacity of a decellularized dental pulp matrix seeded with human dental pulp stem cells: in vitro and preliminary in vivo evaluations. Int Endod J. 2021;54(8):1300-1316. [20] PHAN TV, OO Y, AHMED K, et al. Salivary gland regeneration: from salivary gland stem cells to three-dimensional bioprinting. SLAS Technol. 2023;28(3):199-209. [21] TIEN N, LEE JJ, LEE AK, et al. Additive Manufacturing of Caffeic Acid-Inspired Mineral Trioxide Aggregate/Poly-ε-Caprolactone Scaffold for Regulating Vascular Induction and Osteogenic Regeneration of Dental Pulp Stem Cells. Cells. 2021;10(11):2911. [22] WANG J, QU X, XU C, et al. Thermoplasmonic Regulation of the Mitochondrial Metabolic State for Promoting Directed Differentiation of Dental Pulp Stem Cells. Anal Chem. 2022; 94(27):9564-9571. [23] ZHANG B, YU Q, LIU Y. Polarization of Stem Cells Directed by Magnetic Field-Manipulated Supramolecular Polymeric Nanofibers. ACS Appl Mater Interfaces. 2021;13(8):9580-9588. [24] WANG C, WANG Y, WANG H, et al. SFRP2 enhances dental pulp stem cell-mediated dentin regeneration in rabbit jaw. Oral Dis. 2021;27(7):1738-1746. [25] MACHLA F, SOKOLOVA V, PLATANIA V, et al. Tissue engineering at the dentin-pulp interface using human treated dentin scaffolds conditioned with DMP1 or BMP2 plasmid DNA-carrying calcium phosphate nanoparticles. Acta Biomater. 2023;159:156-172. [26] LI Q, HU Z, LIANG Y, et al. Multifunctional peptide-conjugated nanocarriers for pulp regeneration in a full-length human tooth root. Acta Biomater. 2021;127:252-265. [27] ZHANG W, ZHENG Y, LIU H, et al. A non-invasive monitoring of USPIO labeled silk fibroin/hydroxyapatite scaffold loaded DPSCs for dental pulp regeneration. Mater Sci Eng C Mater Biol Appl. 2019; 103:109736. [28] QIU Y, SAITO T. Novel Bioactive Adhesive Monomer CMET Promotes Odontogenic Differentiation and Dentin Regeneration. Int J Mol Sci. 2021;22(23):12728. [29] AYADILORD M, NASSERI S, EMADIAN RAZAVI F, et al. Immunomodulatory effects of phytosomal curcumin on related-micro RNAs, CD200 expression and inflammatory pathways in dental pulp stem cells. Cell Biochem Funct. 2021;39(7):886-895. [30] JEONG SY, LEE S, CHOI WH, et al. Fabrication of Dentin-Pulp-Like Organoids Using Dental-Pulp Stem Cells. Cells. 2020;9(3):642. [31] MEZA G, URREJOLA D, SAINT JEAN N, et al. Personalized Cell Therapy for Pulpitis Using Autologous Dental Pulp Stem Cells and Leukocyte Platelet-rich Fibrin: A Case Report. J Endod. 2019;45(2):144-149. [32] LIU Y, QIU Y, NI S, et al. Mussel-Inspired Biocoating for Improving the Adhesion of Dental Pulp Stem Cells in Dental Pulp Regeneration. Macromol Rapid Commun. 2020;41(24):e2000102. [33] TERRANOVA L, LOUVRIER A, HÉBRAUD A, et al. Highly Structured 3D Electrospun Conical Scaffold: A Tool for Dental Pulp Regeneration. ACS Biomater Sci Eng. 2021; 7(12):5775-5787. [34] LIU Z, LI S, XU S, et al. Hsa_Circ_0005044 Promotes Osteo/Odontogenic Differentiation of Dental Pulp Stem Cell Via Modulating miR-296-3p/FOSL1. DNA Cell Biol. 2023;42(1): 14-26. [35] MU R, CHEN B, BI B, et al. LIM Mineralization Protein-1 Enhances the Committed Differentiation of Dental Pulp Stem Cells through the ERK1/2 and p38 MAPK Pathways and BMP Signaling. Int J Med Sci. 2022;19(8): 1307-1319. [36] 陈冬梅,徐丽丽,周建伟.姜黄素通过Wnt信号通路调节人牙髓干细胞的成牙本质分化[J].中国组织工程研究,2019,23(25): 4018-4024. [37] LIU C, YU J, LIU B, et al. BACH1 regulates the proliferation and odontoblastic differentiation of human dental pulp stem cells. BMC Oral Health. 2022;22(1):536. [38] LIU Y, JING H, KOU X, et al. PD-1 is required to maintain stem cell properties in human dental pulp stem cells. Cell Death Differ. 