Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (6): 1257-1264.doi: 10.12307/2025.308
Previous Articles Next Articles
General pattern of GSK3/Nrf2-regulated biological rhythms in organismal aging
Chen Yilin, Jiang Xiaobo, Qu Honglin, Liu Ruilian
- School of Physical Education, Yichun University, Yichun 336000, Jiangxi Province, China
-
Received:2024-01-29Accepted:2024-04-09Online:2025-02-28Published:2024-06-22 -
Contact:Jiang Xiaobo, Lecturer, School of Physical Education, Yichun University, Yichun 336000, Jiangxi Province, China -
About author:Chen Yilin, PhD, Lecturer, School of Physical Education, Yichun University, Yichun 336000, Jiangxi Province, China -
Supported by:Science and Technology Program Project of Jiangxi Provincial Health Commission, No. SKJP202212703 (to CYL); Science and Technology Program Project of Jiangxi Provincial Department of Education, No. GJJ190873 (to CYL)
CLC Number:
Cite this article
Chen Yilin, Jiang Xiaobo, Qu Honglin, Liu Ruilian. General pattern of GSK3/Nrf2-regulated biological rhythms in organismal aging [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1257-1264.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
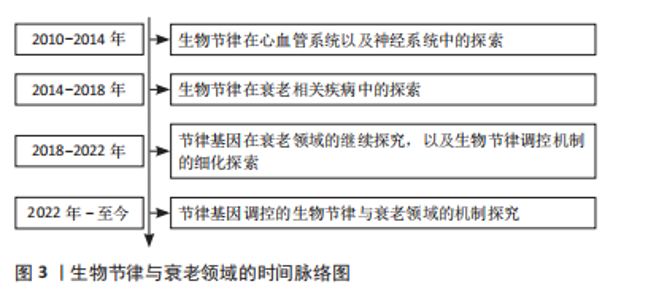
2.1 GSK3/Nrf2与生物节律 生物节律是动物对自然界昼夜变化的适应,动物的行为和生理活动多表现出24 h的周期性变化,如睡眠、进食、营养代谢(血糖、血脂波动)等[7]。动物的生物节律主要由中枢神经的视交叉神经核感受外界昼夜光暗变化来决定,外周组织如肝脏等接受视交叉神经核的信号校准自身节律。 2.1.1 GSK3与生物节律 GSK3已被证实是细胞中底物最多的蛋白激酶之一[8],超过40种蛋白质受GSK3调控,影响各种细胞过程[9]。有研究发现,Cry2和Per2作为昼夜蛋白表达的抑制因子在昼夜节律中发挥着重要作用,并受到GSK3的调控。GSK3在体外和体内均与Per2相互作用,在体外磷酸化Per2,并促进Per2易位至细胞核,它还导致其伴侣蛋白Cry2的蛋白酶体降解[10],并分别通过S557和S553残基磷酸化Cry2和丝氨酸蛋白激酶(DYRK1A)[11]。GSK3磷酸化Bmal1,导致其随后的泛素化和降解[12]。GSK3活性的激酶测定显示,该酶通过修饰特定丝氨酸残基簇来调节Clock(节律基因)磷酸化/降解[13]。GSK3β在S9位点磷酸化的研究表明,GSK3β活性在夜晚结束至清晨达到最大。这导致Cry2在S557位点的磷酸化上调,从而促进该蛋白的节律性降解[13]。此外,GSK3磷酸化Rev-erbα(抑制Bmal1表达的蛋白),这种修饰导致Rev-erbα的激活及其向细胞核的易位[14]。 GSK3α和GSK3β在下丘脑视交叉神经核中表达[15]。GSK3负责影响视交叉神经核神经元分子钟功能的反馈回路[6]。视交叉神经核中GSK3α的表达和GSK3β磷酸化形式的出现具有昼夜节律的特征[16]。有研究表明,在夜间开始时,大鼠视交叉神经核神经元中的GSK活性降低。然而,GSK3β活性在夜间结束时增加[17]。小鼠视交叉神经核的免疫荧光染色显示,在夜间结束时,光照显著增加GSK3活性,即早在光脉冲后30-60 min就降低了磷酸化的GSK3β水平[17]。此外,研究还发现,即使是在黑暗中保存至少2周的小鼠海马区提取物,其特点是在GSK3β磷酸化中存在明显的内源性昼夜节律[18]。在果蝇中,GSK3同源物Sgg在腹侧小神经元中的功能对成年个体的一般节律性运动活动的调节起着关键作用,对于维持正常节律至关重要[19]。Sgg在果蝇中的突变导致昼夜节律周期延长,而其活性上调则缩短昼夜节律周期[20]。Sgg磷酸化Tim (Timeless,果蝇中Cry的同源物)并调节Per/Tim异源二聚体的核易位[20]。值得注意的是,在果蝇和小鼠的时钟结构中,Tim和Cry2分别与Per形成二聚体。很有可能GSK3通过调节与Per蛋白一起运作的组件来促进时钟功能[11]。 2.1.2 Nrf2与生物节律 动物组织和细胞暴露于环境变化中会定期产生活性氧。为了组织和细胞的正常功能,有效清除合成的活性氧是必不可少的。生物节律在维持活性氧正常水平和保护组织和细胞免受氧化损伤方面起着至关重要的作用[21]。氧化应激可能是由生物钟相关基因1发出的活性氧信号和昼夜节律输出之间的协调所控制的。生物节律基因通过转录控制活性氧的产生、反应及其基因调控,生物节律基因及其组成部分的突变导致氧化应激反应的改变[22]。活性氧的内稳态受到Nrf2等抗氧化系统的严格调控,Nrf2的转录调控也依赖于生物节律[21]。谷胱甘肽介导的Nrf2通路的改变在癌症和肺纤维化等其他几种疾病的发病机制中起着重要作用[23]。有证据表明,Nrf2的表达由于ClockΔ19突变而中断,导致低水平的还原性谷胱甘肽和高水平的氧化损伤。BMAL1和CLOCK调节Nrf2的转录,以昼夜节律的形式堆积此类蛋白,并操作参与谷胱甘肽代谢的最重要基因的转录。Nrf2通过有节律地招募到靶基因的AREs来发挥其活性,从而防止氧化损伤[24]。 昼夜节律改变引起的氧化应激引发促炎条件,可能损害关键抗氧化(Nrf2)和炎症(核因子κB)途径之间的交换[25-26]。有研究结果表明,生物钟同步的细胞对抗氧化剂有更有效和更快的反应,并抑制慢性炎症对细胞的损害[26]。另一项研究利用Nrf2/ARE途径通过肾系统缺血再灌注诱导氧化应激来对抗生物钟,发现由内源性昼夜节律基因调控的Nrf2/ARE通路参与了抗氧化应激机制的保护。BMALI调节Nrf2基因,抗氧化途径的节律异常改变抗氧化蛋白的表达,这些抗氧化蛋白参与周期性调节,改变缺血-再灌注应激的敏感性。