Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (36): 7889-7897.doi: 10.12307/2025.542
Previous Articles Next Articles
Astrocytes regulate remyelination in central nervous system
Shui Jing1, He Yu1, Jiang Nan1, Xu Kun2, Song Lijuan2, 3, Ding Zhibin1, 2, Ma Cungen2, Li Xinyi1, 3
- 1Department of Neurology, Third Hospital of Shanxi Medical University (Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital), Taiyuan 030032, Shanxi Province, China; 2Key Research Laboratory of Benefiting Qi for Acting Blood Circulation Method to Treat Multiple Sclerosis of State Administration of Traditional Chinese Medicine/Department of Encephalopathy, First Clinical College, Shanxi University of Chinese Medicine, Jinzhong 030619, Shanxi Province, China; 3Key Laboratory of Cellular Physiology of Ministry of Education, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
-
Received:2024-07-13Accepted:2024-08-24Online:2025-12-28Published:2025-03-25 -
Contact:Li Xinyi, Chief physician, Department of Neurology, Third Hospital of Shanxi Medical University (Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital), Taiyuan 030032, Shanxi Province, China; Key Laboratory of Cellular Physiology of Ministry of Education, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China; Co-corresponding author: Ma Cungen, Junior professor, Key Research Laboratory of Benefiting Qi for Acting Blood Circulation Method to Treat Multiple Sclerosis of State Administration of Traditional Chinese Medicine/Department of Encephalopathy, First Clinical College, Shanxi University of Chinese Medicine, Jinzhong 030619, Shanxi Province, China -
About author:Shui Jing, Master candidate, Physician, Department of Neurology, Third Hospital of Shanxi Medical University (Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital), Taiyuan 030032, Shanxi Province, China. He Yu, Master candidate, Physician, Department of Neurology, Third Hospital of Shanxi Medical University (Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital), Taiyuan 030032, Shanxi Province, China. Shui Jing and He Yu contributed equally to this article. -