2018; 25(7):1350-1360. [39] CHEN J, XU H, XIA K, et al. Resolvin E1 accelerates pulp repair by regulating inflammation and stimulating dentin regeneration in dental pulp stem cells. Stem Cell Res Ther. 2021;12(1):75. [40] KONG Y, HU X, ZHONG Y, et al. Magnesium-enriched microenvironment promotes odontogenic differentiation in human dental pulp stem cells by activating ERK/BMP2/Smads signaling. Stem Cell Res Ther. 2019;10(1):378. [41] XUAN K, LI B, GUO H, et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10(455):eaaf3227. [42] HAN B, CAO C, WANG A, et al. Injectable Double-Network Hydrogel-Based Three-Dimensional Cell Culture Systems for Regenerating Dental Pulp. ACS Appl Mater Interfaces. 2023;15(6):7821-7832. [43] JUN SK, YOON JY, MAHAPATRA C, et al. Ceria-incorporated MTA for accelerating odontoblastic differentiation via ROS downregulation. Dent Mater. 2019;35(9): 1291-1299. [44] WANG L, ZHANG Y, XIA Y, et al. Photocross-linked silk fibroin/hyaluronic acid hydrogel loaded with hDPSC for pulp regeneration. Int J Biol Macromol. 2022;215:155-168. [45] XIA K, CHEN Z, CHEN J, et al. RGD- and VEGF-Mimetic Peptide Epitope-Functionalized Self-Assembling Peptide Hydrogels Promote Dentin-Pulp Complex Regeneration. Int J Nanomedicine. 2020; 15:6631-6647. [46] KHAYAT A, MONTEIRO N, SMITH EE, et al. GelMA-Encapsulated hDPSCs and HUVECs for Dental Pulp Regeneration. J Dent Res. 2017;96(2):192-199. [47] ZHANG Q, YANG T, ZHANG R, et al. Platelet lysate functionalized gelatin methacrylate microspheres for improving angiogenesis in endodontic regeneration. Acta Biomater. 2021;136:441-455. [48] PARK H, COLLIGNON AM, LEPRY WC, et al. Acellular dense collagen-S53P4 bioactive glass hybrid gel scaffolds form more bone than stem cell delivered constructs. Mater Sci Eng C Mater Biol Appl. 2021;120:111743. [49] BAKHTIAR H, ASHOORI A, RAJABI S, et al. Human amniotic membrane extracellular matrix scaffold for dental pulp regeneration in vitro and in vivo. Int Endod J. 2022;55(4): 374-390. [50] LUZURIAGA J, GARCÍA-GALLASTEGUI P, GARCÍA-URKIA N, et al. Osteogenic differentiation of human dental pulp stem cells in decellularised adipose tissue solid foams. Eur Cell Mater. 2022;43:112-129. [51] ATILA D, KESKIN D, LEE YL, et al. Injectable methacrylated gelatin/thiolated pectin hydrogels carrying melatonin/tideglusib-loaded core/shell PMMA/silk fibroin electrospun fibers for vital pulp regeneration. Colloids Surf B Biointerfaces. 2023;222:113078. [52] ALI M, OKAMOTO M, KOMICHI S, et al. Lithium-containing surface pre-reacted glass fillers enhance hDPSC functions and induce reparative dentin formation in a rat pulp capping model through activation of Wnt/β-catenin signaling. Acta Biomater. 2019;96: 594-604. [53] IRFAN M, KIM JH, MARZBAN H, et al. The role of complement C5a receptor in DPSC odontoblastic differentiation and in vivo reparative dentin formation. Int J Oral Sci. 2022;14(1):7. [54] 王舸,谢利,田卫东.牙髓再生中促进血管化策略的新进展[J].中国组织工程研究, 2022,26(30):4904-4911. [55] 张楚晗,张东敏,徐稳安.牙髓再生组织工程中的细胞共培养体系[J].中国组织工程研究,2023,27(15):2379-2384. [56] CATALDI A, AMOROSO R, DI GIACOMO V, et al. The Inhibition of the Inducible Nitric Oxide Synthase Enhances the DPSC Mineralization under LPS-Induced Inflammation. Int J Mol Sci. 2022;23(23):14560. [57] LIANG X, XIE L, ZHANG Q, et al. Gelatin methacryloyl-alginate core-shell microcapsules as efficient delivery platforms for prevascularized microtissues in endodontic regeneration. Acta Biomater. 2022;144: 242-257. [58] 吕继忠.成纤维细胞来源诱导性多能干细胞定向分化为血管内皮样细胞及与牙髓干细胞的共培养[J].中国组织工程研究,2018, 22(21):3371-3375. [59] ITOH Y, SASAKI JI, HASHIMOTO M, et al. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J Dent Res. 2018; 97(10):1137-1143. [60] BINDAL P, GNANASEGARAN N, BINDAL U, et al. Angiogenic effect of platelet-rich concentrates on dental pulp stem cells in inflamed microenvironment. Clin Oral Investig. 