由此得出结论,控制抗氧化途径昼夜节律的生物钟在抗氧化应激调节中起着重要作用[27]。BMAL1的去除导致Nrf2活性改变,并导致活性氧和白细胞介素1β(促炎细胞因子)的积累。因此,生物钟控制抗氧化作用以维持白细胞介素1β和转录Nrf2活性[28]。 另外,研究发现Nrf2蛋白水平会发生节律性变化,并且在Rat1成纤维细胞裂解液和细胞核中也观察到Nrf2表达的节律模式[23],这些发现为Nrf2在细胞水平上的自主、稳定、节律性表达提供了证据。在WT(野生型)小鼠中,D3T诱导Nrf2可激活含有E-box和d-box的节律基因,而Nrf2的缺失则导致Nrf2-/-小鼠胚胎成纤维细胞的昼夜节律中断[29]。这种直接作用表明Nrf2可以参与节律幅度和周期长度的调节[30]。此外,同样有研究表明,敲除小鼠肝脏中的Nrf2基因会改变昼夜周期的长度[31]。并且,Nrf2很可能通过调控Cry2和Rev-erbα的表达而起作用[32]。Nrf2和节律蛋白可能形成一个抑制环,在昼夜节律中整合细胞氧化还原信号。 2.1.3 GSK3对Nrf2的调节 正常情况下,由于其天然抑制剂蛋白Keap1的作用,Nrf2主要保存在细胞质中[33]。然而,Nrf2一旦暴露于药物诱导剂中,就会从Keap1中分离出来,易位到细胞核中,并与AREs (抗氧化反应元件)结合,进一步导致多种具有抗氧化和解毒生物活性的基因的表达[34]。在这个过程中,AMP活化的蛋白激酶可以调节GSK3β的失活,促进Nrf2的核易位[35-36]。在复杂的信号通路中,GSK3、Nrf2和核因子κB轻链增强子构成了细胞凋亡、炎症反应以及活性氧相关的调控回路[37]。多篇报道表明Nrf2/ARE抗氧化通路和Wnt/β-catenin通路之间存在信号协同作用,其中GSK3是中心调节因子[38-40]。GSK3具有抑制Nrf2的神经保护和抗氧化功能[41],并促进核因子κB的炎症作用[42-43]。 GSK3使Nrf2结构域中的特定丝氨酸残基磷酸化,形成β-转录重复包含蛋白(β-transducin repeat containing protein,β-TrCP)识别的降解结构域。泛素化的Nrf2被含有Cullin1和RING-box1蛋白的蛋白酶体复合物降解。此外,GSK3β可以通过β-TrCP独立的方式激活酪氨酸激酶来抑制Nrf2。GSK3β在Y213位点磷酸化Fyn激酶,激活的Fyn在细胞核中积累,磷酸化Nrf2,导致Nrf2的输出和降解。在氧化应激或存在硫醇化合物的情况下,细胞核中的Nrf2水平会升高,从而刺激含ARE基因的表达;在没有应激的情况下,Nrf2主要被Cullin3泛素化[44]。有研究发现,Nrf2的Neh6结构域包含2个β-TrCP结合序列。GSK3介导的Neh6结构域的S338(和S342)磷酸化增强了GSK3与β-TrCP的结合[44]。Nrf2的磷酸化前作用显然是由属于CMGC(CDK/MAPK/GSK3/CLK)家族的激酶介导的,其催化位点被多肽链的柔性部分(T环)阻断,除非该位点被信号激酶磷酸化。与大多数CMGC激酶不同,GSK3的T环主要在Y279 (GSK3α)或Y216 (GSK3β)位点磷酸化。因此,GSK3 能够在没有信号传导的情况下进行基线催化[45],这一特性使得Nrf2的稳定性可以在一个额外的调节点得到控制[46]。另外有研究发现,抑制GSK3和预磷酸化激酶可以稳定Nrf2[47]。有趣的是,Nrf2相关的转录因子Nrf1也被蛋白酶体以β-TrCP依赖的方式降解。在这种情况下,降解依赖于DSGLS序列,它被CK2识别和磷酸化,而不是被GSK3识别和磷酸化[47]。与糖原合成酶一样,β-连环蛋白通过相同的激酶被预磷酸化[47]。因此,这些底物的泛素化和降解速率部分取决于其特异性预磷酸化激酶和GSK3/CK2的调节。 由此结合上文可知,GSK3对Nrf2具有调节作用,这种调节作用至少通过3种不同的途径施加:GSK3直接参与Nrf2的降解(促进Nrf2泛素化);GSK3磷酸化易位到细胞核的Fyn激酶并修饰Nrf2,导致Nrf2从细胞核中移除;GSK3磷酸化生物钟蛋白Bmal1和Clock,导致其蛋白酶体降解,降低Nrf2的表达。并且GSK3/Nrf2与节律基因之间也存在着相互的联系与调节,然而它们之间的关联是否影响衰老的进程以及存在怎样的机制影响衰老仍比较模糊。 2.2 GSK3/Nrf2、生物节律与衰老 2.2.1 生物节律与衰老 在哺乳动物中,生物节律系统控制着每天的行为和生理节律。这种进化上保守的计时机制能够使生物体内部过程与环境时间线索同步,从而确保最佳的有机体适应性[48]。 生物节律对组织稳态、睡眠调节和行为的系统性影响已得到充分证实,和年龄有直接关系[49-50]。随着人们年龄的增长,生物节律发生了变化,这可能会加速衰老过程[51]。在动物模型中,无论食物成分如何,高振幅的昼夜节律都与健康和寿命延长相关[52-53],而昼夜节律的振幅随着正常衰老而下降,并经常表现出相位变化[54]。此外,年老的动物在光/暗周期的引导/同步方面存在缺陷[55-56],这损害了生物体适应环境变化的能力。 通过基因操纵破坏生物钟稳态可导致与年龄相关的症状[57]。例如,缺乏时钟基因的啮齿动物寿命缩短,包括Clock-/-小鼠和Bmal1-/-小鼠[52]。值得注意的是,恢复适当昼夜节律的干预措施可以延长寿命。例如,将胎儿视交叉神经核移植到老年动物体内可以增加节律性并延长寿命[58]。相反,啮齿动物外周组织中昼夜节律基因的遗传扰动与代谢紊乱有关[59]。生活方式造成的生物节律紊乱(如时差反应、倒班工作)与小鼠寿命缩短以及人类患癌症、心血管疾病和代谢紊乱的风险增加有关[60-61]。衰老如何扰乱内部时钟的功能仍然是一个悬而未决的问题,但总的来说,了解生理和代谢的昼夜节律调节可能为设计和实施抗衰老干预提供新的见解,见图3。"
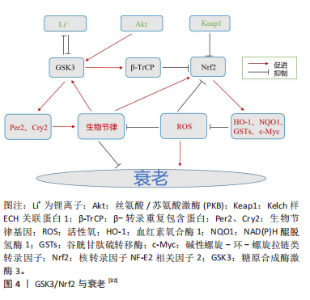
2.2.2 GSK3与衰老 与GSK3相关的衰老是一种由多种不利信号引起的永久性细胞周期停滞状态,包括接触抑制、基因毒性损伤和线粒体应激[62]。将衰老细胞移植到小鼠体内会导致衰老病理,而从药理学上去除这些细胞可以缓解症状[63]。据报道,2种GSK3亚型的N端磷酸化都伴随着肝细胞衰老,用锂(一种GSK3抑制剂,可以降低酶的活性)治疗足以诱导衰老表型,这与增强的合成代谢(包括蛋白质合成和糖生成)相一致[64]。另外,GSK3b被证明在衰老的人类成纤维细胞的细胞核中积累,在那里它与p53形成了稳定的复合物[65]。有趣的是,用锂处理这些细胞阻断了GSK3b与p53的相互作用,减少了与衰老状态相关的年龄依赖性p53积累,以及诱导细胞过渡到可逆的静止状态。此外,在蠕虫中,抑制GSK3导致剂量依赖性的寿命增加,并伴随着染色质重塑[64]。另一项研究发现,锂增加了蠕虫的线粒体能量,并导致功能失调线粒体的选择性自噬[66]。在果蝇中,锂导致三酰甘油的剂量依赖性降低和外源性耐药性增加,锂的寿命促进作用依赖于Nrf1的激活[32]。由此可见,GSK3在不同情况下既能促进细胞衰老又能抑制细胞衰老,其机制与锂发挥的作用有关。 GSK3参与了许多过程的调节,包括代谢、增殖、凋亡、自噬、发育和分化[67]。