Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (7): 1504-1511.doi: 10.12307/2025.016
Previous Articles Next Articles
Mechanism of exosomal miRNA involved in tumor chemotherapy resistance
Weng Zongqin, Zhao Hailong
- Teaching and Research Office of Pathophysiology, Zunyi Medical University, Zunyi 563000, Guizhou Province, China
-
Received:2023-11-16Accepted:2024-02-06Online:2025-03-08Published:2024-06-28 -
Contact:Zhao Hailong, MD, Associate professor, Teaching and Research Office of Pathophysiology, Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
About author:Weng Zongqin, Master candidate, Teaching and Research Office of Pathophysiology, Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
Supported by:National Natural Science Foundation of China, No. 82060503 (to ZHL); Guizhou Provincial Science and Technology Plan Project, No. Qiankehe Foundation-ZK[2022] General 622; Qiankehe Foundation [2019] No. 1334 (to ZHL); Science and Technology Fund Funding Project of Guizhou Provincial Health Commission, No. gzwjkj2019-1-033 (to ZHL)
CLC Number:
Cite this article
Weng Zongqin, Zhao Hailong. Mechanism of exosomal miRNA involved in tumor chemotherapy resistance[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1504-1511.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
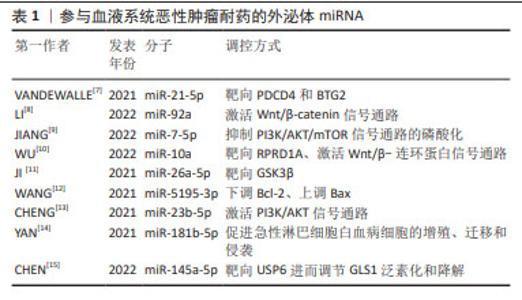
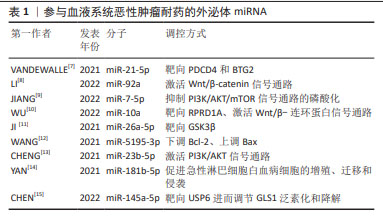
2.1 外泌体miRNA与血液系统恶性肿瘤化疗耐药 外泌体研究的历程始于对细胞间通讯工具的初步认知,见图4,随着研究的深入,逐渐揭示出外泌体在细胞之间传递信息的关键作用。急性髓细胞白血病(acute myeloidleukemia,AML)耐药产生的原因与外泌体源性miRNA表达水平有关,外泌体源性miRNA通过降低AML细胞对化疗药物的敏感性、调节AML细胞周期和抑制AML细胞凋亡介导耐药性的产生,见表1。外泌体miRNA在AML中介导耐药性产生的具体机制主要是相关通路和靶点失调:外泌体miR-21-5p通过靶向PDCD4和BTG2,使得阿糖胞苷处理后AML细胞的凋亡水平降低,促进AML细胞生长[7];外泌体miR-92a在血浆中呈现表达上调,miR-92a可以在受体细胞中转运和下调PTEN并激活Wnt/β-catenin信号通路,引起AML细胞对阿糖胞苷的耐药[8];骨髓间充质干细胞源性外泌体miR-7-5p通过抑制PI3K/AKT/mTOR信号通路的磷酸化来负调节OSBPL11,从而抑制AML细胞增殖并促进细胞凋亡[9];骨髓间充质干细胞是AML骨髓微环境的组成部分,并通过外泌体在微环境中维持与AML细胞之间通讯,促进AML进展,与骨髓间充质干细胞源性外泌体共培养的AML细胞暴露于阿糖胞苷的化学敏感性较低,这表明骨髓间充质干细胞源性外泌体可以保护AML细胞免受阿糖胞苷抑制作用,与此同时,与来自健康供者的骨髓间充质干细胞源性外泌体相比,来自AML患者的骨髓间充质干细胞源性外泌体中miR-10a表达水平显著上调,通过下调miR-10a表达后,AML患者的骨髓间充质干细胞源性外泌体处理的AML细胞的化学敏感性显著增加,提示miR-10a可能介导耐药反应。RPRD1A为miR-10a靶点之一,下调其表达后AML细胞的化学敏感性随之降低,最终导致Wnt/β-连环蛋白信号通路激活。总的来说,miR-10a经由外泌体传递给AML细胞,靶向RPRD1A并激活Wnt/β-catenin信号通路,从而降低AML细胞的化学敏感性[10]。除此之外,miR-26a-5p也具有相同调节方式[11]。 部分外泌体miRNA则可以通过激活细胞周期来模拟肿瘤细胞增殖,如AML细胞衍生的miR-5195-3p通过下调Bcl-2和上调Bax表达来提高肿瘤细胞的抗凋亡水平,而miR-23b-5p 通过 PI3K/AKT 途径减少肿瘤细胞增殖并诱导细胞凋亡[12-13]。外泌体miRNA不仅在AML中被发现,其在急性淋巴细胞白血病(acute lymphoblastic leukemia,ALL)中也具有相关报道,如miR-181b-5p在所有ALL细胞系中高表达,携带这种miRNA的外泌体可以促进ALL细胞的增殖、迁移和侵袭,进而引发耐药产生。miR-181b-5p还可以通过抑制细胞凋亡来促进ALL的进展[14]。慢性髓系白血病(chronic myelogenous leukemia,CML)细胞耐药性也可被外泌体调节,如外泌体miR-145a-5p通过靶向USP6进而调节GLS1泛素化和降解,最终促进耐药细胞凋亡[15]。此外,外泌体miRNA在弥漫性大B细胞淋巴瘤(diffuse large B-cell lymphoma,DLBCL)中也有所涉及,外泌体miR-451a在DLBCL中显著下调,且DLBCL患者血清中外泌体miR-451a水平与无进展生存期和总生存期均显著相关[16]。 综上所述,外泌体源性miRNA与血液系统恶性肿瘤化疗耐药关系密切,并且通过促进癌细胞应对压力和产生化学耐药性来促进癌细胞的存活和转移。"

