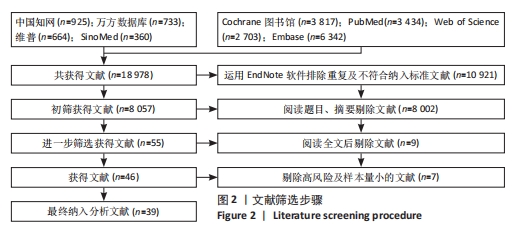
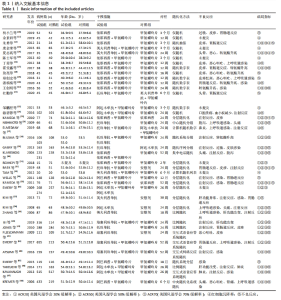
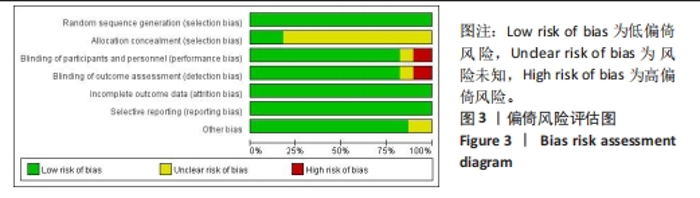
Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (29): 4748-4756.doi: 10.12307/2024.587
Network meta-analysis on efficacy and safety of different biological agents in treatment of rheumatoid arthritis
Jia Hongsheng, Wang Fan, Chen Chun, Sun Bo, Fang Shengqi
- Nanyang TCM Hospital, Nanyang 473000, Henan Province, China
-
Received:2023-11-07Accepted:2023-12-21Online:2024-10-18Published:2024-03-23 -
Contact:Jia Hongsheng, Master, Nanyang TCM Hospital, Nanyang 473000, Henan Province, China -
About author:Jia Hongsheng, Master, Nanyang TCM Hospital, Nanyang 473000, Henan Province, China
CLC Number:
Cite this article
Jia Hongsheng, Wang Fan, Chen Chun, Sun Bo, Fang Shengqi. Network meta-analysis on efficacy and safety of different biological agents in treatment of rheumatoid arthritis[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(29): 4748-4756.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
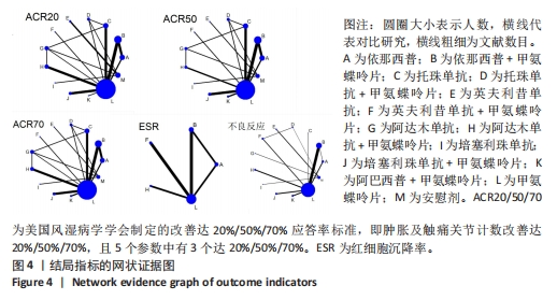
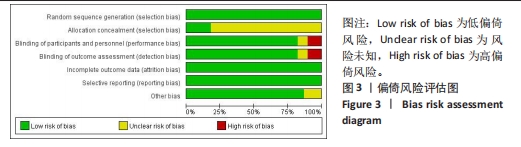
2.3 网状Meta分析网状证据图结果 纳入的研究共包括13种治疗措施,分别是A:依那西普;B:依那西普+甲氨蝶呤片;C:托珠单抗;D:托珠单抗+甲氨蝶呤片;E:英夫利昔单抗;F:英夫利昔单抗+甲氨蝶呤片;G:阿达木单抗;H:阿达木单抗+甲氨蝶呤片;I:培塞利珠单抗;J:培塞利珠单抗+甲氨蝶呤片;K:阿巴西普+甲氨蝶呤片;L:甲氨蝶呤片;M:安慰剂。 结局指标的网状证据图见图4,从网状证据图可以看出:在各结局指标中,甲氨蝶呤片应用人数最多,依那西普+甲氨蝶呤片次之,依那西普+甲氨蝶呤片与甲氨蝶呤片之间对比研究最多,托珠单抗与甲氨蝶呤片、阿达木单抗+甲氨蝶呤片与甲氨蝶呤片之间次之。图中存在三角闭环及四角闭环说明既有研究间直接比较又有间接比较。"
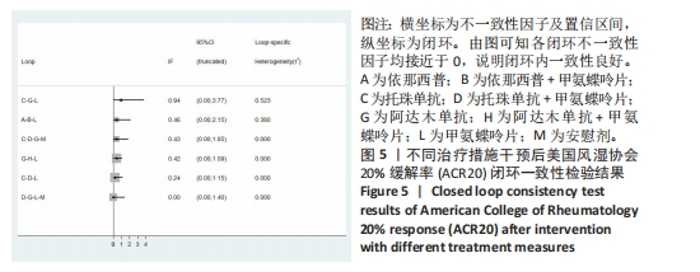
2.4 结局指标直接Meta分析及一致性检验结果 2.4.1 ACR20指标 运用直接Meta分析对各研究间异质性进行分析,结果显示,整体I2=86.3%,P < 0.05,提示各临床研究间具有一定异质性,运用亚组分析,剔除高异质性文献后再次运用直接Meta分析,经与前次对比,结果稳定,并进行敏感性分析,显示结果稳定,故在随机效应模型下进行分析。ACR20指标直接Meta分析结果显示:依那西普、阿达木单抗、培塞利珠单抗+甲氨蝶呤片措施与甲氨蝶呤片之间效果无明显差异(P > 0.05),依那西普+甲氨蝶呤片、托珠单抗、托珠单抗+甲氨蝶呤片、英夫利昔单抗+甲氨蝶呤片、阿达木单抗+甲氨蝶呤片、阿巴西普+甲氨蝶呤片措施治疗效果优于甲氨蝶呤片措施,差异有显著性意义(P < 0.05);依那西普+甲氨蝶呤片治疗措施治疗效果优于依那西普(P < 0.05),托珠单抗、阿达木单抗+甲氨蝶呤片措施治疗效果优于阿达木单抗(P < 0.05),托珠单抗+甲氨蝶呤片、英夫利昔单抗、阿达木单抗、培塞利珠单抗措施治疗效果优于安慰剂(P < 0.05),托珠单抗措施与托珠单抗+甲氨蝶呤片措施之间效果无显著差异(P > 0.05)。 运用不一致性模型进行不一致性检验,结果显示P=0.770 5,P > 0.05,说明研究数据一致性良好;并运用节点分裂法进行局部的一致性检验,检验结果P > 0.05,显示研究间具有一致性,故在一致性模型下进行网状Meta分析,并对6个闭环进行一致性检验,检验结果显示一致性良好。闭环一致性检验结果见图5。"

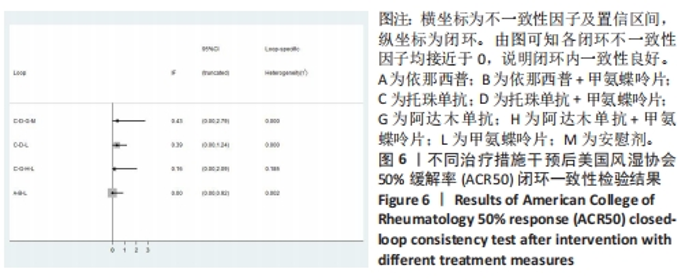