在GSK3磷酸化靶标之前,通常会有启动激酶,如蛋白激酶A(PKA)、蛋白激酶C(PKC)、蛋白激酶CK1和CK2以及细胞周期蛋白依赖性激酶5[68]。GSK3介导的磷酸化经常导致其靶标失活和蛋白酶体降解[67]。基于其广泛的功能,GSK3与多种年龄相关的疾病有关,包括癌症、糖尿病、情绪障碍、动脉粥样硬化、阿尔茨海默病和帕金森病等[69]。在神经元中,GSK3β会选择性地将微管相关的tau蛋白磷酸化,而在阿尔茨海默病患者大脑中,这些位点会过度磷酸化[70]。过度磷酸化的TAU蛋白对微管的亲和力下降,它以螺旋丝的形式积聚,其代表了阿尔茨海默病大脑中神经纤维缠结的主要成分。并且,在肌萎缩性侧索硬化症、帕金森病、痴呆、皮质基底变性、创伤性脑损伤、唐氏综合征、脑炎后帕金森病和尼曼-皮克病患者中同样也可检测到神经原纤维缠结[70]。另外,在阿尔茨海默病患者的脑组织中,GSK3β水平升高了50%。抑制GSK3β可改善与阿尔茨海默病和上述其他疾病相关的认知症状。在神经退行性变的细胞模型(生长因子缺失)和动物模型(脑缺血)中,GSK3β的活性都会增加[71]。通过天然化合物或设计药理学上适用的抑制剂来调节GSK3(尤其是GSK3β)的活性可能仍然是各种治疗方法的一个有效靶点[72]。 2.2.3 Nrf2与衰老 Nrf2蛋白和mRNA的表达在大脑和心脏等几种组织中随着年龄的增长而下降[73-74]。这与Nrf2靶基因NAD(P)H醌脱氢酶1、γ-GCS、血红素氧合酶1的减少以及核因子κB靶基因如细胞间黏附分子1和白细胞介素6的增加有关[74]。据报道,Nrf2活性的增加促进了哺乳动物的健康衰老,然而,该途径的经典诱导剂在不同衰老模型中具有不同的结果[75]。例如,叔丁基对苯二酚在老年大鼠的原代培养星形细胞中诱导Nrf2活性,但对老年大鼠的心脏组织没有影响,揭示了Nrf2调控的复杂性[76-77]。SKN-1是秀丽隐杆线虫中哺乳动物Nrf2的同源基因,被认为是线虫的相关衰老调节剂,其下调会影响生物体的寿命[78]。此外,SKN-1依赖性信号通路的激活保留了蛋白酶平衡网络和/或赋予氧化应激抗性,防止了果蝇和秀丽隐杆线虫的衰老相关损伤[79-80]。这些结果支持了Nrf2激活促进健康衰老的保守机制的前提。 细胞衰老已被认为是衰老的重要标志[81]。众所周知,衰老细胞会分泌多种促炎细胞因子、趋化因子和其他因子,这些因子统称为衰老相关分泌表型,并改变细胞微环境[82-83]。从这个意义上说,转录因子Nrf2已经成为炎症的关键调节因子[84-86]。在几种Nrf2 基因敲除小鼠模型中,都观察到了炎症加剧的现象[87]。此外,研究表明,在慢性炎症组织中,Nrf2通路的激活重新建立氧化还原平衡,促进细胞修复,同时限制自由基的产生和肿瘤坏死因子诱导的炎症[84]。在临床研究中,富马酸二甲酯(一种Nrf2诱导剂)因其抗炎功能已被批准用于多发性硬化症的治疗[88]。此外,在转录因子激活后,促炎因子基因诱导(白细胞介素6和白细胞介素1β)受到抑制[89]。另外,有研究发现衣康酸(脂多糖处理的人巨噬细胞中丰富的代谢物)是一种抗炎代谢物,可以激活Nrf2来应对炎症[90]。这些和其他研究表明,除了Nrf2激活诱导的抗氧化反应外,其参与炎症也可能在细胞保护和稳态中发挥重要作用。 尽管Nrf2被认为是促进生存和延长寿命的分子,但是仍然有一些在体内/外的研究报道,衰老与Nrf2表达呈负相关[91]。此外,有研究发现Nrf2在细胞衰老过程中功能下降,其沉默导致人胚胎成纤维细胞过早衰老[92]。Caveolin-1(内凹陷蛋白)对Nrf2的抑制也观察到同样的结果,这也促进了衰老[93]。与此同时,Nrf2-/-成纤维细胞的预期寿命较短[94]。另外,Nrf2选择性激活剂已被证明可以防止细胞衰老,如雷帕霉素[91],在其他情况下,可以增加正常成纤维细胞的预期寿命[95]。Nrf2调节的酶,如超氧化物歧化酶,在某些情况下能够防止衰老和炎症[96]。因此,尽管Nrf2在炎症和细胞衰老中发挥重要作用似乎是明确的,但需要进行更多的实验来了解该转录因子在这些现象中的确切参与情况。 2.2.4 GSK3/Nrf2与衰老 GSK3参与糖原代谢、细胞增殖、干细胞更新、凋亡和发育[44]。在基础条件下,GSK3磷酸化Tyr279(GSK3α)或Tyr216(GSK3β),其活性通过Ser21(GSK-3α)或Ser9(GSK-3β)的抑制性磷酸化来调节[97]。此外,p38 MAPK(P38丝裂原活化蛋白激酶)诱导Thr390磷酸化和乙酰化也会改变其活性[9]。有报道称,GSK3α基因敲除小鼠的寿命比野生型小鼠短,并且更容易发生与年龄相关的慢性疾病,如心脏肥厚和收缩功能障碍,这种疾病倾向与mTORC1激活(哺乳动物雷帕霉素复合物1的靶点)和自噬标志物的抑制有关。有报道称GSK-3β调节Nrf2向细胞质的重新定位[98]。在化学保护基因被激活后,Nrf2核输出信号开始向细胞质转运。在对应激的延迟反应中,Akt激活GSK3β磷酸化苏氨酸残基处的Fyn蛋白,导致其核积累。反过来,Fyn磷酸化Tyr568中的Nrf2[99],在胞质溶胶中与Crm1结合输出和降解[44]。 研究表明,PI3K/Akt/GSK-3β信号通路的激活与Nrf2核易位降低以及抗氧化和解毒酶在衰老加速小鼠(SAMP8)肝脏中的表达降低一致[100]。添加指向GSK-3β的反义寡核苷酸,改善了与Nrf2核水平升高相关的小鼠记忆和学习缺陷[101],这表明GSK-3有助于维持啮齿动物的健康衰老。有报道显示,氧化应激耐受性的丧失与Akt减少和衰老过程中GSK3β活性增加有关,这使得人们对Nrf2通路中激酶的机制调控有了更多的了解。Nrf2过表达通过PI3K/Akt/GSK-3β/Fyn信号诱导缺氧时的心脏保护支持了这一观点[102]。GSK-3β还磷酸化了Nrf2中的一组丝氨酸/苏氨酸残基,形成一个降解结构域,该降解结构域在Cul-3/Rbx1复合物泛素化之前被β-TrCP的E3连接酶识别[103]。研究发现应激细胞中Nrf2的降解主要是由对氧化还原状态变化不敏感的Neh6降解子进行的,这与稳态细胞中Nrf2降解所必需和充分的Neh2结构域相反[104]。此外,该结构域是GSK3磷酸化的假定序列。因此,GSK3β/β-TrCP介导的负调控可能是导致衰老脆性的原因[105]。见图4。"