2019;23(10):3821-3831. [61] LIANG C, LIANG Q, XU X, et al. Bone morphogenetic protein 7 mediates stem cells migration and angiogenesis: therapeutic potential for endogenous pulp regeneration. Int J Oral Sci. 2022;14(1):38. [62] WU Y, LI B, YU D, et al. CBX7 Rejuvenates Late Passage Dental Pulp Stem Cells by Maintaining Stemness and Pro-angiogenic Ability. Tissue Eng Regen Med. 2023;20(3):473-488. [63] NAKASHIMA M, IOHARA K. Mobilized dental pulp stem cells for pulp regeneration: initiation of clinical trial. J Endod. 2014;40(4 Suppl): S26-32. [64] NAKASHIMA M, FUKUYAMA F, IOHARA K. Pulp Regenerative Cell Therapy for Mature Molars: A Report of 2 Cases. J Endod. 2022;48(10): 1334-1340.e1. [65] ZHANG R, XIE L, WU H, et al. Alginate/laponite hydrogel microspheres co-encapsulating dental pulp stem cells and VEGF for endodontic regeneration. Acta Biomater. 2020;113:305-316. [66] ATILA D, CHEN CY, LIN CP, et al. In vitro evaluation of injectable Tideglusib-loaded hyaluronic acid hydrogels incorporated with Rg1-loaded chitosan microspheres for vital pulp regeneration. Carbohydr Polym. 2022;278:118976. [67] LI L, GE J. Exosome‑derived lncRNA‑Ankrd26 promotes dental pulp restoration by regulating miR‑150‑TLR4 signaling. Mol Med Rep. 2022; 25(5):152. [68] GUO S, DEBBI L, ZOHAR B, et al. Stimulating Extracellular Vesicles Production from Engineered Tissues by Mechanical Forces. Nano Lett. 2021;21(6):2497-2504. [69] HUANG CC, NARAYANAN R, ALAPATI S, et al. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials. 2016;111:103-115. [70] KONG F, WU CT, GENG P, et al. Dental Pulp Stem Cell-Derived Extracellular Vesicles Mitigate Haematopoietic Damage after Radiation. Stem Cell Rev Rep. 2021;17(2): 318-331. [71] MA S, JIANG Y, QIAN Y, et al. The Emerging Biological Functions of Exosomes from Dental Tissue-Derived Mesenchymal Stem Cells. Cell Reprogram. 2023;25(2):53-64. [72] CHEN Y, KOSHY R, GUIRADO E, et al. STIM1 a calcium sensor promotes the assembly of an ECM that contains Extracellular vesicles and factors that modulate mineralization. Acta Biomater. 2021;120:224-239. [73] JIN Q, LI P, YUAN K, et al. Extracellular vesicles derived from human dental pulp stem cells promote osteogenesis of adipose-derived stem cells via the MAPK pathway. J Tissue Eng. 2020;11:2041731420975569. [74] CHEN Y, MA Y, YANG X, et al. The Application of Pulp Tissue Derived-Exosomes in Pulp Regeneration: A Novel Cell-Homing Approach. Int J Nanomedicine. 2022;17:465-476. [75] XIE L, GUAN Z, ZHANG M, et al. Exosomal circLPAR1 Promoted Osteogenic Differentiation of Homotypic Dental Pulp Stem Cells by Competitively Binding to hsa-miR-31. Biomed Res Int. 2020;2020:6319395. [76] ZENG J, HE K, MAI R, et al. Exosomes from human umbilical cord mesenchymal stem cells and human dental pulp stem cells ameliorate lipopolysaccharide-induced inflammation in human dental pulp stem cells. Arch Oral Biol. 2022;138:105411. [77] LI B, LIANG A, ZHOU Y, et al. Hypoxia preconditioned DPSC-derived exosomes regulate angiogenesis via transferring LOXL2. Exp Cell Res. 2023;425(2):113543. [78] 宫晟凯,杨晓姗,窦庚,等.牙髓干细胞来源凋亡小体调节巨噬细胞极化及炎症反应[J].口腔疾病防治,2022,30(1):12-19. [79] LI Z, WU M, LIU S, et al. Apoptotic vesicles activate autophagy in recipient cells to induce angiogenesis and dental pulp regeneration. Mol Ther. 2022;30(10):3193-3208. [80] CHEN WJ, XIE J, LIN X, et al. The Role of Small Extracellular Vesicles Derived from Lipopolysaccharide-preconditioned Human Dental Pulp Stem Cells in Dental Pulp Regeneration. J Endod. 2021;47(6):961-969. [81] LEE AE, CHOI JG, SHI SH, et al. DPSC-Derived Extracellular Vesicles Promote Rat Jawbone Regeneration. J Dent Res. 2023;102(3): 313-321. [82] SWANSON WB, ZHANG Z, XIU K, et al. Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater. 2020;118:215-232. [83] LI B, XIAN X, LIN X, et al. Hypoxia Alters the Proteome Profile and Enhances the Angiogenic Potential of Dental Pulp Stem Cell-Derived Exosomes. Biomolecules. 2022; 12(4):575. [84] 陈婷,李心竹,徐稳安.外泌体和细胞因子促进牙髓血管生成的作用与调控机制[J].中国组织工程研究,2020,24(14):2263-2270. [85] ZHOU H, LI X, WU RX, et al. Periodontitis-compromised dental pulp stem cells secrete extracellular vesicles carrying miRNA-378a promote local angiogenesis by targeting Sufu to activate the Hedgehog/Gli1 signalling. Cell Prolif. 2021;54(5):e13026. [86] 蓝彬园,林熹,陈文瑨,等.脂多糖刺激人牙髓干细胞分泌的外泌体联合基质细胞衍生因子-1对牙髓再生的影响[J].中华口腔医学杂志,2022,57(1):60-67. [87] LI J, JU Y, LIU S, et al. Exosomes derived from lipopolysaccharide-preconditioned human dental pulp stem cells regulate Schwann cell migration and differentiation. Connect Tissue Res. 2021;62(3):277-286. [88] BRUNELLO G, ZANOTTI F, TRENTINI M, et al. Exosomes Derived from Dental Pulp Stem Cells Show Different Angiogenic and Osteogenic Properties in Relation to the Age of the Donor. Pharmaceutics. 2022;14(5):908. [89] MAS-BARGUES C, SANZ-ROS J, ROMERO-GARCÍA N, et al. Small extracellular vesicles from senescent stem cells trigger adaptive mechanisms in young stem cells by increasing antioxidant enzyme expression. Redox Biol. 2023;62:102668. [90] TAKEUCHI H, TAKAHASHI H, TANAKA A. Effects of Human Dental Pulp Stem Cell-Derived Conditioned Medium on Atrophied Submandibular Gland after the Release from Ligation of the Main Excretory Duct in Mice. J Hard Tissue Biol. 2020;29(3):183-192. [91] KICHENBRAND C, VELOT E, MENU P, et al. Dental Pulp Stem Cell-Derived Conditioned Medium: An Attractive Alternative for Regenerative Therapy. Tissue Eng Part B Rev. 2019;25(1):78-88. [92] KAWAMURA R, HAYASHI Y, MURAKAMI H, et al. EDTA soluble chemical components and the conditioned medium from mobilized dental pulp stem cells contain an inductive microenvironment, promoting cell proliferation, migration, and odontoblastic differentiation. Stem Cell Res Ther. 2016; 7(1):77. [93] YAMAMOTO T, OSAKO Y, ITO M, et al. Trophic Effects of Dental Pulp Stem Cells on Schwann Cells in Peripheral Nerve Regeneration. Cell Transplant. 2016;25(1):183-193. [94] SULTAN N, AMIN LE, ZAHER AR, et al. Neurotrophic effects of dental pulp stem cells on trigeminal neuronal cells. Sci Rep. 2020;10(1):19694. [95] ZAYED M, IOHARA K, WATANABE H, et al. Characterization of stable hypoxia-preconditioned dental pulp stem cells compared with mobilized dental pulp stem cells for application for pulp regenerative therapy. Stem Cell Res Ther. 2021;12(1):302. [96] JOO KH, SONG JS, KIM S, et al. Cytokine Expression of Stem Cells Originating from the Apical Complex and Coronal Pulp of Immature Teeth. J Endod. 2018;44(1): 87-92.e1. [97] SARRA G, MACHADO MEL, CABALLERO-FLORES HV, et al. Effect of human dental pulp stem cell conditioned medium in the dentin-pulp complex regeneration: A pilot in vivo study. Tissue Cell. 2021;72:101536. [98] KARIMI-HAGHIGHI S, CHAVOSHINEZHAD S, SAFARI A, et al. Preconditioning with secretome of neural crest-derived stem cells enhanced neurotrophic expression in mesenchymal stem cells. Neurosci Lett. 2022;773:136511. [99] LAMBRICHTS I, DRIESEN RB, DILLEN Y, et al. Dental Pulp Stem Cells: Their Potential in Reinnervation and Angiogenesis by Using Scaffolds. J Endod. 2017;43(9S):S12-S16. [100] ZIAUDDIN SM, NAKASHIMA M, WATANABE H, et al. Biological characteristics and pulp regeneration potential of stem cells from canine deciduous teeth compared with those of permanent teeth. Stem Cell Res Ther. 2022;13(1):439. [101] MURAKAMI M, HORIBE H, IOHARA K, et al. The use of granulocyte-colony stimulating factor induced mobilization for isolation of dental pulp stem cells with high regenerative potential. Biomaterials. 2013;34(36): 9036-9047. [102] NAKASHIMA M, IOHARA K, MURAKAMI M, et al. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Res Ther. 2017; 8(1):61. [103] ZHENG L, LIU Y, JIANG L, et al. Injectable decellularized dental pulp matrix-functionalized hydrogel microspheres for endodontic regeneration. Acta Biomater. 2023;156:37-48. |