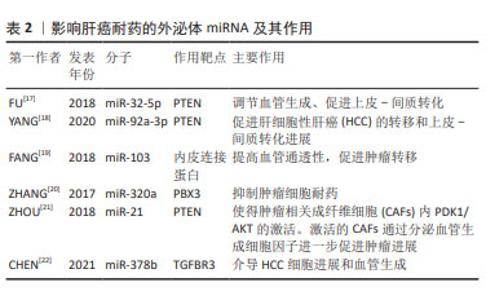
2.2 外泌体miRNA与肝癌化疗耐药 肝细胞性肝癌(hepatocelular carcinoma,HCC)耐药现象的产生,主要表现为治疗过程中肿瘤持续增长和远处转移,如耐药的肝癌细胞外泌体能够将致癌miR-32-5p传递至药物敏感细胞,使 PTEN表达明显下降,并通过调节血管生成和上皮-间质转化诱导敏感细胞多药耐药发生[17],见表2。研究发现,肝癌细胞源性外泌体miR-92a-3p可通过抑制PTEN和激活相关信号通路,进而促进HCC的转移和上皮-间质转化进展[18];肝癌细胞分泌的miR-103能够靶向多个内皮连接蛋白,提高血管通透性,促进肿瘤转移[19]。除此之外,肿瘤相关成纤维细胞在肝癌转移中也扮演重要角色,如外泌体miR-320a通过直接结合下游靶受体PBX3从而抑制肿瘤细胞耐药[20]。 "
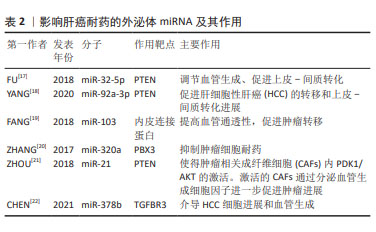
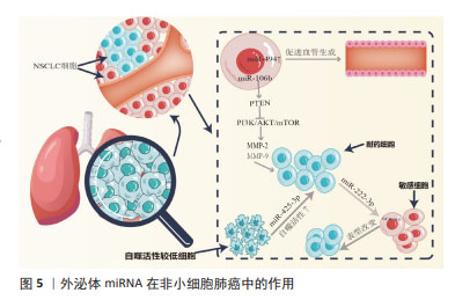
HCC来源外泌体miRNA还能调控并干扰靶基因的表达,诱导肿瘤血管新生,进而促进肝癌侵袭和转移。研究发现,外泌体miR-21可通过调控PTEN,导致肿瘤相关成纤维细胞内PDK1/AKT的激活,激活的肿瘤相关成纤维细胞通过分泌血管生成细胞因子进一步促进肿瘤进展,同时高水平的血清外泌体miR-21与HCC患者的肿瘤相关成纤维细胞活化程度和血管密度高度相关[21]。细胞凋亡的失调在肝癌耐药中同样重要,例如,CHEN等[22]研究表明,miR-378b在 HCC中高表达,而TGFBR3则在HCC中低表达,且二者呈靶向关系,下调miR-378b可干扰HCC细胞迁移并促进细胞凋亡,相反,过表达miR-378b可促进血管生成和肿瘤生长,敲低TGFBR3则逆转了下调miR-378b对HCC细胞的影响,提示外泌体miR-378b介导 HCC细胞进展和血管生成经由TGFBR3实现。上述研究成果呈现出外泌体miRNA多方面参与肝癌发展,这为深入了解其机制提供了重要线索,同时提示外泌体miRNA在肝癌治疗方面的潜在价值,尤其是在干预血管生成和调控细胞凋亡方面。然而,还需要进一步的研究来验证这些发现,并考虑在临床应用中的可行性。整体而言,这个领域的研究为未来肝癌治疗策略的制定提供了有益的信息。 2.3 外泌体miRNA与肺癌化疗耐药 据估计,2015-2030年肺癌死亡率可能会激增近40%[23]。作为肺癌的原发类型,非小细胞肺癌(non-small cell lung cancer,NSCLC)表现出多种组织学类型,如支气管肺泡癌、大细胞癌、鳞状细胞癌和腺癌。迄今为止,铂类化疗是治疗晚期NSCLC患者的最有效疗法,具有更高的生存率和反应率,但同时也具有显著的不良反应,如获得性或内在化学耐药性,严重影响化疗和靶向治疗的疗效。外泌体miRNA可通过促进化疗药物外排或改变药物作用靶点等方式介导肺癌耐药性的产生:WEI等[24]发现,亲代敏感细胞可接受来自吉西他滨耐药细胞的外泌体miR-222-3p,以自身SOCS3的启动子作为miR-222-3p靶点,诱导敏感细胞产生对吉西他滨的耐药性,见图5;SUN等[25]发现外泌体miR-106b可通过靶向PTEN,进而负调控PI3K/AKT/mTOR和增加基质金属蛋白酶2和基质金属蛋白酶9的表达,从而介导耐药产生。DONG等[26]发现缺氧 NSCLC 细胞源性外泌体 miR-21可通过下调磷酸酶和PTEN促进顺铂耐药;SONG等[27]发现与对治疗有反应的NSCLC患者相比,对顺铂耐药的NSCLC患者肿瘤组织来源外泌体中miR-4443的表达更高,且miR-4443的高表达与促进NSCLC细胞的多柔比星耐药性产生相关;MAO等[28]发现miR-494在NSCLC的肿瘤组织中表达上调,miR-494促进A549 NSCLC细胞系的血管生成,并且缺氧可导致其表达增加,miR-494较高的水平与较差的预后和增加顺铂耐药性有关。"

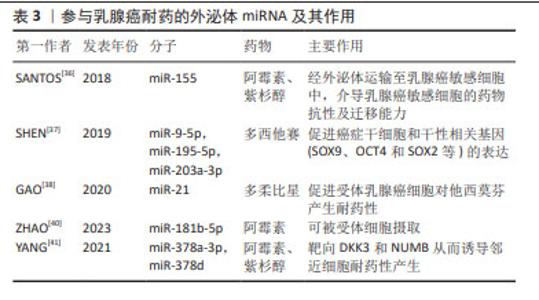
外泌体miRNA还可以作为自噬介质诱导耐药,如来源于顺铂耐药的NSCLC细胞的外泌体miRNA-425-3p通过AKT1/mTOR信号通路导致自噬活性上调,从而增加对顺铂的耐药性[29]。研究证明,NSCLC细胞来源肿瘤相关成纤维细胞对顺铂治疗具有先天耐药性,肿瘤相关成纤维细胞条件培养基显著提高了顺铂治疗后NSCLC细胞的存活率,即赋予NSCLC细胞的顺铂抗性。综上所述,外泌体在耐药性形成中具有多方面潜力。外泌体通过传递miRNA,如miR-222-3p、miR-106b、miR-21、miR-4443和miR-494,能够影响细胞内信号通路,调控基因表达,以及促进血管生成等生物过程,从而导致药物抵抗。此外,外泌体中的miRNA还能作为自噬递质,通过调控自噬活性影响细胞的药物敏感性,这表明外泌体在传递信息、调控基因表达和影响细胞行为方面具有重要的作用。 EGFR也称为ErbB-1或HER1[30],与多种转录因子的激活有关。吉非替尼耐药的细胞及其衍生的外泌体miR-214水平分别显著高于吉非替尼敏感的细胞及其衍生的外泌体,如外泌体miR-214从耐药细胞转移到敏感细胞时,通过体外抑制细胞凋亡和体内抑制肿瘤生长,使EGFR突变型肺癌对吉非替尼耐药。然而,当耐药细胞衍生外泌体用miR-214 antagomir转染时,吉非替尼耐药性被逆转[31], 提示miR-214可能参与了复杂的基因表达调控并决定敏感细胞行为等的变化。此外,研究表明外泌体miR-21可以从对吉非替尼耐药细胞转移到对吉非替尼敏感细胞,随后激活AKT信号并导致吉非替尼耐药[32],外泌体miR-21还参与了肺癌细胞对顺铂暴露的敏感性调节,这表明miR-21可作为一个治疗靶点,反义抑制miR-21可能提高EGFR-TKI的疗效。 2.4 外泌体miRNA与乳腺癌化疗耐药 外泌体主要通过介导药物直接外排、影响肿瘤干细胞和介导抗凋亡信号增强乳腺癌耐药[33]。