2.4.2 ACR50指标直接Meta分析及一致性检验结果 运用直接Meta分析对各研究间异质性进行分析,结果显示,整体I2=88.3%,P < 0.05,提示各临床研究间具有一定异质性,运用亚组分析,剔除高异质性文献后再次分析,经与前次对比,结果稳定,并对结果进行敏感性分析,显示结果稳定,故在随机效应模型下进行分析。ACR50直接Meta分析结果显示:依那西普、阿达木单抗、阿巴西普+甲氨蝶呤片措施与甲氨蝶呤片之间效果无明显差异(P > 0.05),依那西普+甲氨蝶呤片、托珠单抗、托珠单抗+甲氨蝶呤片、英夫利昔单抗+甲氨蝶呤片、阿达木单抗+甲氨蝶呤片、培塞利珠单抗+甲氨蝶呤片措施治疗效果优于甲氨蝶呤片措施,差异有显著性意义(P < 0.05);依那西普+甲氨蝶呤片治疗措施治疗效果优于依那西普(P < 0.05),托珠单抗、阿达木单抗+甲氨蝶呤片措施治疗效果优于阿达木单抗(P < 0.05),托珠单抗+甲氨蝶呤片、英夫利昔单抗、阿达木单抗、培塞利珠单抗措施治疗效果优于安慰剂(P < 0.05),托珠单抗措施与托珠单抗+甲氨蝶呤片措施之间效果无显著差异(P > 0.05)。 对纳入研究进行网状Meta不一致性检验,结果显示P=0.991 5,P > 0.05,说明研究数据一致性良好;并运用节点分裂法进行局部的一致性检验,检验结果P > 0.05,显示研究间具有一致性,故在一致性模型下进行网状Meta分析,并对6个闭环进行一致性检验,检验结果显示一致性良好。闭环一致性检验结果见图6。"

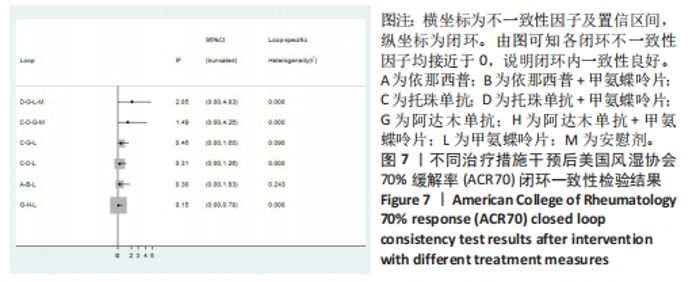
2.4.3 ACR70指标直接Meta分析及一致性检验结果 运用直接Meta分析对各研究间异质性进行分析,结果显示,整体I2=83.3%,P < 0.05,提示各临床研究间具有轻度异质性,运用亚组分析,剔除高异质性文献后再次运用直接Meta分析,经与前次对比,结果稳定,并对结果进行敏感性分析,经对比显示结果稳定,故在随机效应模型下进行分析。ACR70直接Meta分析结果显示:依那西普、阿达木单抗、培塞利珠单抗+甲氨蝶呤片措施与甲氨蝶呤片之间效果无明显差异(P > 0.05),依那西普+甲氨蝶呤片、托珠单抗、托珠单抗+甲氨蝶呤片、阿达木单抗+甲氨蝶呤片、阿巴西普+甲氨蝶呤片措施治疗效果优于甲氨蝶呤片措施,差异有显著性意义(P < 0.05);依那西普+甲氨蝶呤片治疗措施治疗效果优于依那西普(P < 0.05),托珠单抗、阿达木单抗+甲氨蝶呤片措施治疗效果优于阿达木单抗(P < 0.05),英夫利昔单抗、阿达木单抗措施治疗效果优于安慰剂(P < 0.05),托珠单抗、托珠单抗+甲氨蝶呤片措施之间效果无显著差异(P > 0.05)。 对纳入研究进行不一致性检验,结果显示P=0.171 2,P > 0.05说明研究数据一致性良好;并运用节点分裂法进行局部的一致性检验,检验结果P > 0.05,显示研究间具有一致性,故在一致性模型下进行网状Meta分析。并对6个闭环进行一致性检验,检验结果显示一致性良好。闭环一致性检验结果见图7。"


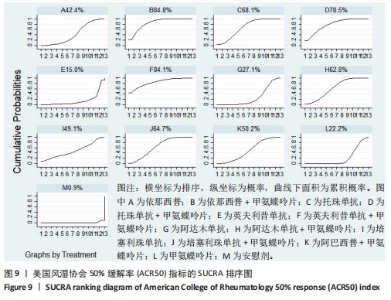
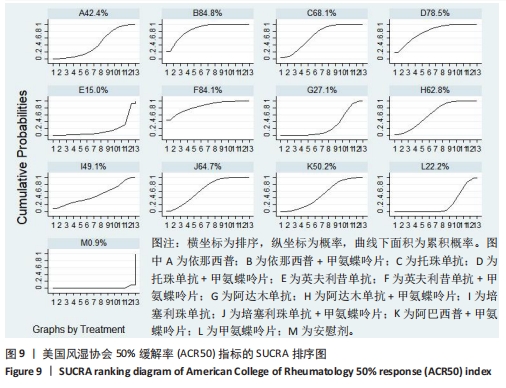
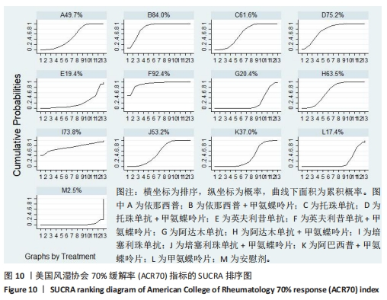
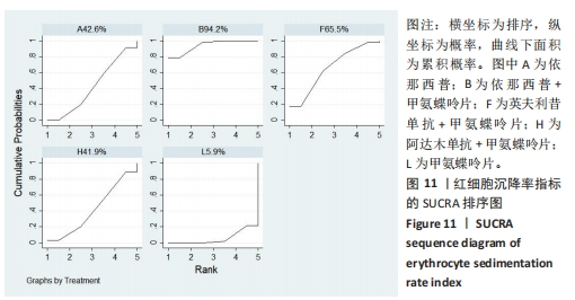
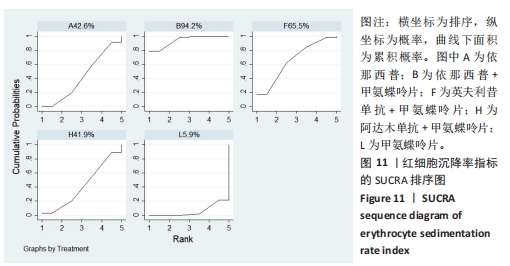
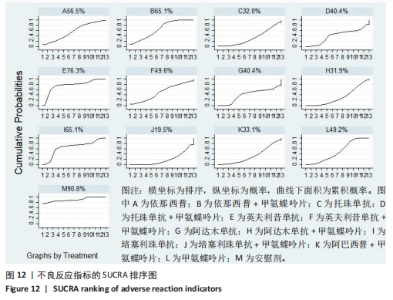
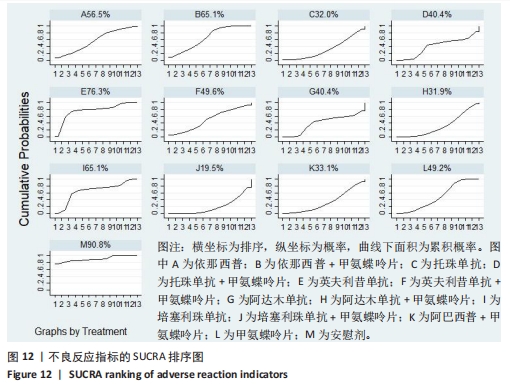
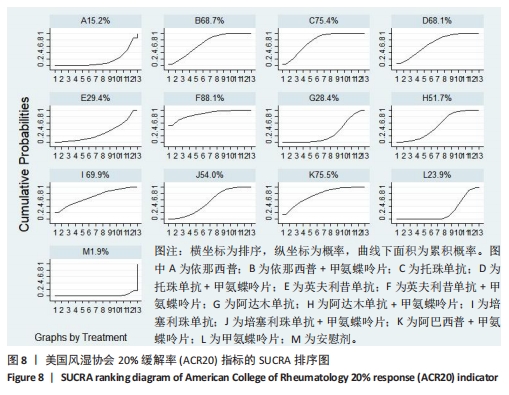
2.4.4 红细胞沉降率指标直接Meta分析及一致性检验结果 运用直接Meta分析及亚组分析对各研究间异质性进行分析,结果显示,整体I2=6.5%,P > 0.05,提示各临床研究间一致性良好,故在固定效应模型下进行分析。红细胞沉降率直接Meta分析结果显示:依那西普、依那西普+甲氨蝶呤片、英夫利昔单抗+甲氨蝶呤片治疗措施效果优于甲氨蝶呤片,差异有显著性意义(P < 0.05),阿达木单抗+甲氨蝶呤片措施与甲氨蝶呤片措施之间无显著差异(P > 0.05),依那西普+甲氨蝶呤片治疗措施效果优于依那西普(P < 0.05)。 对纳入研究进行不一致性检验,结果显示P=0.059 4,P > 0.05说明研究数据一致性良好;并运用节点分裂法进行局部的一致性检验,检验结果P > 0.05,显示研究间具有一致性,故在一致性模型下进行网状Meta分析。并对闭环进行一致性检验,闭环IF=13.24,95%CI:0.00-27.00,检验结果显示一致性良好。 2.4.5 不良反应指标直接Meta分析及一致性检验结果 运用直接Meta分析及亚组分析对各研究间异质性进行分析,结果显示,整体I2=42.9%,P > 0.05,提示各临床研究间一致性良好,故在固定效应模型下进行分析。不良反应指标直接Meta 分析结果显示:依那西普措施不良反应发生情况优于甲氨蝶呤片,差异有显著性意义(P < 0.05),依那西普+甲氨蝶呤片、托珠单抗、英夫利昔单抗+甲氨蝶呤片、阿达木单抗+甲氨蝶呤片、阿巴西普+甲氨蝶呤片治疗措施不良反应发生情况与甲氨蝶呤片措施无显著差异(P > 0.05),甲氨蝶呤片措施在不良反应发生情况方面优于培塞利珠单抗+甲氨蝶呤片(P < 0.05),依那西普+甲氨蝶呤片措施在不良反应发生情况方面优于依那西普(P < 0.05),托珠单抗+甲氨蝶呤片措施与英夫利昔单抗+甲氨蝶呤片措施无显著差异(P > 0.05),托珠单抗+甲氨蝶呤片,英夫利昔单抗,阿达木单抗,培塞利珠单抗措施在不良反应发生情况方面不如安慰剂安全,差异有显著性意义。 对文献进行一致性检验,P=0.210,P > 0.05,研究间无明显异质性,对局部进行节点分裂法进行不一致性检验,结果显示P > 0.05,无明显异质性,故在一致性模型下进行网状分析。对闭环依那西普-依那西普+甲氨蝶呤片-甲氨蝶呤片进行不一致性检验,IF=1.96,95%CI:0.67-3.25,结果显示一致性良好。 