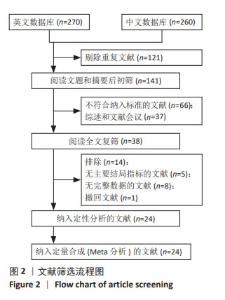
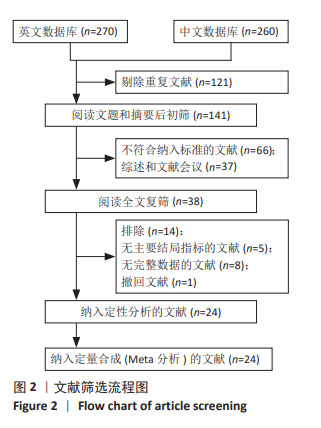
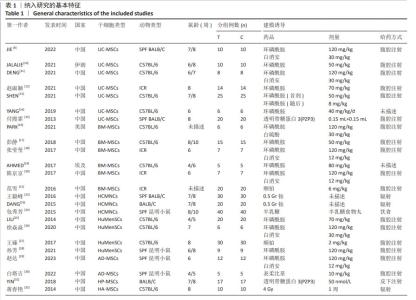
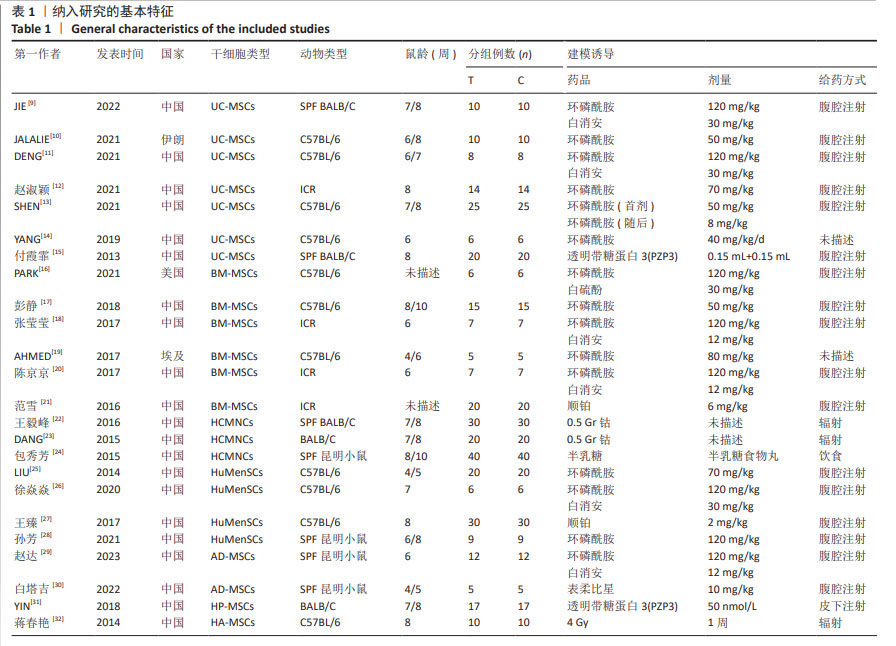
Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (36): 7898-7908.doi: 10.12307/2025.533
Previous Articles Next Articles
A network meta-analysis of efficacy of mesenchymal stem cells from different sources in treatment of premature ovarian failure animal models
Li Zhe1, Li Ping1, Zhang Chao2, Guo Guangling1
- 1Anti-Aging Center, 2Evidence-Based Medicine Center, Affiliated Hospital of Hubei University of Medicine (Taihe Hospital), Shiyan 442000, Hubei Province, China
-
Received:2024-06-04Accepted:2024-07-22Online:2025-12-28Published:2025-03-25 -
Contact:Guo Guangling, MS, Associate professor, Master’s supervisor, Anti-Aging Center, Affiliated Hospital of Hubei University of Medicine (Taihe Hospital), Shiyan 442000, Hubei Province, China -
About author:Li Zhe, Master candidate, Anti-Aging Center, Affiliated Hospital of Hubei University of Medicine (Taihe Hospital), Shiyan 442000, Hubei Province, China -
Supported by:Shiyan Science and Technology Bureau Research Project, No. 22Y46 (to GGL)
CLC Number:
Cite this article
Li Zhe, Li Ping, Zhang Chao, Guo Guangling. A network meta-analysis of efficacy of mesenchymal stem cells from different sources in treatment of premature ovarian failure animal models[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7898-7908.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
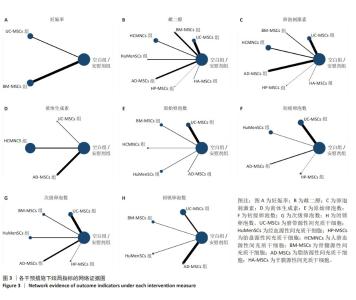
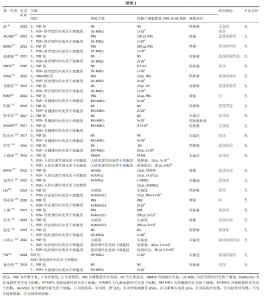
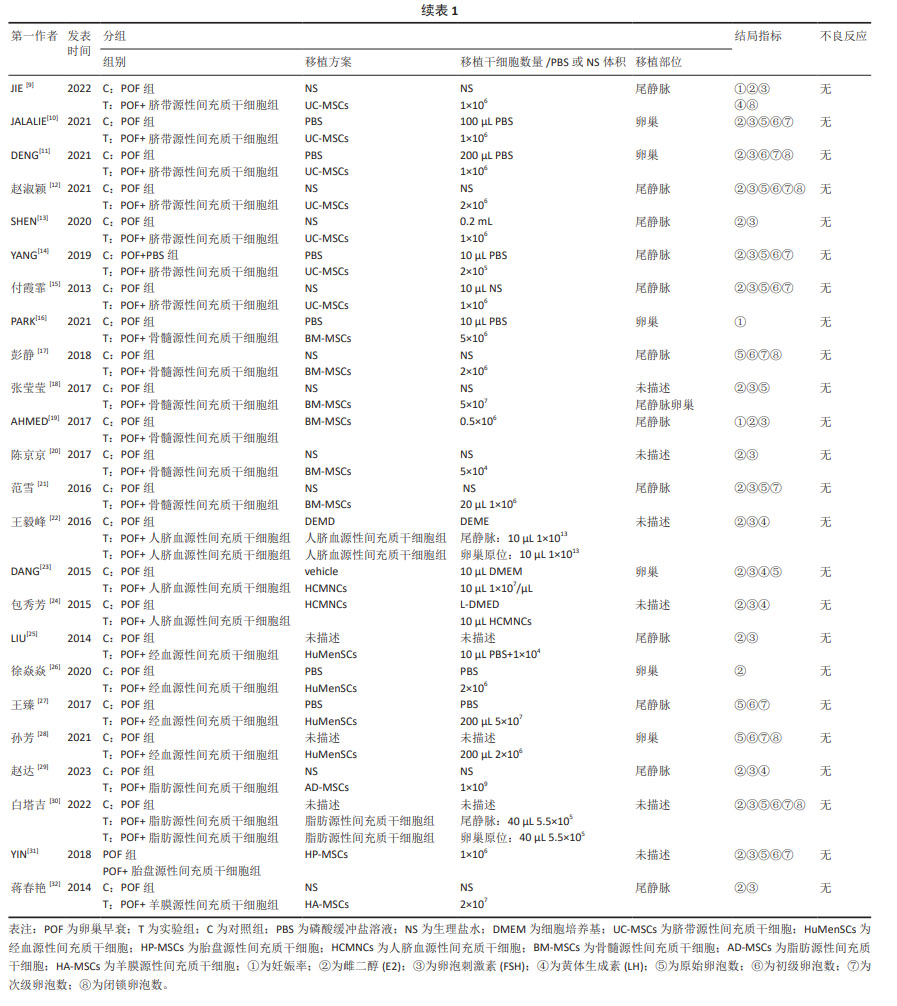
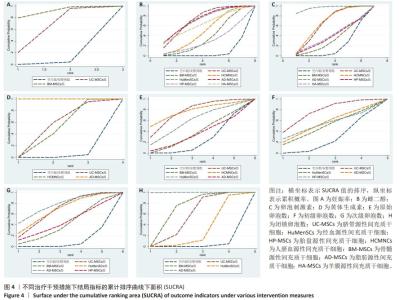
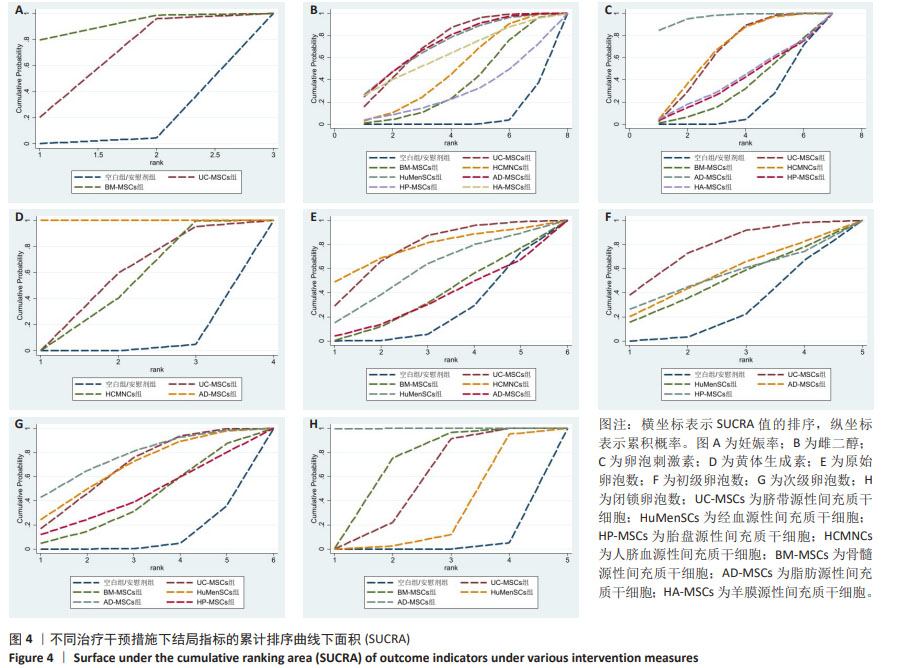
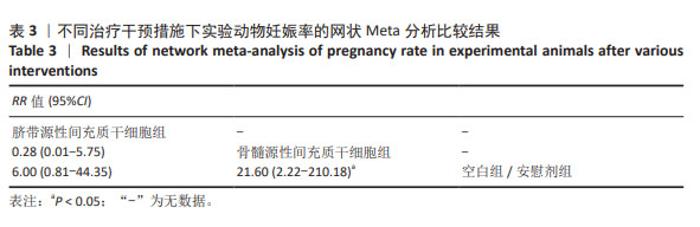
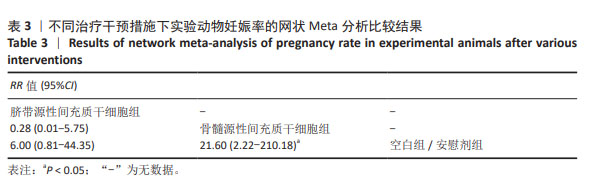
2.3 不同干预措施治疗卵巢早衰结局指标变化情况 2.3.1 不同干预措施下实验动物的妊娠率的网状Meta分析结果 共有3篇研究[9,16,19],不同干预措施的网络证据图,如图3A。因为各研究之间未形成闭合环,故不进行一致性分析。SUCRA概率排序结果依次为:骨髓源性间充质干细胞组(89.7%) >脐带源性间充质干细胞组(58.1%) >空白组/安慰剂组(2.3%)。结果表明,BM-MSCs在提高实验动物妊娠率方面,效果可能最好,见图4A。网状Meta分析结果表明,共产生3组两两比较,其中骨髓源性间充质干细胞组与空白组/安慰剂组比较差异有显著性意义(P < 0.05),脐带源性间充质干细胞组与骨髓源性间充质干细胞组,脐带源性间充质干细胞组与空白组/安慰剂组差异没有显著性意义,见表3。 "