| [1] ZINOVKIN RA, KONDRATENKO ND, ZINOVKINA LA. Does Nrf2 Play a Role of a Master Regulator of Mammalian Aging? Biochemistry (Mosc). 2022;87(12):1465-1476. [2] LIU F, CHANG HC. Physiological links of circadian clock and biological clock of aging. Protein Cell. 2017;8(7):477-488. [3] MATTIS J, SEHGAL A. Circadian Rhythms, Sleep, and Disorders of Aging. Trends Endocrinol Metab. 2016;27(4):192-203. [4] KONDRATOVA AA, KONDRATOV RV. The circadian clock and pathology of the ageing brain. Nat Rev Neurosci. 2012;13(5):325-335. [5] SHILOVSKY GA. Lability of the Nrf2/Keap/ARE Cell Defense System in Different Models of Cell Aging and Age-Related Pathologies. Biochemistry(Mosc). 2022;87(1):70-85. [6] BESING RC, PAUL JR, HABLITZ LM, et al. Circadian rhythmicity of active GSK3 isoforms modulates molecular clock gene rhythms in the suprachiasmatic nucleus. J Biol Rhythms. 2015;30(2):155-160. [7] ALESSANDRO MS, GOLOMBEK DA, CHIESA JJ. Protein Kinases in the Photic Signaling of the Mammalian Circadian Clock. Yale J Biol Med. 2019;92(2):241-250. [8] WANG L, LI J, DI LJ. Glycogen synthesis and beyond, a comprehensive review of GSK3 as a key regulator of metabolic pathways and a therapeutic target for treating metabolic diseases. Med Res Rev. 2022;42(2):946-982. [9] ROBERTSON H, HAYES JD, SUTHERLAND C. A partnership with the proteasome; the destructive nature of GSK3. Biochem Pharmacol. 2018;147:77-92. [10] LELOUP JC, GOLDBETER A. Modelling the dual role of Per phosphorylation and its effect on the period and phase of the mammalian circadian clock. IET Syst Biol. 2011;5(1):44. [11] LIU T, WANG Y, WANG J, et al. DYRK1A inhibitors for disease therapy: Current status and perspectives. Eur J Med Chem. 2022;229:114062. [12] SAHAR S, ZOCCHI L, KINOSHITA C, et al. Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS One. 2010;5(1):e8561. [13] MATSUMURA R, TSUCHIYA Y, TOKUDA I, et al. The mammalian circadian clock protein period counteracts cryptochrome in phosphorylation dynamics of circadian locomotor output cycles kaput (CLOCK). J Biol Chem. 2014;289(46):32064-32072. [14] DONG H, LI D, YANG R, et al. GSK3 phosphorylates and regulates the Green Revolution protein Rht-B1b to reduce plant height in wheat. Plant Cell. 2023;35(6):1970-1983. [15] SINTUREL F, GOS P, PETRENKO V, et al. Circadian hepatocyte clocks keep synchrony in the absence of a master pacemaker in the suprachiasmatic nucleus or other extrahepatic clocks. Genes Dev. 2021;35(5-6):329-334. [16] 徐成伟,梁杞梅,谢秋幼.昼夜节律与意识障碍的关系研究进展[J].中国康复医学杂志,2023,38(10):1468-1473. [17] PAUL JR, MCKEOWN AS, DAVIS JA, et al. Glycogen synthase kinase 3 regulates photic signaling in the suprachiasmatic nucleus. Eur J Neurosci. 2017;45(8):1102-1110. [18] BESING RC, ROGERS CO, PAUL JR, et al. GSK3 activity regulates rhythms in hippocampal clock gene expression and synaptic plasticity. Hippocampus. 2017;27(8):890-898. [19] TOP D, HARMS E, SYED S, et al. GSK-3 and CK2 Kinases Converge on Timeless to Regulate the Master Clock. Cell Rep. 2016;16(2):357-367. [20] TROSTNIKOV MV, ROSHINA NV, BOLDYREV SV, et al. Disordered Expression of shaggy, the Drosophila Gene Encoding a Serine-Threonine Protein Kinase GSK3, Affects the Lifespan in a Transcript-, Stage-, and Tissue-Specific Manner. Int J Mol Sci. 2019;20(9):2200. [21] PATEL SA, VELINGKAAR NS, KONDRATOV RV. Transcriptional control of antioxidant defense by the circadian clock. Antioxid Redox Signal. 2014;20(18):2997-3006.