研究表明,肿瘤相关脂肪细胞不仅可以增加乳腺癌细胞外泌体的数量,还能促进外泌体介导阿霉素的直接外排,有助于阿霉素治疗的乳腺癌细胞产生耐药性[34]。外泌体是上皮-间质转化的重要中介物,发生上皮-间质转化的癌细胞会更具有侵略性,如外泌体可以将pro-EMT因子传递给乳腺癌受体细胞,使其在耐药方面的水平增加,从而促进乳腺癌进展[35]。SANTOS等[36]发现,阿霉素和紫杉醇耐药细胞中miR-155显著增高,且与上皮-间质转化密切相关,阿霉素和紫杉醇敏感细胞与肿瘤干细胞或阿霉素和紫杉醇耐药细胞的外泌体共同培养后,导致miR-155表达明显增加并诱导化疗耐药性,见表3。SHEN等[37]提出,乳腺癌细胞经化疗诱导后可释放多种含miRNA的外泌体,包括miR-9-5p、miR-195-5p和miR-203a-3p,这3个miRNA同时靶向转录因子ONECUT2,进而促进肿瘤干细胞和干性相关基因(SOX9、OCT4和SOX2等)的表达,针对这个适应机制,可以通过阻断外泌体 miRNA-ONECUT2 轴,最大限度发挥化疗的抗肿瘤作用并降低肿瘤治疗中的化疗耐药性。肿瘤相关成纤维细胞分泌的外泌体中富含miR-21,miR-21可下调其靶点ERα和PTEN,最终促进受体乳腺癌细胞对他西莫芬产生耐药性[38]。除miRNA外,肿瘤分泌的外泌体也通过转移特定蛋白来介导耐药性。含有UCH-L1和P-gp蛋白的外泌体来源于对多柔比星耐药的乳腺癌细胞,它们被分泌到细胞外微环境中,并被对多柔比星敏感的细胞吸收,从而导致耐药性[39]。"
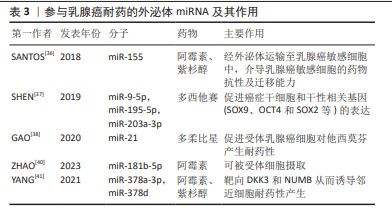
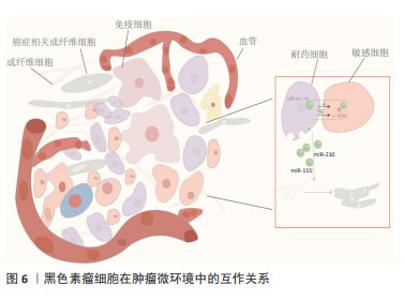
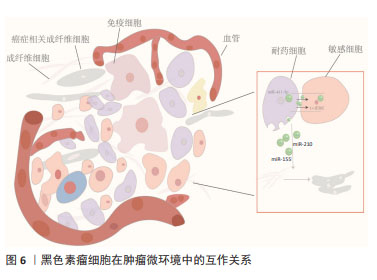
外泌体miRNA还可因受体细胞具有高摄取率能力而成为乳腺癌细胞群耐药性特征的关键递质并且有效传递耐药性。例如,ZHAO等[40]发现阿霉素耐药的乳腺癌细胞中miR-181b-5p呈高水平表达,主要通过靶向BCLAF1从而下调p53/p21水平并抑制阿霉素诱导的G1停滞。野生型乳腺癌细胞经乳腺癌耐药细胞外泌体孵育后,野生型乳腺癌细胞中miR-181b-5p水平显著上调,通过检测野生型细胞的细胞周期、Cyclin D1表达水平、β-半乳糖苷酶活性以及p53/p21水平,结果显示野生型乳腺癌细胞具有耐药表型,而当使用miR-181b-5p抑制剂后逆转了由乳腺癌耐药细胞外泌体引起的细胞周期停滞减少等表型改变,以及SASP基因的变化。此外,YANG等[41]经临床水平证明,乳腺癌细胞在用阿霉素或紫杉醇刺激后产生的外泌体将miR-378a-3p和miR-378d传递给邻近细胞并通过靶向DKK3和NUMB从而诱导耐药性,而将化疗药物与miR-378a-3p和miR-378d表达抑制剂联合使用则逆转了外泌体诱导的耐药性。总之,针对以上耐药诱导机制,在使用miRNA分子抑制剂后细胞的耐药表型发生改变,这可能会为临床小分子抑制剂的使用提供新方向,但从机体多维度调控角度而言,仅单一细胞层面研究尚缺乏有力支撑来推动该领域进展。 2.5 外泌体miRNA与黑色素瘤化疗耐药 黑色素瘤是皮肤癌中最具侵袭性和危及生命的恶性肿瘤[42]。大多数黑色素瘤都携带原癌基因突变,以BRAF突变型(约占所有种类的50%)最常见。BRAF突变促使MAPK活化及下游靶位基因MEK和ERK的激活,导致黑色素瘤细胞的生长和侵袭增强[43]。因此,临床上BRAF突变型黑色素瘤患者的主要靶向治疗方法是BRAF通路抑制剂和MEK抑制剂联合,进而诱导肿瘤细胞凋亡发生,在一定程度上提高了患者的生存率,但这种化疗常常进一步增强肿瘤细胞的防御系统,从而产生耐药性。某些抗性驱动因子,如PDGFRβ可通过外泌体转移到受体黑色素瘤细胞,从而导致PI3K/AKT信号通路的剂量依赖性激活,并改变转移性黑色素瘤对BRAF抑制剂敏感的表型[44]。经LUNAVAT等[45]证实,在BRAF V600E突变的黑色素瘤细胞中,维莫非尼(BRAF抑制剂)显著增加了外泌体RNA总量和蛋白质含量,其中miR-211-5p增加最为显著,主要是通过MITF调节TRPM1基因,从而激活黑色素瘤细胞存活途径来诱导的,而下调miR-211-5p表达导致维莫非尼耐药细胞系的增殖能力受到抑制。除此之外,GEBHARDT等[46]证明了BRAF抑制剂治疗期间,miR-129-5p表达增加,将miR-129-5p 过表达后,黑色素瘤细胞增殖减少并且对BRAF抑制的反应有所改善,该研究还证实,SOX4 是miR-129-5p在BRAF突变型黑色素瘤治疗中发挥潜在作用的靶点。 肿瘤微环境是肿瘤异质性维持、肿瘤进展和耐药性的关键因素。在肿瘤微环境中外泌体miRNA可促进黑色素瘤耐药性的形成,主要通过细胞间的直接接触和分泌可溶性分子而发生:高转移性黑色素瘤细胞通过递送外泌体miR-411-5p进入低转移性黑色素瘤细胞,随后激活ERK信号通路,增强低转移性黑色素瘤细胞转移定植能力[47],见图6;癌症相关成纤维细胞是肿瘤微环境中最丰富的基质细胞,它们在肿瘤生长、转移、免疫抑制和耐药性中起重要作用。黑色素瘤源性外泌体miRNA可以促进癌症相关成纤维细胞分化,而肿瘤微环境中癌症相关成纤维细胞释放的外泌体miRNA在治疗耐药性中发挥着重要作用[48]。肿瘤干细胞具有强大的增殖和自我修复能力,比其他类型的恶性细胞更难以消灭,因此也形成了肿瘤药物治疗的主要障碍,在肿瘤微环境中肿瘤干细胞通过释放靶向抗凋亡和免疫抑制途径的外泌体miRNA来转移邻近敏感细胞并增强耐药性[49]。黑色素瘤不是一个孤立的实体,而是依赖于邻近的微环境,包括成纤维细胞、免疫细胞、血管和细胞外基质,这种微环境是支持黑色素瘤存活、局部侵袭和转移性播散的活性启动子[50],与外泌体衍生miRNA密不可分。外泌体衍生miRNA不仅诱导成纤维细胞和巨噬细胞形成癌症相关成纤维细胞和肿瘤相关巨噬细胞以促进黑色素瘤进展,而且还通过循环系统传播以诱导远处器官中微环境的形成,称为转移前生态位。黑色素瘤外泌体miR-210和miR-155诱导成人成纤维细胞的代谢变化,包括增加有氧糖酵解代谢和减少氧化磷酸化,从而促进转移前微环境形成[51]。因此,与传统黑色素瘤细胞基因的不稳定性相比,基质成纤维细胞等在基因上更稳定,靶向肿瘤微环境相关细胞或许会更有优势。"