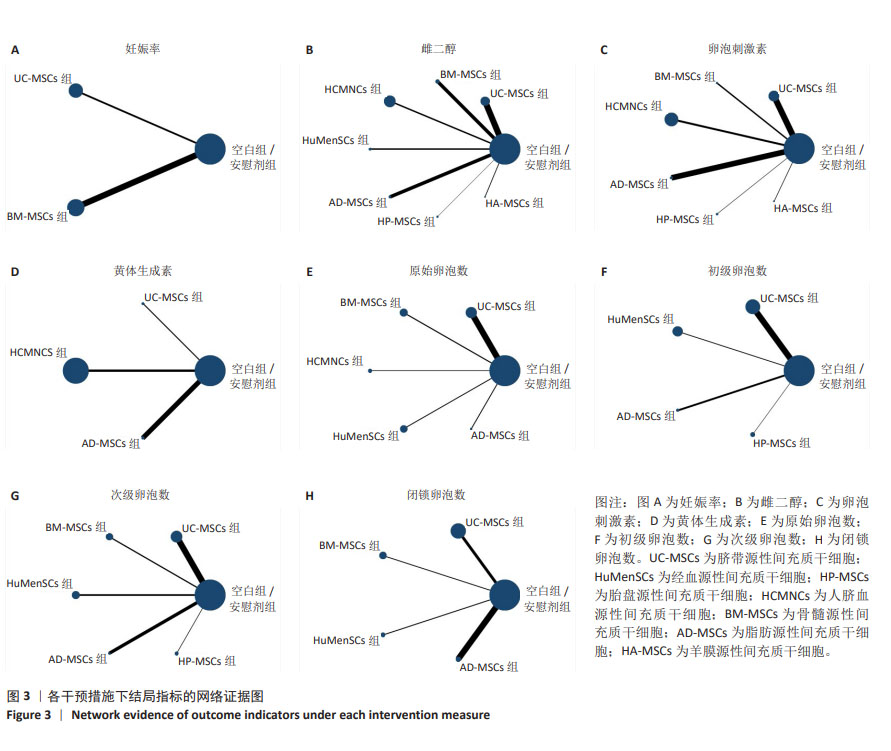
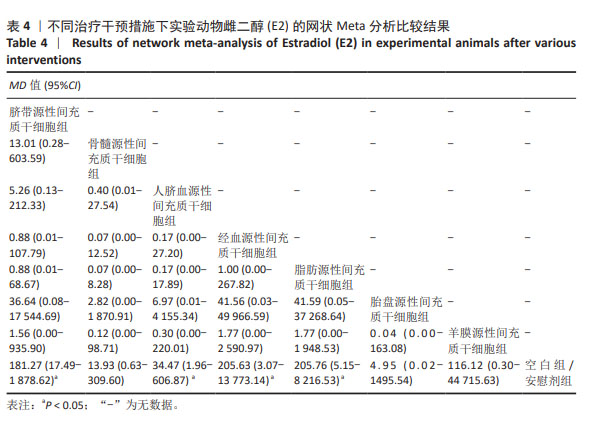
2.3.2 不同干预措施下实验动物雌二醇的网状Meta分析结果 共有20篇研究[9-15,18-26,29-32],不同干预措施网络证据,如图3B。因为各研究之间未形成闭合环,故不进行一致性分析。SUCRA概率排序结果依次为:脐带源性间充质干细胞组(72.7%) >脂肪源性间充质干细胞组(72.6%) >经血源性间充质干细胞组(71.7%) >羊膜源性间充质干细胞组(62.4%) >人脐血源性间充质干细胞组(48.9%) >骨髓源性间充质干细胞组(36.6%) >胎盘源性间充质干细胞组(29.2%) >空白组/安慰剂组(5.9%)。结果表明,脐带源性间充质干细胞在提高实验动物雌二醇方面,效果可能最好,见图4B。网状Meta分析结果表明,共产生28组两两比较,其中脐带源性间充质干细胞组与空白组/安慰剂组比较,人脐血源性间充质干细胞与空白组/安慰剂组比较,经血源性间充质干细胞组与空白组/安慰剂组比较,脂肪源性间充质干细胞组与空白组/安慰剂组比较,差异均有显著性意义(P < 0.05),其他各组之间差异没有显著性意义,见表4。 "
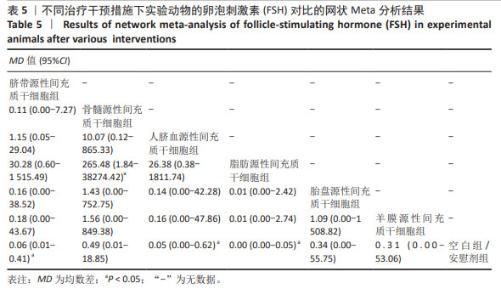
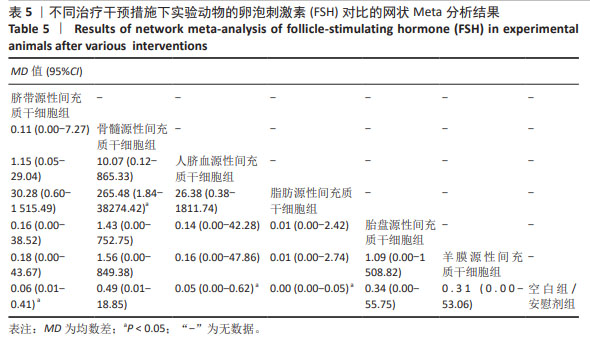
2.3.3 不同干预措施下实验动物卵泡刺激素的网状Meta分析结果 共有16篇研究[9-15,18,20,22-24,29-30],不同干预措施网络证据,如图3C。因为各研究之间未形成闭合环,故不进行一致性分析。SUCRA概率排序结果依次为:脂肪源性间充质干细胞组(96.3%) >人脐血源性间充质干细胞组(65.4%) >脐带源性间充质干细胞组(63.9%) >羊膜源性间充质干细胞组(39.0%) >胎盘源性间充质干细胞组(37.0%) >骨髓源性间充质干细胞组(31.2%) >空白组/安慰剂组(17.2%)。结果表明,脂肪源性间充质干细胞在降低实验动物卵泡刺激素方面,效果可能最好,见图4C。网状Meta分析结果表明,共产生21组两两比较,其中脐带源性间充质干细胞组与空白组/安慰剂组比较,骨髓源性间充质干细胞组与人脐血源性间充质干细胞组比较,人脐血源性间充质干细胞组与空白组/安慰剂组比较,脂肪源性间充质干细胞组与空白组/安慰剂组比较,差异均有显著性意义(P < 0.05),其他各组之间差异没有显著性意义,见表5。"
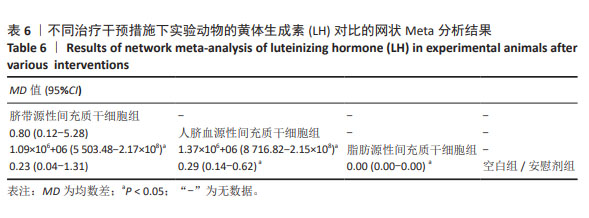
2.3.4 不同干预措施下实验动物黄体生成素的网状Meta分析结果 共有4篇研究[9,22-24,29],不同干预措施网络证据,见图3D。因为各研究之间未形成闭合环,故未进行一致性分析。SUCRA概率排序结果依次为:脂肪源性间充质干细胞组(100.0%) >脐带源性间充质干细胞组(51.6%) >人脐血源性间充质干细胞组(46.8%) >空白组/安慰剂组(1.6%)。结果表明,脂肪源性间充质干细胞在降低实验动物黄体生成素方面,效果可能最好,见图4D。网状Meta分析结果表明,共产生6组两两比较,其中脐带源性间充质干细胞组与人脐血源性间充质干细胞组比较,人脐血源性间充质干细胞组与脂肪源性间充质干细胞组比较,人脐血源性间充质干细胞组与空白组/安慰剂组比较,脂肪源性间充质干细胞组与空白组/安慰剂组比较,差异有显著性意义(P < 0.05),其他各组差异无显著性意义,见表6。 "
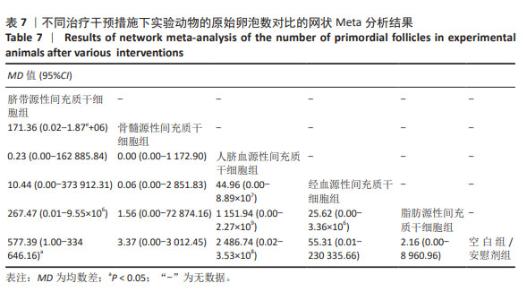
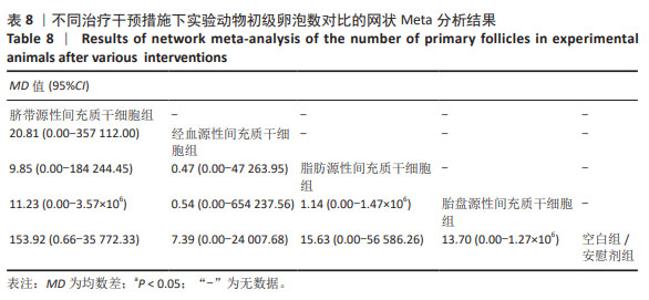
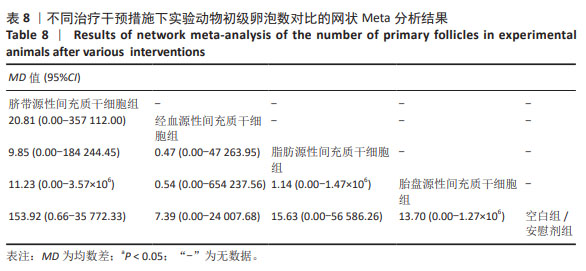
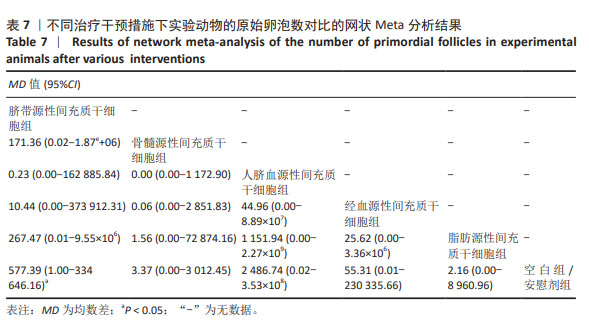
2.3.5 不同干预措施下实验动物原始卵泡数的网状Meta分析结果 共有12篇研究[9-10,12,14-15,17-18,21,23,27-28,30], 不同干预措施网络证据,见图3E。SUCRA概率排序结果依次为:人脐血源性间充质干细胞组(76.3%) >脐带源性间充质干细胞组(75.5%) >经血源性间充质干细胞组(57.5%) >骨髓源性间充质干细胞组(36.0%) >脂肪源性间充质干细胞组(33.0%) >空白组/安慰剂组(21.8%)。结果表明,人脐血源性间充质干细胞组在提高实验动物原始卵泡数可能最好,见图4E。网状Meta分析结果表明,脐带源性间充质干细胞组与空白组/安慰剂组比较,差异有显著性意义(P < 0.05),其他各组之间差异均无显著性意义,见表7。"
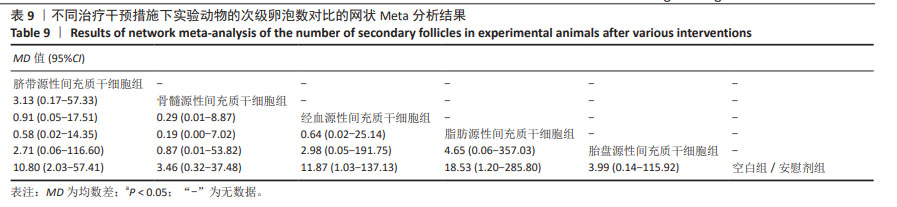