Supported by:Youth Fund of National Natural Science Foundation of China, No. 82301579 (to DZB); Talent Introduction Program of Scientific Research Foundation of Third Hospital of Shanxi Medical University, No. 2021RC033 (to DZB); Grant of Innovative Young Talent Team of Shanxi Science and Technology in 2022, No. 202204051001028 (to SLJ)
CLC Number:
Cite this article
Shui Jing, He Yu, Jiang Nan, Xu Kun, Song Lijuan, Ding Zhibin, Ma Cungen, Li Xinyi. Astrocytes regulate remyelination in central nervous system[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7889-7897.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

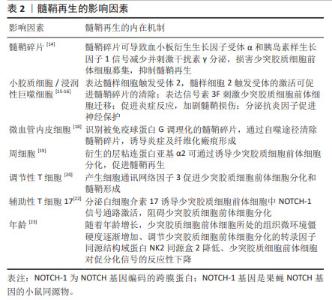
髓鞘碎片可抑制中枢神经系统髓鞘再生。脱髓鞘病变产生的髓鞘碎片含有髓鞘再生的抑制分子,过多的髓鞘碎片可导致血小板衍生生长因子受体α和胰岛素样生长因子1信号减少并刺激干扰素γ分泌,损害少突胶质细胞前体细胞募集、增殖和成熟[14]。小胶质细胞和浸润性巨噬细胞是中枢神经系统发挥吞噬功能的主要细胞,通过表达髓样细胞2触发受体促进髓鞘碎片的清除,增加脱髓鞘区域少突胶质细胞前体细胞的密度及少突胶质细胞的形成,增强中枢神经系统髓鞘再生[15]。 除了参与吞噬髓鞘碎片以促进髓鞘再生外,小胶质细胞/巨噬细胞还可表达信号素3F以神经纤毛蛋白2依赖的方式刺激少突胶质细胞前体细胞迁移,增强少突胶质细胞前体细胞募集,加速髓鞘再生的发生[16]。当小胶质细胞被激活时,它们会分泌白细胞介素1、白细胞介素12、白细胞介素23、肿瘤坏死因子α和诱导型一氧化氮合酶等因子促进炎症反应,加剧髓鞘损伤;也可分泌白细胞介素4、白细胞介素10、白细胞介素13、转化生长因子β和精氨酸酶1等因子来促进神经保护[17]。促炎型小胶质细胞/巨噬细胞在髓鞘再生的募集阶段普遍存在,而抗炎型小胶质细胞/巨噬细胞在分化阶段占主导地位,从促炎状态到抗炎状态的及时过渡对快速有效的髓鞘再生至关重要。 研究表明,微血管内皮也具备髓鞘碎片吞噬作用。微血管内皮细胞作为“业余”吞噬细胞,可识别被免疫球蛋白G调理化的髓鞘碎片,而后通过自噬途径清除髓鞘碎片。内皮细胞通过吞噬被免疫球蛋白G调理化的髓鞘碎片,并将髓鞘抗原呈递给淋巴细胞,生成特异性抗体可以进一步调理髓鞘碎片并促进其吞噬,内皮细胞对髓鞘碎片的摄取和处理可诱导炎症及纤维化瘢痕形成[18]。周细胞作为中枢神经系统的血管周围细胞,其功能并不局限于血管稳态,也参与调节中枢神经系统髓鞘再生过程。在中枢神经系统脱髓鞘后,周细胞具有高度增殖反应,其衍生的层粘连蛋白亚基α2可通过诱导少突胶质细胞前体细胞向少突胶质细胞分化,加速髓鞘再生[19]。 外周免疫细胞也可参与调节髓鞘再生,其中,调节性T细胞促进髓鞘再生,辅助性T细胞17抑制髓鞘再生。体外研究表明,调节性T细胞通过产生细胞通讯网络因子3促进少突胶质细胞前体细胞分化和髓鞘形成[20]。需注意的是,细胞通讯网络因子3并不是中枢神经系统有效髓鞘形成或髓鞘再生所必需的[21]。辅助性T细胞17浸润与自发髓鞘再生受损有关,其分泌的白细胞介素17可诱导少突胶质细胞前体细胞中NOTCH-1信号通路激活,损害少突胶质细胞前体细胞分化为成熟少突胶质细胞,导致中枢神经系统髓鞘再生失败。白细胞介素17受体衔接蛋白Act 1具有泛素连接酶活性,其可与NOTCH-1的胞内结构域(NOTCH-1 intracellular domain,NICD1)结合,使 NICD1 泛素化,形成Act1-NICD1复合物并易位到细胞核中,与转录因子免疫球蛋白κJ区的重组信号结合蛋白形成稳定的复合物,促进基因转录共激活因子的募集,诱导少突胶质细胞前体细胞的增殖及相关基因的表达,干扰少突胶质细胞前体细胞的正常分化程序导致脱髓鞘[22]。 衰老是影响中枢系统髓鞘再生的因素之一。随着年龄增长,机体内烟酰胺腺嘌呤二核苷酸(NAD)水平逐渐下降、少突胶质细胞前体细胞所处的组织微环境僵硬度逐渐增加、调节少突胶质细胞前体细胞分化的转录因子同源结构域蛋白NK2同源盒2降低、少突胶质细胞前体细胞对促分化信号的反应性下降等均可导致少突胶质细胞前体细胞分化潜力减低[23]。NAD-SIRT2-H3K18Ac-ID4轴可以介导髓鞘再生,其中,NAD依赖性去乙酰酶SIRT2是恢复少突胶质细胞前体细胞分化潜力所必需的,通过补充NAD+前体β-烟酰胺单核苷酸,诱导少突胶质细胞前体细胞中SIRT2进入细胞核,促进少突胶质细胞前体细胞分化为成熟的少突胶质细胞,进而增强中枢神经系统髓鞘再生[24];老年小鼠少突胶质细胞前体细胞表达的压电型机械感应离子通道1可通过感知细胞周围环境的硬度,抑制少突胶质细胞前体细胞的分化[25],GsMTx4(一种蜘蛛毒肽)通过抑制压电型机械感应离子通道1可恢复少突胶质细胞前体细胞的分化活性,促进髓鞘再生;二甲双胍或间断禁食治疗可以恢复老年小鼠少突胶质细胞前体细胞对促分化信号的反应,从而改善髓鞘再生[26]。 2.2 星形胶质细胞调节髓鞘再生 星形胶质细胞在髓鞘再生过程中起着双重作用。星形胶质细胞可通过转化为少突胶质谱系细胞、支持营养轴突及释放神经生长因子等途径实现有效的髓鞘再生;还可通过分泌神经毒性因子、炎性递质和细胞外基质成分等抑制少突胶质细胞前体细胞增殖、迁移和分化等过程,限制髓鞘修复,具体机制见图3。"