| [1] SUNG H, FERLAY J, SIEGEL RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. [2] ZHANG A, MIAO K, SUN H, et al. Tumor heterogeneity reshapes the tumor microenvironment to influence drug resistance. Int J Biol Sci. 2022;18(7):3019-3033. [3] HAN QF, LI WJ, HU KS, et al. Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol Cancer. 2022; 21(1):207. [4] HOSSEINIKHAH SM, GHEYBI F, MOOSAVIAN SA, et al. Role of exosomes in tumour growth, chemoresistance and immunity: state-of-the-art. J Drug Target. 2023;31(1):32-50. [5] HILL M, TRAN N. miRNA interplay: mechanisms and consequences in cancer. Dis Model Mech. 2021;14(4):dmm047662. [6] JAFARI A, BABAJANI A, ABDOLLAHPOUR-ALITAPPEH M, et al. Exosomes and cancer: from molecular mechanisms to clinical applications. Med Oncol. 2021;38(4):45. [7] VANDEWALLE V, ESSAGHIR A, BOLLAERT E, et al. miR-15a-5p and miR-21-5p contribute to chemoresistance in cytogenetically normal acute myeloid leukaemia by targeting PDCD4, ARL2 and BTG2. J Cell Mol Med. 2021;25(1):575-585. [8] LI H, XIE C, LU Y, et al. Exosomal miR92a Promotes Cytarabine Resistance in Myelodysplastic Syndromes by Activating Wnt/β-catenin Signal Pathway. Biomolecules. 2022;12(10):1448. [9] JIANG D, WU X, SUN X, et al. Bone mesenchymal stem cell-derived exosomal microRNA-7-5p inhibits progression of acute myeloid leukemia by targeting OSBPL11. J Nanobiotechnology. 2022;20(1):29. [10] WU J, ZHANG Y, LI X, et al. Exosomes from bone marrow mesenchymal stem cells decrease chemosensitivity of acute myeloid leukemia cells via delivering miR-10a. Biochem Biophys Res Commun. 2022;622: 149-156. [11] JI D, HE Y, LU W, et al. Small-sized extracellular vesicles (EVs) derived from acute myeloid leukemia bone marrow mesenchymal stem cells transfer miR-26a-5p to promote acute myeloid leukemia cell proliferation, migration, and invasion. Hum Cell. 2021;34(3):965-976. [12] WANG D, MING X, XU J, et al. Circ_0009910 shuttled by exosomes regulates proliferation, cell cycle and apoptosis of acute myeloid leukemia cells by regulating miR-5195-3p/GRB10 axis. Hematol Oncol. 2021;39(3):390-400. [13] CHENG H, DING J, TANG G, et al. Human mesenchymal stem cells derived exosomes inhibit the growth of acute myeloid leukemia cells via regulating miR-23b-5p/TRIM14 pathway. Mol Med. 2021; 27(1):128. [14] YAN W, SONG L, WANG H, et al. Extracellular vesicles carrying miRNA-181b-5p affects the malignant progression of acute lymphoblastic leukemia. J Transl Med. 2021;19(1):511. [15] CHEN X, CHEN Y, ZHANG M, et al. HucMSC exosomes promoted imatinib-induced apoptosis in K562-R cells via a miR-145a-5p/USP6/GLS1 axis. Cell Death Dis. 2022;13(1):92. [16] CAO D, CAO X, JIANG Y, et al. Circulating exosomal microRNAs as diagnostic and prognostic biomarkers in patients with diffuse large B-cell lymphoma. Hematol Oncol. 