2.5 结局指标两两比较结果 2.5.1 ACR20指标 在改善病情达到ACR20方面:与安慰剂措施相比,依那西普+甲氨蝶呤片和甲氨蝶呤片治疗效果与其无显著差异,剩余治疗措施治疗效果优于安慰剂;与依那西普相比,依那西普+甲氨蝶呤片、托珠单抗、托珠单抗+甲氨蝶呤片、英夫利昔单抗+甲氨蝶呤片、阿巴西普+甲氨蝶呤片的治疗效果优于依那西普,其他治疗措施治疗效果与其无显著差异;与甲氨蝶呤片相比,英夫利昔单抗、阿达木单抗、阿达木单抗+甲氨蝶呤片、培塞利珠单抗的效果与其无显著差异,其他治疗措施治疗效果明显优于甲氨蝶呤片;依那西普+甲氨蝶呤片、托珠单抗、托珠单抗+甲氨蝶呤片、英夫利昔单抗、英夫利昔单抗+甲氨蝶呤片、阿达木单抗、阿达木单抗+甲氨蝶呤片、培塞利珠单抗、培塞利珠单抗+甲氨蝶呤片、阿巴西普+甲氨蝶呤片之间,除了英夫利昔单抗+甲氨蝶呤片、托珠单抗的治疗效果优于阿达木单抗,其他治疗措施之间无显著差异。由此可见,不同生物制剂间的疗效差距不明显,联合用药的效果要优于单独用药。 2.5.2 ACR50指标 在改善病情达到ACR50方面:与安慰剂措施相比,除英夫利昔单抗治疗效果无区别外,其他治疗措施治疗效果均优于安慰剂。与英夫利昔单抗相比,依那西普+甲氨蝶呤片、英夫利昔单抗+甲氨蝶呤片、托珠单抗+甲氨蝶呤片措施治疗效果优于英夫利昔单抗,其他治疗措施效果同英夫利昔单抗无显著差异。与甲氨蝶呤片相比,依那西普、阿达木单抗、培塞利珠单抗、阿巴西普+甲氨蝶呤片治疗效果同甲氨蝶呤片无显著差异,安慰剂差于甲氨蝶呤片、剩余治疗措施均优于甲氨蝶呤片。与阿达木单抗相比,依那西普+甲氨蝶呤片、托珠单抗、托珠单抗+甲氨蝶呤片,英夫利昔单抗+甲氨蝶呤片、培塞利珠单抗+甲氨蝶呤片、阿达木单抗+甲氨蝶呤片的治疗效果优于阿达木单抗,阿达木单抗优于安慰剂,剩余治疗措施同阿达木单抗无显著差异。依那西普、依那西普+甲氨蝶呤片、托珠单抗、托珠单抗+甲氨蝶呤片、英夫利昔单抗+甲氨蝶呤片、阿达木单抗+甲氨蝶呤片、培塞利珠单抗、培塞利珠单抗+甲氨蝶呤片、阿巴西普+甲氨蝶呤片治疗措施之间比较,依那西普+甲氨蝶呤片优于依那西普,剩余措施均无显著差异。由此可见,不同生物制剂间的疗效差异不显著,联合用药的效果要优于单独用药。 2.5.3 ACR70指标 在改善病情达到ACR70方面:与安慰剂措施相比,英夫利昔单抗、阿达木单抗、培塞利珠单抗、甲氨蝶呤片治疗效果与安慰剂无显著差异,剩余治疗措施治疗效果均好于安慰剂。与甲氨蝶呤片相比,英夫利昔单抗、阿达木单抗、培塞利珠单抗,安慰剂与甲氨蝶呤片无显著差异,剩余治疗措施效果优于甲氨蝶呤片。与英夫利昔单抗相比,依那西普+甲氨蝶呤片、英夫利昔单抗+甲氨蝶呤片治疗措施效果优于英夫利昔单抗,剩余治疗措施与英夫利昔单抗无显著差异。与阿达木单抗相比,依那西普、英夫利昔单抗、培塞利珠单抗、阿巴西普+甲氨蝶呤片、甲氨蝶呤片、安慰剂治疗效果同阿达木单抗无显著差异,依那西普+甲氨蝶呤片、托珠单抗、托珠单抗+甲氨蝶呤片、英夫利昔单抗+甲氨蝶呤片、阿达木单抗+甲氨蝶呤片、培塞利珠单抗+甲氨蝶呤片治疗措施优于阿达木单抗。依那西普、依那西普+甲氨蝶呤片、托珠单抗+甲氨蝶呤片、英夫利昔单抗+甲氨蝶呤片、阿达木单抗、阿达木单抗+甲氨蝶呤片、培塞利珠单抗、培塞利珠单抗+甲氨蝶呤片、阿巴西普+甲氨蝶呤片治疗措施之间比较,依那西普+甲氨蝶呤片优于依那西普和阿巴西普+甲氨蝶呤片、英夫利昔单抗+甲氨蝶呤片优于阿巴西普+甲氨蝶呤片,剩余治疗措施的治疗效果无显著差异。由此可见,不同生物制剂间的疗效差异不显著,联合用药的效果要优于单独用药。 2.5.4 红细胞沉降率指标 在改善红细胞沉降率指标方面:依那西普、依那西普+甲氨蝶呤片、英夫利昔单抗+甲氨蝶呤片、阿达木单抗+甲氨蝶呤片、甲氨蝶呤片措施之间比较,依那西普+甲氨蝶呤片措施优于依那西普和甲氨蝶呤片,剩余措施间治疗效果无显著差异。 2.5.5 不良反应指标两两比较结果 在不良反应发生情况方面:与安慰剂措施相比,安慰剂措施的不良反应发生情况好于托珠单抗+甲氨蝶呤片,英夫利昔单抗,阿达木单抗治疗措施,剩余治疗措施同安慰剂无显著差异。与英夫利昔单抗相比,安慰剂措施优于英夫利昔单抗措施,英夫利昔单抗措施优于托珠单抗+甲氨蝶呤片、阿达木单抗措施,剩余治疗措施同英夫利昔单抗无显著差异。与依那西普+甲氨蝶呤片措施相比,依那西普+甲氨蝶呤片措施优于培塞利珠单抗+甲氨蝶呤片措施,剩余治疗措施同依那西普+甲氨蝶呤片措施无显著差异。依那西普、托珠单抗、托珠单抗+甲氨蝶呤片、英夫利昔单抗+甲氨蝶呤片、阿达木单抗、阿达木单抗+甲氨蝶呤片、培塞利珠单抗、培塞利珠单抗+甲氨蝶呤片、阿巴西普+甲氨蝶呤片、甲氨蝶呤片治疗措施间不良反应发生情况无显著差异。由此可知,不同生物制剂治疗类风湿性关节炎的不良反应发生情况没有明显差异。 2.6 结局指标网状Meta分析SUCRA 排序结果 2.6.1 ACR20指标 不同生物制剂改善ACR20的排序结果,见图8,英夫利昔单抗+甲氨蝶呤片(88.1%)>阿巴西普+甲氨蝶呤片(75.5%)>托珠单抗(75.4%)>培塞利珠单抗(69.9%)>依那西普+甲氨蝶呤片(68.7%)>托珠单抗+甲氨蝶呤片(68.1%)>培塞利珠单抗+甲氨蝶呤片(54.0%)>阿达木单抗+甲氨蝶呤片(51.7%)>英夫利昔单抗(29.4%)>阿达木单抗(28.4%)>甲氨蝶呤片(23.9%)>依那西普(15.2%)>安慰剂(1.9%)。由此可知,生物制剂联合用药在临床应用时可作为首选应用以提高疗效。"
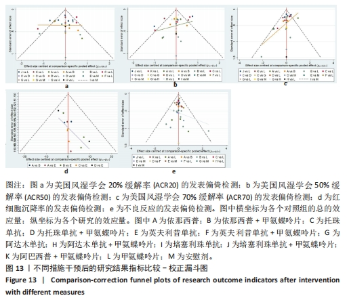
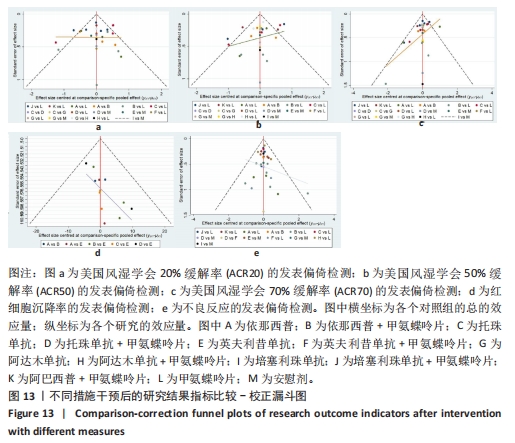