22] LAI AG, DOHERTY CJ, MUELLER-ROEBER B, et al. CIRCADIAN CLOCK-ASSOCIATED 1 regulates ROS homeostasis and oxidative stress responses. Proc Natl Acad Sci U S A. 2012;109(42):17129-17134. [23] PEKOVIC-VAUGHAN V, GIBBS J, YOSHITANE H, et al. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014;28(6):548-560. [24] SHIM JS, IMAIZUMI T. Circadian clock and photoperiodic response in Arabidopsis: from seasonal flowering to redox homeostasis. Biochemistry. 2015;54(2):157-170. [25] RAMOS-TOVAR E, MURIEL P. Free radicals, antioxidants, nuclear factor-E2-related factor-2 and liver damage. J Appl Toxicol. 2020;40(1):151-168. [26] FRIGATO E, BENEDUSI M, GUIOTTO A, et al. Circadian Clock and OxInflammation: Functional Crosstalk in Cutaneous Homeostasis. Oxid Med Cell Longev. 2020;2020:2309437. [27] SUN Q, ZENG C, DU L, et al.Mechanism of circadian regulation of the NRF2/ARE pathway in renal ischemia-reperfusion. Exp Ther Med. 2021;21(3):190. [28] EARLY JO, MENON D, WYSE CA, et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc Natl Acad Sci U S A. 2018;115(36):E8460-E8468. [29] SPIERS JG, BREDA C, ROBINSON S, et al. Drosophila Nrf2/Keap1 Mediated Redox Signaling Supports Synaptic Function and Longevity and Impacts on Circadian Activity. Front Mol Neurosci. 2019;12:86. [30] WIBLE RS, RAMANATHAN C, SUTTER CH, et al. NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. Elife. 2018;7:e31656. [31] XU YQ, ZHANG D, JIN T, et al. Diurnal variation of hepatic antioxidant gene expression in mice. PLoS One. 2012;7(8):e44237. [32] SHILOVSKY GA, PUTYATINA TS, MORGUNOVA GV, et al. A Crosstalk between the Biorhythms and Gatekeepers of Longevity: Dual Role of Glycogen Synthase Kinase-3. Biochemistry (Mosc). 2021;86(4):433-448. [33] MALHOTRA D, PORTALES-CASAMAR E, SINGH A, et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010;38(17):5718-5734. [34] NISO-SANTANO M, GONZÁLEZ-POLO RA, BRAVO-SAN PEDRO JM, et al. Activation of apoptosis signal-regulating kinase 1 is a key factor in paraquat-induced cell death: modulation by the Nrf2/Trx axis. Free Radic Biol Med. 2010;48(10):1370-1381. [35] JOO MS, KIM WD, LEE KY, et al. AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol Cell Biol. 2016;36(14): 1931-1942. [36] LV H, HONG L, TIAN Y, et al. Corilagin alleviates acetaminophen-induced hepatotoxicity via enhancing the AMPK/GSK3β-Nrf2 signaling pathway. Cell Commun Signal. 2019;17(1):2. [37] MARCHETTI B. Nrf2/Wnt resilience orchestrates rejuvenation of glia-neuron dialogue in Parkinson’s disease. Redox Biol. 2020;36:101664. [38] L’EPISCOPO F, TIROLO C, TESTA N, et al. Plasticity of subventricular zone neuroprogenitors in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of Parkinson’s disease involves cross talk between inflammatory and Wnt/β-catenin signaling pathways: functional consequences for neuroprotection and repair. J Neurosci. 2012;32(6):2062-2085. [39] L’EPISCOPO F, SERAPIDE MF, TIROLO C, et al. A Wnt1 regulated Frizzled-1/β-Catenin signaling pathway as a candidate regulatory circuit controlling mesencephalic dopaminergic neuron-astrocyte crosstalk: Therapeutical relevance for neuron survival and neuroprotection. Mol Neurodegener. 