2.3.7 不同干预措施下实验动物次级卵泡数的网状Meta分析结果 共有11篇研究[10-12,14-15,17,21,27-28,30-31],不同干预措施网络证据,如图3G。因为各研究之间未形成闭合环,故不进行一致性分析。SUCRA概率排序结果依次为:脂肪源性间充质干细胞组(76.1%) >经血源性间充质干细胞组(66.8%) >脐带源性间充质干细胞组(66.5%) >胎盘源性间充质干细胞组(43.0%) >骨髓源性间充质干细胞组(39.5%) >空白组/安慰剂组(8.2%)。结果表明,脂肪源性间充质干细胞在提高实验动物次级卵泡数可能最好,见图4G。网状Meta分析结果表明,共产生15组两两比较,其中脐带源性间充质干细胞组与空白组/安慰剂组比较,经血源性间充质干细胞组与空白组/安慰剂组比较,脂肪源性间充质干细胞组与空白组/安慰剂组比较,差异均有显著性意义(P < 0.05),其他各组之间差异无显著性意义,见表9。 "
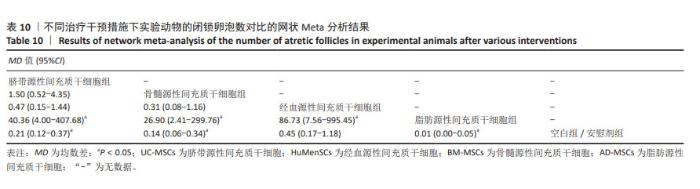
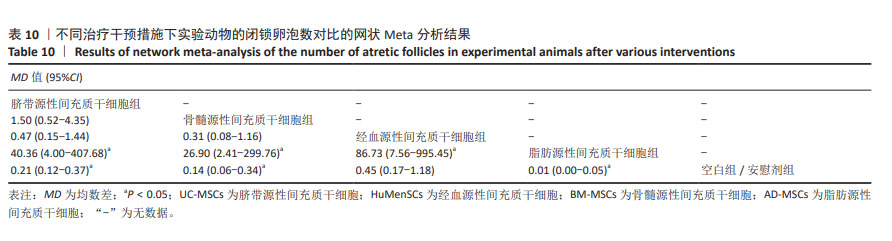
2.3.8 不同干预措施下实验动物闭锁卵泡数的网状Meta分析结果 共有6篇研究[9,11-12,17,28,30],不同干预措施网络证据,如图3H。因为各研究之间未形成闭合环,故不进行一致性分析。SUCRA概率排序结果依次为:脂肪源性间充质干细胞组(99.9%) >骨髓源性间充质干细胞组(68.1%) >脐带源性间充质干细胞组(53.4%) >经血源性间充质干细胞组(27.3%) >空白组/安慰剂组(1.2%)。结果表明,脂肪源性间充质干细胞在降低实验动物闭锁卵泡数可能最好,见图4H。网状Meta分析结果表明,共产生15组两两比较,其中脐带源性间充质干细胞组与空白组/安慰剂组比较,脐带源性间充质干细胞组与脂肪源性间充质干细胞组比较,骨髓源性间充质干细胞组与空白组/安慰剂组比较,骨髓源性间充质干细胞组与脂肪源性间充质干细胞组比较,经血源性间充质干细胞组与脂肪源性间充质干细胞组比较,脂肪源性间充质干细胞组与空白组/安慰剂组比较,差异均有显著性意义(P < 0.05),其他各组之间差异无显著性意义,见表10。 "
| [1] ALI I, PADHIAR AA, WANG T, et al. Stem cell-based therapeutic strategies for premature ovarian insufficiency and infertility: a focus on aging. Cells. 2022;11(23):3713. [2] KIRSHENBAUM M, ORVIETO R. Premature ovarian insufficiency (POI) and autoimmunity-an update appraisal. J Assist Reprod Genet. 2019;36:2207-2215. [3] SHEIKHANSARI G, AGHEBATI-MALEKI L, NOURI M, et al. Current approaches for the treatment of premature ovarian failure with stem cell therapy. Biomed Pharmacother. 2018;102:254-262. [4] VOLAREVIC V, BOJIC S, NURKOVIC J, et al. Stem cells as new agents for the treatment of infertility: current and future perspectives and challenges. BioMed Res. Int. 2014;2014:507234. [5] ZHENG Q, FU X, JIANG J, et al. Umbilical cord mesenchymal stem cell transplantation prevents chemotherapy-induced ovarian failure via the NGF/TrkA pathway in rats. Biomed Res Int. 2019; 2019:6539294. [6] LI Z, ZHANG M, TIAN Y, et al. Mesenchymal stem cells in premature ovarian insufficiency: mechanisms and prospects. Front Cell Dev Biol. 2021;9:718192. [7] 陈匡阳,马彬,王亚楠,等.SYRCLE动物实验偏倚风险评估工具简介[J].中国循证医学杂志, 2014,14(10):1281-1285. [8] 文进,李幼平.Meta分析中效应尺度指标的选择[J].