2.7 结局指标病程的亚组分析结果 临床研究中,药物的疗程对研究结果有一定影响,现对各结局指标药物疗程进行亚组分析发现:在ACR20、ACR50方面,3个月及以下疗程治疗效果同3-6个月、6个月以上之间无明显差异(P > 0.05),3-6个月疗程与6个月以上之间有明显差异(P < 0.05)。在ACR70方面,3个月及以下疗程同3-6个月、6个月以上之间有明显差异(P < 0.05),3-6个月、6个月以上疗程之间差异无显著性意义(P > 0.05)。在红细胞沉降率方面,3个月及以下疗程治疗效果同3-6个月疗程无明显差异(P > 0.05),与6个月以上疗程有明显差异(P < 0.05);3-6个月、6个月以上疗程之间差异无显著性意义(P > 0.05)。在不良反应发生情况方面,3个月及以下疗程不良反应发生情况同3个月至6个月、6个月以上疗程之间有明显差异(P < 0.05),3个月至6个月、6个月以上疗程之间差异无显著性意义(P > 0.05)。 2.8 结局指标发表偏倚分析结果 发表偏倚检测的比较-校正漏斗图见图13。图中治疗措施两两比较用不同颜色的点代替。图13a-e中图形基本对称且在上方聚集,说明研究间存在发表偏倚的可能性较小;图13d中对称性欠佳,考虑为纳入研究中关于红细胞沉降率的研究数量较少导致。"
| [1] 王馨苑,黄夏冰,李娟,等.桃红四物汤“异病同治”类风湿性关节炎和骨关节炎的网络药理学及分子对接分析[J].中国组织工程研究,2022,26(15): 2419-2425. [2] 符译尹,蒲梅婷,潘田芬,等.白芍水煎液对类风湿性关节炎患者的临床疗效[J].中成药,2021, 43(12):3346-3350. [3] 许海艳,况南珍,张瑜娟,等.类风湿性关节炎治疗方法的研究进展[J].南昌大学学报(医学版), 2020,60(5):97-102. [4] 杨腾蛟,朱珊珊,陆艳丽,等.免疫抑制剂结合生物制剂治疗类风湿关节炎效果[J].实用中西医结合临床,2023,23(6):6-10. [5] 刘念,张秀灵,尚静静,等.甲氨蝶呤联合传统DMARDs与联合生物制剂治疗RA的疗效比较[J].南昌大学学报(医学版),2017,57(6):32-36. [6] GRAUDAL N, KAAS-HANSEN BS, GUSKI L, et al. Different original and biosimilar tnf inhibitors similarly reduce joint destruction in rheumatoid arthritis-a network meta-analysis of 36 randomized controlled trials. Int J Mol Sci. 2019;20(18):4350. [7] MA K, LI L, LIU C, et al. Efficacy and safety of various anti-rheumatic treatments for patients with rheumatoid arthritis: a network meta-analysis. Arch Med Sci. 2019;15(1):33-54. [8] ARNETT FC, EDWORTHY SM, BLOCH DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315-325. [9] 陈薇,方赛男,刘建平,等.国际循证医学证据分级体系的发展与现状[J].中国中西医结合杂志, 2017,37(12):1413-1419. [10] 杨小兰,高薇.注射用重组人Ⅱ型肿瘤坏死因子受体-抗体融合蛋白治疗类风湿关节炎患者疗效评价[J].临床研究,2019,27(5):69-70. [11] 余素君,纳世丽,康丽.注射用重组人Ⅱ型肿瘤坏死因子受体-抗体融合蛋白联合甲氨蝶呤治疗类风湿关节炎的效果研究[J].药物评价研究,2019, 42(5):940-943. [12] 朱勇,吴旭强,刘怡,等.重组人肿瘤坏死因子受体-抗体融合蛋白治疗类风湿关节炎65例的疗效与安全性观察[J].实用医院临床杂志,2012,9(2):97-99. [13] 庞金奎,杨秀峰.英夫利西单抗联合甲氨蝶呤治疗活动性类风湿关节炎疗效分析[J].中外医疗, 2015,34(6):125-126. [14] 夏志高,柳彩阳.英夫利西单抗联合甲氨蝶呤治疗活动性类风湿关节炎的效果及安全性研究[J].中国当代医药,2017,24(19):151-153. [15] 陈士军,朱卫民.依那西普治疗活动性类风湿关节炎患者的临床疗效探讨[J].陕西医学杂志,2013, 42(8):1065-1066. [16] 丁菱,何善智,钟伟秋.依那西普治疗活动性类风湿关节炎的临床疗效分析[J].医学综述,2012, 18(19):3293-3294,3299. [17] 霍爱鑫,高鹏,刘宇宏,等.依那西普联合甲氨蝶呤治疗类风湿关节炎的临床效果[J].延安大学学报(医学科学版),2017,15(1):20-23. [18] 敖亮,刘瑞,李静.依那西普联合甲氨蝶呤治疗类风湿关节炎的临床疗效及安全性[J].临床医学研究与实践,2018,3(24):51-52. [19] 郑创史,邱钊禹.依那西普联合甲氨蝶呤治疗类风湿关节炎的疗效及对血清TL1A、TNF-α、IL-6以及IL-17水平的影响[J].中国药师,2016,19(4):715-717. [20] 潘楠楠,蒋艳.依那西普联合甲氨蝶呤对类风湿关节炎患者外周血T淋巴细胞亚群及血清炎症因子水平的影响[J].菏泽医学专科学校学报,2020, 32(4):4-7. [21] 王创明,郑洵,陈海波.依那西普、甲氨蝶呤联合治疗对类风湿关节炎患者血清IL-1、COX-2、TNF-α的影响[J].北方药学,2016,13(4):117-118. [22] 杜勤,徐红娣.托珠单抗对类风湿关节炎患者疗效、免疫球蛋白及辅助性T细胞水平的影响[J].药学实践杂志,2020,38(1):71-73, 87. [23] 邹鹏程,叶金宝,翁文翔,等.甲氨蝶呤与阿达木单抗联合治疗对类风湿关节炎患者关节症状及血清抗CCP抗体、炎症因子水平的影响[J].武警后勤学院学报(医学版),2021,30(9):90-91, 94. [24] 游碧蓉,田新玮,薛冰,等.阿达木单抗联合甲氨蝶呤治疗类风湿关节炎的疗效研究[J].