2011;6:49. [40] L’EPISCOPO F, TIROLO C, TESTA N, et al. Reactive astrocytes and Wnt/β-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neurobiol Dis. 2011;41(2):508-527. [41] CUADRADO A, KÜGLER S, LASTRES-BECKER I. Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol. 2018;14:522-534. [42] HOFFMEISTER L, DIEKMANN M, BRAND K, et al. GSK3: A Kinase Balancing Promotion and Resolution of Inflammation. Cells. 2020;9(4):820. [43] YOUSEF MH, SALAMA M, EL-FAWAL HAN, et al. Selective GSK3β Inhibition Mediates an Nrf2-Independent Anti-inflammatory Microglial Response. Mol Neurobiol. 2022;59(9):5591-5611. [44] CUADRADO A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic Biol Med. 2015;88(Pt B):147-157. [45] BEUREL E, GRIECO SF, JOPE RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114-131. [46] TEBAY LE, ROBERTSON H, DURANT ST, et al. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol Med. 2015;88(Pt B):108-146. [47] TSUCHIYA Y, TANIGUCHI H, ITO Y, et al. The casein kinase 2-nrf1 axis controls the clearance of ubiquitinated proteins by regulating proteasome gene expression. Mol Cell Biol. 2013;33(17):3461-3472. [48] RIJO-FERREIRA F, TAKAHASHI JS, FIGUEIREDO LM. Circadian rhythms in parasites. PLoS Pathog. 2017;13(10):e1006590. [49] FROY O. Circadian aspects of energy metabolism and aging. Ageing Res Rev. 2013;12(4):931-940. [50] HOOD S, AMIR S. The aging clock: circadian rhythms and later life. J Clin Invest. 2017;127(2):437-446. [51] WELZ PS, BENITAH SA. Molecular Connections Between Circadian Clocks and Aging. J Mol Biol. 2020;432(12):3661-3679. [52] DUBROVSKY YV, SAMSA WE, KONDRATOV RV. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging (Albany NY). 2010;2(12):936-944. [53] KATEWA SD, AKAGI K, BOSE N, et al. Peripheral Circadian Clocks Mediate Dietary Restriction-Dependent Changes in Lifespan and Fat Metabolism in Drosophila. Cell Metab. 2016;23(1):143-154. [54] 何本进,梁庆华,胡才友,等.昼夜节律与衰老的研究[J].中国老年保健医学,2015,13(4):20-28. [55] CHANG HC, GUARENTE L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell. 2013;153(7): 1448-1460. [56] SELLIX MT, EVANS JA, LEISE TL, et al. Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. J Neurosci. 2012;32(46):16193-16202. [57] LANANNA BV, MUSIEK ES. The wrinkling of time: Aging, inflammation, oxidative stress, and the circadian clock in neurodegeneration. Neurobiol Dis. 2020;139:104832. [58] 秦景梅,赵芳,宋涛.老年慢性心力衰竭患者应用不同剂量他汀干预对心率变异性、昼夜节律的影响[J].中国老年学杂志,2021, 41(13):2695-2697. [59] PASCHOS GK, IBRAHIM S, SONG WL, et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med. 2012 18(12):1768-1777. [60] YU EA, WEAVER DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY). 2011;3(5):479-493. [61] MORRIS CJ, PURVIS TE, HU K, et al. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016;113(10):E1402-E1411. [62] WILEY CD, VELARDE MC, LECOT P, et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016;23(2):303-314. [63] XU M, PIRTSKHALAVA T, FARR JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246-1256.
[64] SOUDER DC, ANDERSON RM. An expanding GSK3 network: implications for aging research. Geroscience. 2019;41(4):369-382.