中国循证医学杂志,2007;7(8):606-613. [9] JIE H, JINXIANG W, YE L, et al. Effects of umbilical cord mesenchymal stem cells on expression of CYR61, FSH and AMH in mice with premature ovarian failure. Cell Mol Biol (Noisy-le-grand). 2022;67(4):358-366. [10] JALALIE L, REZAEE MA, REZAIE MJ, et al. Human umbilical cord mesenchymal stem cells improve morphometric and histopathologic changes of cyclophosphamide-injured ovarian follicles in mouse model of premature ovarian failure. Acta Histochem. 2021;123(1):151658. [11] DENG T, HE J, YAO Q, et al. Human umbilical cord mesenchymal stem cells improve ovarian function in chemotherapy-induced premature ovarian failure mice through inhibiting apoptosis and inflammation via a paracrine mechanism. Reprod Sci. 2021;28(6):1718-1732. [12] 赵淑颖,郭广玲,董斯睿,等.脐带间充质干细胞移植对卵巢早衰模型小鼠卵巢功能的影响[J].生物医学工程与临床,2022,26(1):15-21. [13] SHEN J, CAO D, SUN JL. Ability of human umbilical cord mesenchymal stem cells to repair chemotherapy-induced premature ovarian failure. World J Stem Cells. 2020;12(4):277-287. [14] YANG Y, LEI L, WANG S, et al. Transplantation of umbilical cord-derived mesenchymal stem cells on a collagen scaffold improves ovarian function in a premature ovarian failure model of mice. In Vitro Cell Dev Biol Anim. 2019;55(4):302-311. [15] 付霞霏,何援利.脐带间充质干细胞移植对免疫性卵巢早衰的影响[J].广东医学,2013,34(23): 3535-3538. [16] PARK HS, CHUGH RM, ELSHAROUD A, et al. Safety of intraovarian injection of human mesenchymal stem cells in a premature ovarian insufficiency mouse model. Cell Transplant. 2021;30:963689720988502. [17] 彭静,肖娜,程腊梅.骨髓来源间充质干细胞对卵巢早衰小鼠的修复作用[J].中南大学学报(医学版),2018,43(1):7-13. [18] 张莹莹,殷慧群,倪丰,等.骨髓间充质干细胞移植在卵巢早衰小鼠卵巢功能重建中应用[J].安徽农业大学学报,2017,44(1):44-49. [19] BADAWY A, SOBH MA, AHDY M, et al. Bone marrow mesenchymal stem cell repair of cyclophosphamide-induced ovarian insufficiency in a mouse model. Int J Womens Health. 2017;9:441-447. [20] 陈京京,殷慧群,汪存利,等.骨髓间充质干细胞移植在卵巢早衰小鼠卵巢及生育功能重建中的作用[J].安徽医科大学学报,2017,52(11):1611-1615. [21] 范雪,王爱娟,张云,等.骨髓间充质干细胞移植对卵巢早衰模型小鼠卵巢功能的影响[J].中国老年学杂志,2016,36(21):5222-5224. [22] 王毅峰,宋文广,刘淑霞.人脐血单个核细胞移植治疗裸鼠放射性卵巢早衰[J].