现代生物医学进展,2016,16(35):6906-6909. [25] KAMEDA H, UEKI Y, SAITO K, et al. Japan biological agent study integrated consortium. Etanercept (ETN) with methotrexate (MTX) is better than ETN monotherapy in patients with active rheumatoid arthritis despite MTX therapy: a randomized trial. Mod Rheumatol. 2010;20(6):531-538. [26] NISHIMOTO N, MIYASAKA N, YAMAMOTO K, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol. 2009;19(1):12-19. [27] ELMEDANY SH, MOHAMED AE, GALIL SMA. Efficacy and safety profile of intravenous tocilizumab versus intravenous abatacept in treating female Saudi Arabian patients with active moderate-to-severe rheumatoid arthritis. Clin Rheumatol. 2019; 38(8):2109-2117. [28] BIJLSMA JWJ, WELSING PMJ, WOODWORTH TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388(10042):343-355. [29] GABAY C, EMERY P, VAN VOLLENHOVEN R, et al. ADACTA Study Investigators. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381(9877):1541-1550. [30] KLARESKOG L, VAN DER HEIJDE D, DE JAGER JP, et al. TEMPO (Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes) study investigators. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004; 363(9410):675-681. [31] REXHEPI S, REXHEPI M, REXHEPI B, et al. Evaluation of the efficacy of combined therapy of methotrexate and etanercept versus methotrexate as a mono-therapy. Open Access Maced J Med Sci. 2018;6(5):786-789. [32] BAEK HJ, LIM MJ, PARK W, et al. Efficacy and safety of tocilizumab in Korean patients with active rheumatoid arthritis. Korean J Intern Med. 2019;34(4):917-931. [33] TAM LS, SHANG Q, LI EK, et al. Infliximab is associated with improvement in arterial stiffness in patients with early rheumatoid arthritis -- a randomized trial. J Rheumatol. 2012;39(12):2267-2275. [34] WELLS AF, WESTHOVENS R, REED DM, et al.Abatacept plus methotrexate provides incremental clinical benefits versus methotrexate alone in methotrexate-naive patients with early rheumatoid arthritis who achieve radiographic nonprogression. J Rheumatol. 2011;38(11):2362-2368. [35] KAMEDA H, KANBE K, SATO E, et al. Continuation of methotrexate resulted in better clinical and radiographic outcomes than discontinuation upon starting etanercept in patients with rheumatoid arthritis: 52-week results from the JESMR study. J Rheumatol. 2011;38(8):1585-1592. [36] EMERY P, GENOVESE MC, VAN VOLLENHOVEN R, et al. Less radiographic progression with adalimumab plus methotrexate versus methotrexate monotherapy across the spectrum of clinical response in early rheumatoid arthritis. J Rheumatol. 