2022;40(2):172-180. [17] FU X, LIU M, QU S, et al. Exosomal microRNA-32-5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway. J Exp Clin Cancer Res. 2018;37(1):52. [18] YANG B, FENG X, LIU H, et al. High-metastatic cancer cells derived exosomal miR92a-3p promotes epithelial-mesenchymal transition and metastasis of low-metastatic cancer cells by regulating PTEN/Akt pathway in hepatocellular carcinoma. Oncogene. 2020;39(42): 6529-6543. [19] FANG JH, ZHANG ZJ, SHANG LR, et al. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology. 2018;68(4): 1459-1475. [20] ZHANG Z, LI X, SUN W, et al. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 2017;397:33-42. [21] ZHOU Y, REN H, DAI B, et al. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J Exp Clin Cancer Res. 2018;37(1):324. [22] CHEN W, HUANG L, LIANG J, et al. Hepatocellular carcinoma cells-derived exosomal microRNA-378b enhances hepatocellular carcinoma angiogenesis. Life Sci. 2021;273:119184. [23] MARTÍN-SÁNCHEZ JC, LUNET N, GONZÁLEZ-MARRÓN A, et al. Projections in Breast and Lung Cancer Mortality among Women: A Bayesian Analysis of 52 Countries Worldwide. Cancer Res. 2018; 78(15):4436-4442. [24] WEI F, MA C, ZHOU T, et al. Correction to: Exosomes derived from gemcitabine resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol Cancer. 2021;20(1):35. [25] SUN S, CHEN H, XU C, et al. Exosomal miR-106b serves as a novel marker for lung cancer and promotes cancer metastasis via targeting PTEN. Life Sci. 2020;244:117297. [26] DONG C, LIU X, WANG H, et al. Hypoxic non-small-cell lung cancer cell-derived exosomal miR-21 promotes resistance of normoxic cell to cisplatin. Onco Targets Ther. 2019;12:1947-1956. [27] SONG Z, JIA G, MA P, et al. Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci. 2021;276:119399. [28] MAO G, LIU Y, FANG X, et al. Tumor-derived microRNA-494 promotes angiogenesis in non-small cell lung cancer. Angiogenesis. 2015;18(3): 373-382. [29] MA Y, YUWEN D, CHEN J, et al. Exosomal Transfer Of Cisplatin-Induced miR-425-3p Confers Cisplatin Resistance In NSCLC Through Activating Autophagy. Int J Nanomedicine. 2019;14:8121-8132. [30] SCHRAMM F, SCHAEFER L, WYGRECKA M. EGFR Signaling in Lung Fibrosis. Cells. 2022;11(6):986. [31] ZHANG Y, LI M, HU C. Exosomal transfer of miR-214 mediates gefitinib resistance in non-small cell lung cancer. Biochem Biophys Res Commun. 2018;507(1-4):457-464. [32] LEONETTI A, CAPULA M, MINARI R, et al. Dynamic Evaluation of Circulating miRNA Profile in EGFR-Mutated NSCLC Patients Treated with EGFR-TKIs. Cells. 2021;10(6):1520. [33] XAVIER CPR, BELISARIO DC, REBELO R, et al. The role of extracellular vesicles in the transfer of drug resistance competences to cancer cells. Drug Resist Updat. 