中国组织工程研究,2016,20(36):5398-5404. [23] DANG J, JIN Z, LIU X, et al. Human cord blood mononuclear cell transplantation for the treatment of premature ovarian failure in nude mice. Int J Clin Exp Med. 2015;8(3):4122-4127. [24] 包秀芳,孙萍.人脐血单个核细胞对半乳糖卵巢早衰小鼠卵巢功能的影响[J].中国医药生物技术,2015,10(5):424-427. [25] LIU T, HUANG Y, ZHANG J, et al. Transplantation of human menstrual blood stem cells to treat premature ovarian failure in mouse model. Stem Cells Dev. 2014;23(13):1548-1557. [26] 徐焱焱.经血间充质干细胞通过IGF-1/AKT/FOXO3a信号通路改善化疗源小鼠卵巢早衰的实验研究[D].银川:宁夏医科大学,2020. [27] 王臻,黄康榕,王月玲,等.经血来源干细胞移植在小鼠卵巢早衰模型中的定位分布[J].西安交通大学学报(医学版),2017,38(6):803-808. [28] 孙芳,韦伟.GnRH激动剂和经血源性干细胞联合治疗对小鼠卵巢功能的影响[J].南方医科大学学报,2021,41(12):1850-1856. [29] 赵达,包利利,王晓黎,等.脂肪间充质干细胞对卵巢早衰小鼠卵巢内分泌功能及颗粒细胞凋亡的影响[J].医学研究杂志,2023,52(8):173-177. [30] 白塔吉,马玉珍.脂肪间充质干细胞对小鼠化疗性卵巢功能不全的治疗作用及机制研究[J].中国妇产科临床杂志,2022,23(6):617-621. [31] YIN N, ZHAO W, LUO Q, et al. Restoring ovarian function with human placenta-derived mesenchymal stem cells in autoimmune-induced premature ovarian failure mice mediated by treg cells and associated cytokines. Reprod Sci. 2018; 25(7):1073-1082. [32] 蒋春艳.人羊膜间充质干细胞修复卵巢功能的实验研究[D].南京:南京医科大学,2014. [33] 唐华均,李成志.卵巢早衰的病因及治疗进展[J].重庆医学,2018,47(13):1777-1780. [34] CORDTS EB, CHRISTOFOLINI DM, DOS SANTOS AA, et al. Genetic aspects of premature ovarian faillure:a literature review. Arch Gynecol Obstet. 2011;283(3):635-643. [35] 邵华.中药联合激素替代疗法治疗卵巢早衰的研究进展[J].中国城乡企业卫生,2022,37(5):13-15. [36] 陈醒,周应芳,白文佩.绝经期激素替代治疗的相关肿瘤风险研究进展[J].国际妇产科学杂志,2016,43(5):489-492,496. [37] 刘紫君,常惠,王宇,等.干细胞治疗生殖系统疾病的研究进展[J].生殖医学杂志,2021, 30(6):836-841. [38] KISIEL AH, MCDUFFEE LA, MASAOUD E, et al. Isolation, characterization, and in vitro proliferation of canine mesenchymal stem cells derived from bone marrow, adipose tissue, muscle, and periosteum. Am J Vet Res. 2012;73(8):1305-1317. [39] 赵淑颖,郭广玲,刘晨晨,等.干细胞移植治疗卵巢早衰:基于13篇动物实验的Meta分析[J].中国组织工程研究,2022,26(25):4084-4092. [40] 李萍,郭广玲,董斯睿,等.脐带间充质干细胞移植治疗卵巢早衰有效性动物实验的Meta分析[J].中国循证医学杂志,2022,22(9): 1041-1047. [41] 刘荣霞,杨炳,余丽梅,等.不同来源间充质干细胞治疗卵巢早衰的作用及机制研究进展[J].