2009;36(7): 1429-1441. [37] KIM J, RYU H, YOO DH, et al. A clinical trial and extension study of infliximab in Korean patients with active rheumatoid arthritis despite methotrexate treatment. J Korean Med Sci. 2013;28(12):1716-1722. [38] KIM HY, LEE SK, SONG YW. A randomized, double-blind, placebo-controlled, phase III study of the human anti-tumor necrosis factor antibody adalimumab administered as subcutaneous injections in Korean rheumatoid arthritis patients treated with Methotrexate. J Rheumatol. 2008;10(1):9-16. [39] ZHANG FC, HOU Y, HUANG F, et al. Infliximab versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a preliminary study from China. Rheumatol. 2006;9(2):127-130. [40] BI L, LI Y, HE L, XU H, et al. Efficacy and safety of certolizumab pegol in combination with methotrexate in methotrexate-inadequate responder Chinese patients with active rheumatoid arthritis: 24-week results from a randomised, double-blind, placebo-controlled phase 3 study. Clin Exp Rheumatol. 2019; 37(2):227-234. [41] JONES G, SEBBA A, GU J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69(1):88-96. [42] FLEISCHMANN R, VENCOVSKY J, VAN VOLLENHOVEN RF, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis. 2009;68(6):805-811. [43] EMERY P, BINGHAM CO 3RD, BURMESTER GR, et al. Certolizumab pegol in combination with dose-optimised methotrexate in DMARD-naïve patients with early, active rheumatoid arthritis with poor prognostic factors: 1-year results from C-EARLY, a randomised, double-blind, placebo-controlled phase III study. Ann Rheum Dis. 2017;76(1):96-104. [44] ATSUMI T, YAMAMOTO K, TAKEUCHI T, et al. The first double-blind, randomised, parallel-group certolizumab pegol study in methotrexate-naive early rheumatoid arthritis patients with poor prognostic factors, C-OPERA, shows inhibition of radiographic progression. Ann Rheum Dis. 2016;75(1):75-83. [45] EMERY P, BURMESTER GR, BYKERK VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74(1):19-26. [46] TAKEUCHI T, YAMANAKA H, ISHIGURO N, et al.Adalimumab, a human anti-TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: the HOPEFUL 1 study. Ann Rheum Dis. 2014; 73(3):536-543. [47] KAVANAUGH A, FLEISCHMANN RM, EMERY P, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis. 2013;72(1):64-71. [48] KREMER JM, GENANT HK, MORELAND LW, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144(12):865-876. [49] 李达,姜泉,徐浩东,等.基于络病理论之中医药防治类风湿关节炎证治研究[J].辽宁中医药大学学报,2024,26(1):153-156. [50] 张佳琳,王晓非.生物制剂对类风湿性关节炎患者血脂水平影响的研究进展[J].