| [1] | Liu Yang, Liu Donghui , Xu Lei, Zhan Xu, Sun Haobo, Kang Kai. Role and trend of stimuli-responsive injectable hydrogels in precise myocardial infarction therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2072-2080. |
| [2] | Lai Yu, Chen Yueping, Zhang Xiaoyun. Research hotspots and frontier trends of bioactive materials in treating bone infections [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2132-2144. |
| [3] | Wang Qisa, Lu Yuzheng, Han Xiufeng, Zhao Wenling, Shi Haitao, Xu Zhe. Cytocompatibility of 3D printed methyl acrylated hyaluronic acid/decellularized skin hydrogel scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1912-1920. |
| [4] | Wang Songpeng, Liu Yusan, Yu Huanying, Gao Xiaoli, Xu Yingjiang, Zhang Xiaoming, Liu Min. Bidirectional regulation of reactive oxygen species based on zeolitic imidazolate framework-8 nanomaterials: from tumor therapy and antibacterial activity to cytoprotection [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2033-2013. |
| [5] | Wang Mingqi, Feng Shiya, Han Yinhe, Yu Pengxin, Guo Lina, Jia Zixuan, Wang Xiuli. Construction and evaluation of a neuralized intestinal mucosal tissue engineering model in vitro [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 892-900. |
| [6] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [7] | Yu Shiyu, Yu Sutong, Xu Yang, Zhen Xiangyan, Han Fengxuan. Advances in research and application of tissue engineering therapeutic strategies in oral submucous fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 936-948. |
| [8] | Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Mechanism by which vascular endothelial growth factor A targets regulation of angiogenesis in the treatment of steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 671-679. |
| [9] | Yang Hu, Zheng Yu, Jia Chengming, Wang Tong, Zhang Guangfei, Ji Yaoyao. Immune microenvironment regulates bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 701-710. |
| [10] | Gu Jianmei, Yuan Kunshan, Zhou Qiang, Zhang Haijun, , . Application of laser microporous decellularized scaffolds in tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 499-507. |
| [11] | Yang Fengli, Zhou Chao, Xiong Wei, Zhou Yuxiang, Li Dengshun, Wang Xin, Li Zhanzhen. 3D printed poly-L-lactic acid bone scaffolds in repair of bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 507-515. |
| [12] | Jiang Kan, Alimujiang·Abudourousuli, Shalayiding·Aierxiding, Aikebaierjiang·Aisaiti, Kutiluke·Shoukeer, Aikeremujiang·Muheremu. Biomaterials and bone regeneration: research hotspots and analysis of 500 influential papers [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 528-536. |
| [13] | Yan Qiquan, Yang Libin, Li Mengjun, Ni Yazhuo, Chen Keying, Xu Bo, Li Yaoyang, Ma Shiqing, Li Rui, Li Jianwen. Preparation and antibacterial properties of porcine small intestinal submucosal composite nanohydroxyapatite bioscaffold loaded with antimicrobial peptide KR-12-a5 [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 384-394. |
| [14] | Yuan Qian, Zhang Hao, Pang Jie. Characterization and biological properties of naringin-loaded chitosan/beta-tricalcium phosphate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 424-432. |
| [15] | Wang Zhuo, Sun Panpan, Cheng Huanzhi, Cao Tingting. Application of chitosan in repair and regeneration of oral hard and soft tissues [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 459-468. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||