山东医药,2018,58(40):106-110. [42] LEVY O, KUAI R, SIREN EMJ, et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6:eaba6884. [43] LU X, CUI J, CUI L, et al. The effects of human umbilical cord-derived mesenchymal stem cell transplantation on endometrial receptivity are associated with Th1/Th2 balance change and uNK cell expression of uterine in autoimmune premature ovarian failure mice. Stem Cell Res Ther. 2019;10(1):214. [44] ZHENG Q, FU X, JIANG J, et al., Umbilical cord mesenchymal stem cell transplantation prevents chemotherapy-induced ovarian failure via the NGF/TrkA pathway in rats. Biomed Res Int. 2019; 2019:6539294. [45] 陈英霞,王换换,戴晓宇,等. GDF-9转染的脂肪间充质干细胞治疗化疗性卵巢早衰大鼠[J].现代妇产科进展,2017,26(11):822-828. [46] TAKEHARA Y, YABUUCHI A, EZOE K, et al., The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab Invest. 2013;93(2):181-193. [47] 阴春霞,曲红光,曹阳,等.脂肪干细胞对顺铂所致卵巢早衰大鼠细胞因子的影响[J].中国妇幼保健,2016,31(24):5480-5482. [48] UZBAS E, MAY ID, PARISI AM, et al. Molecular physiognomies and applications of adipose-derived stem cells. Stem Cell Rev Rep. 2015;2:298-308. [49] 许思娟.月经血源性间充质干细胞在女性生殖系统疾病中的应用进展[J].实用妇产科杂志, 2021,37(5):354-357. [50] 闫忠蕊.Gadd45b在MB-MSCs修复表柔比星致GCs损伤机制中的实验研究[D].天津:天津医科大学,2019. [51] ELFAYOMY AK, ALMASRY SM, EL-TARHOUNY SA, et al. Human umbilical cord blood-mesenchymal stem cells transplantation renovates the ovarian surface epithelium in a rat model of premature ovarian failure: possible direct and indirect effects. Tissue Cell. 2016;48(4):370-382. [52] FU YX, JI J, SHAN F, et al. Human mesenchymal stem cell treatment of premature ovarian failure: new challenges and opportunities. Stem Cell Res Ther. 2021;12:161. [53] MOHAMED S, SHALABY S, BRAKTA S, et al. Umbilical cord blood mesenchymal stem cells as an infertility treatment for chemotherapy induced premature ovarian insufficiency. Biomedicines. 2019;7(1):7. [54] ELFAYOMY KA, ALMASRY MS, EL-TARHOUNY AS, et al. Human umbilical cord blood-mesenchymal stem cells transplantation renovates the ovarian surface epithelium in a rat model of premature ovarian failure: possible direct and indirect effects. Tissue and Cell. 2016;48(4):370-382. [55] 侯巧妮,马会明,相丽,等.人胎盘间充质干细胞移植对化疗所致卵巢早衰大鼠卵巢功能的影响[J].山东大学学报(医学版),2019,57(2):52-60. [56] 尹娜.人胎盘间充质干细胞移植能够修复自身免疫性卵巢早衰小鼠卵巢功能[D].滨州:滨州医学院,2018. [57] 李永丽,陈冬梅,徐仙,等.胎盘间充质干细胞培养液对卵巢早衰模型大鼠卵巢BCL-2表达的影响[J].