[65] DAS G, MISRA AK, DAS SK, et al. Role of tau kinases (CDK5R1 and GSK3B) in Parkinson’s disease: a study from India. Neurobiol Aging. 2012;33(7):1485.e9-15. [66] TAM ZY, GRUBER J, NG LF, et al. Effects of lithium on age-related decline in mitochondrial turnover and function in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2014;69(7):810-820. [67] CORMIER KW, WOODGETT JR. Recent advances in understanding the cellular roles of GSK-3. F1000Res. 2017;6:F1000 Faculty Rev-167. [68] KAIDANOVICH-BEILIN O, WOODGETT JR. GSK-3: Functional Insights from Cell Biology and Animal Models. Front Mol Neurosci. 2011;4:40. [69] MCCUBREY JA, RAKUS D, GIZAK A, et al. Effects of mutations in Wnt/β-catenin, hedgehog, Notch and PI3K pathways on GSK-3 activity-Diverse effects on cell growth, metabolism and cancer. Biochim Biophys Acta. 2016;1863(12):2942-2976. [70] 汪盛,李越然,刘俊,等.阿尔茨海默病诊断和治疗研究进展[J].中国药理学与毒理学杂志,2023,37(7):490. [71] SALCEDO-TELLO P, ORTIZ-MATAMOROS A, ARIAS C. GSK3 Function in the Brain during Development, Neuronal Plasticity, and Neurodegeneration. Int J Alzheimers Dis. 2011;2011:189728. [72] WALZ A, UGOLKOV A, CHANDRA S, et al. Molecular Pathways: Revisiting Glycogen Synthase Kinase-3β as a Target for the Treatment of Cancer. Clin Cancer Res. 2017;23(8):1891-1897. [73] 李泽龙,王茂,鄢东海,等.Nrf2与心脏衰老的相关研究进展[J]. 西南国防医药,2019,29(1):91-93. [74] UNGVARI Z, BAILEY-DOWNS L, SOSNOWSKA D, et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011; 301(2):H363-372. [75] LEWIS KN, WASON E, EDREY YH, et al. Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proc Natl Acad Sci U S A. 2015;112(12):3722-3727. [76] ALARCÓN-AGUILAR A, LUNA-LÓPEZ A, VENTURA-GALLEGOS JL, et al. Primary cultured astrocytes from old rats are capable to activate the Nrf2 response against MPP+ toxicity after tBHQ pretreatment. Neurobiol Aging. 2014;35(8):1901-1912. [77] SILVA-PALACIOS A, OSTOLGA-CHAVARRÍA M, BUELNA-CHONTAL M, et al. 3-NP-induced Huntington’s-like disease impairs Nrf2 activation without loss of cardiac function in aged rats. Exp Gerontol. 2017;96:89-98. [78] BLACKWELL TK, STEINBAUGH MJ, HOURIHAN JM, et al. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic Biol Med. 2015;88(Pt B):290-301. [79] TSAKIRI EN, SYKIOTIS GP, PAPASSIDERI IS, et al. Proteasome dysfunction in Drosophila signals to an Nrf2-dependent regulatory circuit aiming to restore proteostasis and prevent premature aging. Aging Cell. 2013; 12(5):802-813. [80] EWALD CY, LANDIS JN, PORTER ABATE J, et al. Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity. Nature. 2015;519(7541):97-101. [81] MITTELBRUNN M, KROEMER G. Hallmarks of T cell aging. Nat Immunol. 2021;22(6):687-698. [82] COPPÉ JP, DESPREZ PY, KRTOLICA A, et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99-118. [83] PRATTICHIZZO F, DE NIGRIS V, MANCUSO E, et al. Short-term sustained hyperglycaemia fosters an archetypal senescence-associated secretory phenotype in endothelial cells and macrophages. Redox Biol. 2018;15: 170-181. [84] RUSHWORTH SA, SHAH S, MACEWAN DJ. TNF mediates the sustained activation of Nrf2 in human monocytes. J Immunol. 2011;187(2):702-707. [85] KOBAYASHI E, SUZUKI T, YAMAMOTO M. Roles nrf2 plays in myeloid cells and related disorders. Oxid Med Cell Longev. 2013;2013:529219. [86] PRATTICHIZZO F, DE NIGRIS V, SPIGA R, et al. Inflammageing and metaflammation: The yin and yang of type 2 diabetes. Ageing Res Rev. 2018;41:1-17. [87] 易宇光,何佳佳,吴俊波.基于Nrf2/ARE信号通路探讨抑制miR-27b对HICH大鼠模型神经细胞凋亡和炎症因子的影响[J].中国老年学杂志,2023,43(16):4001-4005. [88] BURNESS CB, DEEKS ED. Dimethyl fumarate: a review of its use in patients with relapsing-remitting multiple sclerosis. CNS Drugs. 2014; 28(4):373-387. [89] CHOI MJ, LEE EJ, PARK JS, et al. Anti-inflammatory mechanism of galangin in lipopolysaccharide-stimulated microglia: Critical role of PPAR-γ signaling pathway. Biochem Pharmacol. 2017;144:120-131. [90] MILLS EL, RYAN DG, PRAG HA, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018; 556(7699):113-117. [91] WANG R, YU Z, SUNCHU B, et al. Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell. 2017;16(3):564-574. [92] KAPETA S, CHONDROGIANNI N, GONOS ES. Nuclear erythroid factor 2-mediated proteasome activation delays senescence in human fibroblasts. J Biol Chem. 2010;285(11):8171-8184. [93] VOLONTE D, LIU Z, MUSILLE PM, et al. Inhibition of nuclear factor-erythroid 2-related factor (Nrf2) by caveolin-1 promotes stress-induced premature senescence. Mol Biol Cell. 2013;24(12):1852-1862. [94] JÓDAR L, MERCKEN EM, ARIZA J, et al. Genetic deletion of Nrf2 promotes immortalization and decreases life span of murine embryonic fibroblasts. J Gerontol A Biol Sci Med Sci. 2011;66(3):247-256. [95] LERNER C, BITTO A, PULLIAM D, et al. Reduced mammalian target of rapamycin activity facilitates mitochondrial retrograde signaling and increases life span in normal human fibroblasts. Aging Cell. 2013;12(6): 966-977. [96] ZHANG Y, UNNIKRISHNAN A, DEEPA SS, et al. A new role for oxidative stress in aging: The accelerated aging phenotype in Sod1-/- mice is correlated to increased cellular senescence. Redox Biol. 2017;11:30-37. [97] MA T. GSK3 in Alzheimer’s disease: mind the isoforms. J Alzheimers Dis. 2014;39(4):707-710. [98] 吴梅君,叶晓莉,张珏.