实用药物与临床, 2020,23(7):660-665. [51] 陈兰芳,强孚勇,徐亮.托珠单抗联合改善病情抗风湿药治疗中重度类风湿关节炎短期临床观察[J].中国临床药理学与治疗学,2018,23(12):1386-1391. [52] GILLESPIE J, SAVIC S, WONG C, et al. Histone deacetylases are dysregulated in rheumatoid arthritis and a novel histone deacetylase 3-selective inhibitor reduces interleukin-6 production by peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Rheum. 2012;64(2):418-422. [53] 刘昱廷,曾令伟,王柠柠.托珠单抗联合塞来昔布对中重度类风湿关节炎患者炎症反应的影响研究[J].中国处方药,2023,21(7):103-106. [54] 伍俊妍,邱凯锋.类风湿关节炎TNF-α抑制剂药品临床快速评价与遴选广东共识[J/OL].今日药学:1-26[2023-12-19]. http://kns.cnki.net/kcms/detail/44.1650.R.20230529.1003.002.html. [55] 罗绮雯,陈国强,张红卫,等.依那西普注射剂联合雷公藤多苷片治疗强直性脊柱炎的临床研究[J].中国临床药理学杂志,2019,35(15):1578-1580. [56] VAN DARTEL SA, FRANSEN J, KIEVIT W, et al. Difference in the risk of serious infections in patients with rheumatoid arthritis treated with adalimumab, infliximab and etanercept: results from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Ann Rheum Dis. 2013;72(6):895-900. [57] 蔡俊,卫菁,聂力等.英夫利昔单抗致结核感染文献分析[J].药物流行病学杂志,2018,27(11):765-768. |
| [1] | Zhong Jun, Wang Wen. Network meta-analysis of different anatomical repair strategies to improve chronic lateral ankle instability [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1470-1476. |
| [2] | Hu Zhixing, Li Qun, Yang Chao, Wang Xiaoxiao, Fang Luochangting, Hou Wuqiong, Lin Na, Chen Weiheng, Liu Chunfang, Lin Ya. Network meta-analysis of the modeling effects of different factors on rabbit models of steroid-induced osteonecrosis of femoral head [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 976-984. |
| [3] | Chang Ying, Xia Yuan, Sun Yundi, Cheng Lulu, Xiong Wenjuan, Zhao Xianghu. Effectiveness of different specific exercise therapies in treatment of adolescent idiopathic scoliosis: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(36): 5899-5904. |
| [4] | Zhang Xiurong, Cui Xinmei, Zhao Haiyan, Dai Jin, Fu Jiaxin, Wang Bo. Effect of Tiaodu Tongmai acupuncture therapy on rheumatoid arthritis at different ages by generalized estimating equation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5584-5590. |
| [5] | Zhou Erye, Zeng Keqin, Wu Jian, Ren Tian, He Michun. Osteoclast differentiation induced by total immune complex in serum of patients with rheumatoid arthritis leads to osteoporosis and its factors analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5606-5611. |
| [6] | Lu Xiaoling, Liu Bin, Xu Bin. Bioinformatics identification and validation of genes related to fatty acid metabolism in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(32): 5116-5121. |