宁夏医学杂志,2020,42(8):673-676, 672. [58] SEOK J, PARK H, CHOI HJ, et al. Placenta-derived mesenchymal stem cells restore the ovary function in an ovariectomized rat model via an antioxidant effect. Antioxidants. 2020;9(7):591. [59] LAI D, WANG F, YAO X, et al. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J Transl. Med. 2015;13:155. [60] 余丽梅,刘荣霞,张小雨,等.人羊膜间充质干细胞治疗卵巢早衰的作用和机制[J].中国药理学与毒理学杂志, 2019,33(10):919-920. |
| [1] | Zhang Xinxin, Gao Ke, Xie Shidong, Tuo Haowen, Jing Feiyue, Liu Weiguo. Network meta-analysis of non-surgical treatments for foot and ankle ability and dynamic balance in patients with chronic ankle instability [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1931-1944. |
| [2] | Wang Juan, Wang Guanglan, Zuo Huiwu. Efficacy of exercise therapy in the treatment of anterior cruciate ligament reconstruction patients: #br# a network meta-analysis #br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1714-1726. |
| [3] | Wang Kaigang, Hao Dongsheng, Ma Pei, Zhou Shuo, Li Ruimin. Comparison of efficacy of different biological scaffolds for pulp regeneration therapy in immature permanent teeth: a Bayesian network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7447-7460. |
| [4] | Li Jia, Liu Qianru, Xing Mengnan, Chen Bo, Jiao Wei, Meng Zhaoxiang. A network meta-analysis on therapeutic effect of different types of exercise on knee osteoarthritis patients [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 609-616. |
| [5] | Zhao Yuxin, Zhang Deqi, Bi Hongyan. Effect of different stimulation modalities of non-invasive brain stimulation on cognitive function in patients with Parkinson’s disease: a network Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5212-5223. |
| [6] | Hu Tong, Li Xuan, Yuan Jing, Wang Wei. Different electromagnetic stimulation programs improve post-stroke dysphagia: a network Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5224-5236. |
| [7] | Liu Xingzhao, Hu Tong, Ma Yan, Wang Qian, Wei Xiaohui, Chang Wanpeng, Yu Shaohong. Efficacy of rehabilitation robots on lower limb motor function in patients with cerebral palsy: a Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3925-3933. |