2022;62:100833. [34] LEHUÉDÉ C, LI X, DAUVILLIER S, et al. Adipocytes promote breast cancer resistance to chemotherapy, a process amplified by obesity: role of the major vault protein (MVP). Breast Cancer Res. 2019;21(1):7. [35] BIGAGLI E, CINCI L, D’AMBROSIO M, et al. Transcriptomic Characterization, Chemosensitivity and Regulatory Effects of Exosomes in Spontaneous EMT/MET Transitions of Breast Cancer Cells. Cancer Genomics Proteomics. 2019;16(3):163-173. [36] SANTOS JC, LIMA NDS, SARIAN LO, et al. Exosome-mediated breast cancer chemoresistance via miR-155 transfer. Sci Rep. 2018;8(1):829. [37] SHEN M, DONG C, RUAN X, et al. Chemotherapy-Induced Extracellular Vesicle miRNAs Promote Breast Cancer Stemness by Targeting ONECUT2. Cancer Res. 2019;79(14):3608-3621. [38] GAO Y, LI X, ZENG C, et al. CD63+ Cancer-Associated Fibroblasts Confer Tamoxifen Resistance to Breast Cancer Cells through Exosomal miR-22. Adv Sci (Weinh). 2020;7(21):2002518. [39] NING K, WANG T, SUN X, et al. UCH-L1-containing exosomes mediate chemotherapeutic resistance transfer in breast cancer. J Surg Oncol. 2017;115(8):932-940. [40] ZHAO S, PAN T, DENG J, et al. Exosomal transfer of miR-181b-5p confers senescence-mediated doxorubicin resistance via modulating BCLAF1 in breast cancer. Br J Cancer. 2023;128(4):665-677. [41] YANG Q, ZHAO S, SHI Z, et al. Chemotherapy-elicited exosomal miR-378a-3p and miR-378d promote breast cancer stemness and chemoresistance via the activation of EZH2/STAT3 signaling. J Exp Clin Cancer Res. 2021;40(1):120. [42] BOUSSIOS S, RASSY E, SAMARTZIS E, et al. Melanoma of unknown primary: New perspectives for an old story. Crit Rev Oncol Hematol. 2021;158:103208. [43] ZHANG Z, RICHMOND A, YAN C. Immunomodulatory Properties of PI3K/AKT/mTOR and MAPK/MEK/ERK Inhibition Augment Response to Immune Checkpoint Blockade in Melanoma and Triple-Negative Breast Cancer. Int J Mol Sci. 2022;23(13):7353. [44] VELLA LJ, BEHREN A, COLEMAN B, et al. Intercellular Resistance to BRAF Inhibition Can Be Mediated by Extracellular Vesicle-Associated PDGFRβ. Neoplasia. 2017;19(11):932-940. [45] LUNAVAT TR, CHENG L, EINARSDOTTIR BO, et al. BRAFV600 inhibition alters the microRNA cargo in the vesicular secretome of malignant melanoma cells. Proc Natl Acad Sci U S A. 2017;114(29):E5930-E5939. [46] GEBHARDT K, EDEMIR B, GROß E, et al. BRAF/EZH2 Signaling Represses miR-129-5p Inhibition of SOX4 Thereby Modulating BRAFi Resistance in Melanoma. Cancers (Basel). 2021;13(10):2393. [47] CHEN H, ZENG B, LI X, et al. High-Metastatic Melanoma Cells Promote the Metastatic Capability of Low-Metastatic Melanoma Cells via Exosomal Transfer of miR-411-5p. Front Oncol. 