| [7] | Fan Yidong, Qin Gang, He Kaiyi, Gong Yufang, Li Weicai, Wu Guangtao. Expression of immune-related genes in rheumatoid arthritis and a two-sample Mendelian randomization study of immune cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(27): 4312-4318. |
| [8] | Xu Chenghan, Zhuo Hanjie, Chai Xubin, Huang Yong, Zhang Bowen, Chen Qin, Hao Yupeng, Li Lin, Zhou Yingjie. Meta-analysis of the incidence and related factors for cervical spine instability in patients with rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(24): 3922-3929. |
| [9] | Wu Ruiqi, Zhou Yi, Xia Tian, Zhang Chi, Yang Qipei, Zhang Xuan, Zhang Yazhong, Cui Wei. Mendelian randomization study on the association between rheumatoid arthritis and osteoporosis and bone mineral density [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(23): 3715-3721. |
| [10] | Chang Wanpeng, Zhang Zhongwen, Yang Yulin, Zi Yang, Yang Mengqi, Du Bingyu, Wang Nan, Yu Shaohong. Efficacy of rehabilitation exoskeleton robots on post-stroke lower limb motor dysfunction: a Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 321-328. |
| [11] | Ran Lei, Han Haihui, Xu Bo, Wang Jianye, Shen Jun, Xiao Lianbo, Shi Qi. Molecular docking analysis of the anti-inflammatory mechanism of Cibotium barometz and Epimedium for rheumatoid arthritis: animal experiment validation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 208-215. |
| [12] | Xia Tian, Li Binglin, Xiao Fayuan, Zheng Enze, Chen Yueping. CeRNA interaction network and immune manifestation of ferroptosis-related signature genes in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(16): 2561-2567. |
| [13] | Hu Mengfan, Yan Qiuhui, Deng Mengran, Liang Meimei, Liang Liang, Yi Sisi, Deng Jiagang, Yun Chenxia. Mangiferin inhibits proliferation, migration and inflammatory factor expression of fibroblast-like synoviocytes in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(11): 1690-1695. |
| [14] | Yang Yulin, Chang Wanpeng, Ding Jiangtao, Xu Hongli, Wu Xiao, Xiao Boheng, Ma Lihong. Transcranial direct current stimulation at different targets for Parkinson’s disease: a network Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(11): 1797-1804. |
| [15] | Li Wenjie, You Aijia, Zhou Junli, Fang Sujuan, Li Chun. Effects of different dressings in the treatment of burn wounds: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1141-1148. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||