鸢尾苷元通过GSK-3β/Nrf2信号通路减轻Aβ1-42诱导的SH-SY5Y细胞损伤[J].中药材,2023,46(6):1513-1518. [99] CULBRETH M, ZHANG Z, ASCHNER M. Methylmercury augments Nrf2 activity by downregulation of the Src family kinase Fyn. Neurotoxicology. 2017;62:200-206.. [100] TOMOBE K, SHINOZUKA T, KUROIWA M, et al. Age-related changes of Nrf2 and phosphorylated GSK-3β in a mouse model of accelerated aging (SAMP8). Arch Gerontol Geriatr. 2012;54(2):e1-e7. [101] FARR SA, RIPLEY JL, SULTANA R, et al. Antisense oligonucleotide against GSK-3β in brain of SAMP8 mice improves learning and memory and decreases oxidative stress: Involvement of transcription factor Nrf2 and implications for Alzheimer disease. Free Radic Biol Med. 2014;67: 387-395. [102] ZHOU S, YIN X, JIN J, et al. Intermittent hypoxia-induced cardiomyopathy and its prevention by Nrf2 and metallothionein. Free Radic Biol Med. 2017;112:224-239. [103] WARDYN JD, PONSFORD AH, SANDERSON CM. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans. 2015;43(4):621-626. [104] KARUNATILLEKE NC, FAST CS, NGO V, et al. Nrf2, the Major Regulator of the Cellular Oxidative Stress Response, is Partially Disordered. Int J Mol Sci. 2021;22(14):7434. [105] RADA P, ROJO AI, CHOWDHRY S, et al. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011;31(6):1121-1133. |
| [1] | Yang Lixia, Diao Liqin, Li Hua, Feng Yachan, Liu Xin, Yu Yuexin, Dou Xixi, Gu Huifeng, Xu Lanju. Regulatory mechanism of recombinant type III humanized collagen protein improving photoaging skin in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1988-2000. |
| [2] | Liu Huan, Zeng Shaopeng, Chen Jun, He Linqian, Yang Ying, Zhang Jing. Aging-related dysregulation of glucose metabolism: crossroads of cancer and neurodegenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1527-1538. |
| [3] | Hou Chaowen, Li Zhaojin, Kong Jianda, Zhang Shuli. Main physiological changes in skeletal muscle aging and the multimechanism regulatory role of exercise [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1464-1475. |
| [4] | Peng Tuanhui, Song Hongming, Yang Ling, Ding Xiaoge, Meng Pengjun. Effects of long-term endurance exercise on kl/FGF23 axis and calcium-phosphorus metabolism in naturally aging mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1089-1095. |
| [5] | Zhou Feng, Fu Pengfei, Qian Yufan, Xu Pingcheng, Guo Jiongjiong, Zhang Lei. Correlation between spinal sagittal imbalance and knee joint parameters detected by whole-body EOS imaging [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 596-603. |
| [6] | Zhang Qian, Wang Fuxia, Wang Wen, Zhang Kun. Characteristic analysis of nanogel composite system and its application strategies in visualization of diagnostic imaging and therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 480-488. |
| [7] | Tan Fengyi, Xie Jiamin, Pan Zhenfeng, Zhang Xinxu, Zheng Zetai, Zeng Zhiying, Zhou Yanfang. Effect and mechanism of collagen combined with microneedles in treatment of skin photoaging [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 451-458. |
| [8] | Wang Yaping, Gao Tianyun, Wang Bin. Senescence of human bone marrow mesenchymal stromal cells with increasing age is not dependent on the mediation of endogenous retroviruses [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 10-20. |
| [9] | Yuan Weiyuan, Lei Qinhui, Li Xiuqi, Lu Tiezhu, Fu Ziwen, Liang Zhili, Ji Shaoyang, Li Yijia, Ren Yu . Therapeutic effects of adipose-derived mesenchymal stem cells and their exosomes on dexamethasone-induced sarcopenia in mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 58-67. |
| [10] | Miao Jiahang, Ma Sheng, Li Qupeng, Yu Huilin, Hu Tianyu, Gao Xiao, Feng Hu. Cervical lordosis ratio can be used as a decision-making indicator for selection of posterior surgical approach for multi-level cervical spondylotic myelopathy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1796-1802. |
| [11] | Lyu Liting, Yu Xia, Zhang Jinmei, Gao Qiaojing, Liu Renfan, Li Meng, Wang Lu. Bibliometric analysis of research process and current situation of brain aging and exosomes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1457-1465. |
| [12] | Wen Zixing, Xu Xin, Zhu Shengqun. Correlations between gastrocnemius morphology parameters and physical activity capacity in elderly females under high-frequency ultrasound [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1058-1063. |
| [13] | Zhang Xiongjinfu, Chen Yida, Cheng Xinyi, Liu Daihui, Shi Qin . Exosomes derived from bone marrow mesenchymal stem cells of young rats to reverse senescence in aged rat bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7709-7718. |
| [14] | Sima Xinli, Liu Danping, Qi Hui. Effect and mechanism of metformin-modified bone marrow mesenchymal stem cell exosomes on regulating chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7728-7734. |
| [15] | Wang Simin, Zhang Dezhou, Zhao Jing, Wang Chaoqun, Li Kun, Chen Jie, Bai Xue, Zhao Hailong, Zhang Shaojie, Ma Yuan, Hao Yunteng, Yang Yang, Li Zhijun, Shi Jun, Wang Xing. Artificial intelligence and cervical spine image recognition: application prospects and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(33): 7231-7240. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||