2022;12:895164. [48] SHELTON M, ANENE CA, NSENGIMANA J, et al. The role of CAF derived exosomal microRNAs in the tumour microenvironment of melanoma. Biochim Biophys Acta Rev Cancer. 2021;1875(1):188456. [49] PALACIOS-FERRER JL, GARCÍA-ORTEGA MB, GALLARDO-GÓMEZ M, et al. Metabolomic profile of cancer stem cell-derived exosomes from patients with malignant melanoma. Mol Oncol. 2021;15(2):407-428. [50] TRUFFI M, SORRENTINO L, CORSI F. Fibroblasts in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1234:15-29. [51] SHU S, YANG Y, ALLEN CL, et al. Publisher Correction: Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment. Sci Rep. 2019;9(1):4959. [52] ARNOLD M, SIERRA MS, LAVERSANNE M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017; 66(4):683-691. [53] LA VECCHIA S, SEBASTIÁN C. Metabolic pathways regulating colorectal cancer initiation and progression. Semin Cell Dev Biol. 2020;98:63-70. [54] MALEKI M, GOLCHIN A, JAVADI S, et al. Role of exosomal miRNA in chemotherapy resistance of Colorectal cancer: A systematic review. Chem Biol Drug Des. 2023;101(5):1096-1112. [55] ZHANG HW, SHI Y, LIU JB, et al. Cancer-associated fibroblast-derived exosomal microRNA-24-3p enhances colon cancer cell resistance to MTX by down-regulating CDX2/HEPH axis. J Cell Mol Med. 2021; 25(8):3699-3713. [56] HU JL, WANG W, LAN XL, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol Cancer. 2019;18(1):91. [57] SANTOS P, ALMEIDA F. Role of Exosomal miRNAs and the Tumor Microenvironment in Drug Resistance. Cells. 2020;9(6):1450. [58] GAO R, FANG C, XU J, et al. LncRNA CACS15 contributes to oxaliplatin resistance in colorectal cancer by positively regulating ABCC1 through sponging miR-145. Arch Biochem Biophys. 2019; 663:183-191. [59] JIN G, LIU Y, ZHANG J, et al. A panel of serum exosomal microRNAs as predictive markers for chemoresistance in advanced colorectal cancer. Cancer Chemother Pharmacol. 2019;84(2):315-325. [60] YAO S, YIN Y, JIN G, et al. Exosome-mediated delivery of miR-204-5p inhibits tumor growth and chemoresistance. Cancer Med. 2020; 9(16):5989-5998. |
| [1] | Cao Yue, Ye Xinjian, Li Biyao, Zhang Yining, Feng Jianying. Effect of extracellular vesicles for diagnosis and therapy of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1523-1530. |
| [2] | Wang Sifan, He Huiyu, Yang Quan, Han Xiangzhen. miRNA-378a overexpression of macrophage cell line composite collagen sponge: anti-inflammation and tissue repair promotion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 789-799. |
| [3] | Zhang Xiongjinfu, Chen Yida, Cheng Xinyi, Liu Daihui, Shi Qin . Exosomes derived from bone marrow mesenchymal stem cells of young rats to reverse senescence in aged rat bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7709-7718. |