| [8] | Liu Yanyan, Ma Yuanyuan, Huang Xianghua, Zhang Jingkun. Mechanism of different sources of mesenchymal stem cells in treatment of premature ovarian failure [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2764-2773. |
| [9] |
Zhong Jiawen, Jiang Bo, Zhang Wenyan, Li Xiaorong, Qin Ling, Gao Ting .
Liuwei Dihuang Wan inhibits oxidative stress in premature ovarian failure mice by regulating intestinal microbiota #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(11): 2285-2293.
|
| [10] | Zhong Jun, Wang Wen. Network meta-analysis of different anatomical repair strategies to improve chronic lateral ankle instability [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1470-1476. |
| [11] | Hu Zhixing, Li Qun, Yang Chao, Wang Xiaoxiao, Fang Luochangting, Hou Wuqiong, Lin Na, Chen Weiheng, Liu Chunfang, Lin Ya. Network meta-analysis of the modeling effects of different factors on rabbit models of steroid-induced osteonecrosis of femoral head [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 976-984. |
| [12] | Chang Ying, Xia Yuan, Sun Yundi, Cheng Lulu, Xiong Wenjuan, Zhao Xianghu. Effectiveness of different specific exercise therapies in treatment of adolescent idiopathic scoliosis: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(36): 5899-5904. |
| [13] | Jing Tianyuan, Wang Ping, Wang Yi, Hu Yanan, Liu Shanxin, Sun Guodong, Du Haitao. Luzhongjiangu decoction for the treatment of femoral head necrosis in rats: changes in intestinal flora and serum hormones [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5598-5605. |
| [14] | Jia Hongsheng, Wang Fan, Chen Chun, Sun Bo, Fang Shengqi. Network meta-analysis on efficacy and safety of different biological agents in treatment of rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(29): 4748-4756. |
| [15] | Li Xun, Zhang Weichao, Li Yingjie, Liu Rong, Tian Xuewen, Zhang Pengyi, Wang Xiaoqiang. Effect of moderate-intensity exercise on the level of autophagy in bone tissue of ovariectomized rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(20): 3130-3136. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||