| [4] | Guo Jia, Ren Yafeng, Li Bing, Huang Jing, Shang Wenya, Yang Yike, Liu Huiyao. Action mechanism of mesenchymal stem cell-derived exosomes carrying miRNAs in improving spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7827-7838. |
| [5] | Zhao Yihan, Sun Xuhang, Zhao Lin, Jiang Shiqing. Effects and mechanisms of exosomal miRNA in treatment of multiple myeloma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6743-6752. |
| [6] | Wang Lei, Wang Baiyan, Zhou Chunguang, Ren Xiaoyun, Dai Yueyou, Feng Shuying. Role of different cell-derived exosomal miRNAs in progression, diagnosis, and prognosis of gastric cancer [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5434-5442. |
| [7] | Xu Yan, Wang Xuesong, Zhou Lin, Zhou Xiaolei, Jin Yu, Ye Junsong. Different strategies to enhance mesenchymal stem cells in treatment of liver fibrosis: analysis of efficacy and potential risks [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 5002-5012. |
| [8] | Wu Ao, Yu Peng, Teng Jiawen, Kong Peng, Bian Sishan. Analysis of oxidative stress-related genes and immune infiltration in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(2): 302-311. |
| [9] | Liu Xiaoming, Cheng Jinlai, Li Rushuang, Li Niuniu, Qin Qiuyun, Xia Meng, Yao Chun. Jiawei Xiaoyao San exerts anti-liver cancer effects via exosomal miRNA pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4052-4062. |
| [10] | Liu Jianhong, Liao Shijie, Li Boxiang, Tang Shengping, Wei Zhendi, Ding Xiaofei. Extracellular vesicles carrying non-coding RNA regulate the activation of osteoclasts [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1076-1082. |
| [11] | Liu Tao, He Zhijun, Li Jinpeng, Song Yuan, Yao Xingzhang, Chen Wen, Li Yan, Bai Bihui. Role and mechanism of noncoding RNA in diabetic peripheral neuropathy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1124-1129. |
| [12] | Guo Lei, Qi Yansong, Niu Xiaobo. Regulatory role of transforming growth factor beta subfamily in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5695-5701. |
| [13] | Li Yue, Qiao Hua. miRNA derived from mesenchymal stem cells and its derivatives in treatment of pathological scar [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 5042-5047. |
| [14] | Zhou Shuliang, Xu Liang, Qian Xuefeng, Zeng Jincai, Zhu Lifan. Correlation between the expression of miRNA-142-3p, mixed lineage kinase 3 and interleukin-1beta in nucleus pulposus and the degree of lumbar intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 165-171. |
| [15] | Guo Xiangying, Peng Zifu, He Yimin, Fang Hongbo, Jiang Ning. MiRNA-122 contributes to the effect of exercise on non-alcoholic fatty liver [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 272-279. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||