2.2.1 星形胶质细胞吞噬髓鞘碎片影响髓鞘再生 脱髓鞘区域需要有效清除髓鞘碎片,这是髓鞘再生的关键步骤,星形胶质细胞参与清除退化的髓鞘碎片以加速髓鞘再生。星形胶质细胞通过分泌趋化因子C-X-C配体10和C-C基序配体2募集小胶质细胞/巨噬细胞到脱髓鞘部位吞噬髓鞘碎片,还可通过低密度脂蛋白受体相关蛋白1介导的内吞作用摄取髓鞘碎片,再将其转运至溶酶体进行降解,以有效地补充吞噬功能[27]。髓磷脂的摄取诱导星形胶质细胞活化和增殖,导致磷酸核转录因子kappa B的激活及趋化因子的分泌,有趣的是,并未检测到白细胞介素的释放,这表明髓鞘摄取诱导了一种特定的星形胶质细胞激活模式,主要支持免疫细胞的募集[28]。然而,在缺血性损伤所致的继发性脱髓鞘病变中,表达脂质运载蛋白2的星形胶质细胞可获得吞噬表型并摄取髓磷脂,脂质运载蛋白2可与低密度脂蛋白受体相关蛋白1结合,导致进行性脱髓鞘。敲除低密度脂蛋白受体相关蛋白1可降低脂质运载蛋白2诱导的星形胶质细胞对髓鞘的吞噬作用,减少髓鞘脱失[29]。吞噬作用不足会导致髓鞘碎片堆积及髓鞘再生受损,对髓鞘的过度吞噬则会引发神经功能进一步恶化[30],因此,探索星形胶质细胞吞噬髓鞘碎片的内在机制以及保持星形胶质细胞适度的吞噬功能可能是未来有潜力的研究方向。 2.2.2 星形胶质细胞参与炎性反应影响髓鞘再生 星形胶质细胞可分泌炎性细胞因子导致髓鞘脱失。当中枢神经系统受到损伤刺激时,星形胶质细胞可由“静息态”转化为“激活态”,成为反应性星形胶质细胞。反应性星形胶质细胞是一种高度异质的状态,可向相对破坏性或相对保护性的方向上极化:A1型星形胶质细胞释放细胞毒性化学物质,导致神经元和少突胶质细胞死亡并发挥有害作用;A2型星形胶质细胞可以产生神经营养因子并起到神经保护作用。在衰老、损伤或炎症等情况下,活化的小胶质细胞通过分泌白细胞介素1α、肿瘤坏死因子α和补体C1q诱导A1型反应性星形胶质细胞,后者可通过分泌白细胞介素1β、白细胞介素6及Fas配体等神经毒性因子,从而减少少突胶质细胞前体细胞募集,诱导少突胶质细胞凋亡及髓鞘损伤[31]。当反应性星形胶质细胞的形成被阻断时,活化的小胶质细胞不足以诱导神经元或少突胶质细胞的死亡[32],表明星形胶质细胞在小胶质细胞发挥促炎反应过程中具有不可或缺的作用。抑制A1型星形胶质细胞的产生或促进A1型星形胶质细胞向A2型星形胶质细胞转化可能是充满前景的治疗方法。 2.2.3 星形胶质细胞转化为少突胶质细胞前体细胞促进髓鞘再生 星形胶质细胞具备向少突胶质谱系细胞转化的能力。体外研究表明,表皮生长因子可靶向EGF-EGFR-Erk1/2信号转导轴,通过细胞外信号调节激酶1和2依赖性方式激活丝裂原活化蛋白激酶通路,在体内和体外促进SRY-box转录因子10诱导的星形胶质细胞转化为少突胶质细胞谱系细胞,表现为少突胶质细胞转录因子1和2的上调[33]。膜结合神经调节蛋白1的所有亚型都含有一个表皮生长因子样结构域,对于介导生物信号转导至关重要。研究表明,肿瘤坏死因子α可诱导星形胶质细胞表达未成熟细胞标志物分化簇44和RNA结合蛋白Musashi1,成为具备干细胞特性的反应性星形胶质细胞。在肿瘤坏死因子α刺激下,膜结合神经调节蛋白1可使反应性星形胶质细胞在mRNA和蛋白质水平上表达少突胶质谱系细胞标志物血小板衍生生长因子受体α和O4,通过表皮生长因子受体靶向PI3K-AKT-mTOR信号通路调节髓鞘相关基因的表达,促进少突胶质细胞形成及髓鞘再生[34]。星形胶质细胞可向少突胶质细胞谱系细胞转分化,这一发现为脱髓鞘疾病进行髓鞘再生提供了新途径。 2.2.4 星形胶质细胞为髓鞘再生提供营养支持 少突胶质细胞前体细胞向少突胶质细胞分化形成髓鞘的过程中,需要大量的胆固醇、乳酸等营养物质。星形胶质细胞是中枢神经系统胆固醇的主要提供者,通过向少突胶质细胞提供脂质调节髓鞘再生。其中,在溶血卵磷脂诱导的髓鞘损伤动物模型中,星形胶质细胞核转录因子红系2相关因子2通路的持续激活会抑制胆固醇的生物合成/输出,木犀草素可通过下调核转录因子红系2相关因子2通路,上调胆固醇生物合成通路支持成熟少突胶质细胞存活,促进髓鞘形成[35]。在实验性自身免疫性脑脊髓炎或双环己酮草酰二腙脱髓鞘模型中,促炎细胞因子可通过胆固醇转运蛋白——ATP结合盒转运蛋白A1依赖性途径抑制星形胶质细胞的胆固醇外排[36],从膳食中补充的外源性胆固醇可通过受损的血脑屏障进入中枢神经系统,支持少突胶质细胞前体细胞增殖和分化,恢复生长因子的平衡,为髓鞘再生创造有利的环境[37]。低浓度葡萄糖会抑制少突胶质细胞谱系细胞的发育及髓鞘的生成,当提供外源性L-乳酸时,髓鞘形成得以恢复,表明乳酸可以在葡萄糖水平有限时支持少突胶质细胞的发育和髓鞘形成。中枢神经系统少突胶质细胞是大脑中以最高速率消耗乳酸的细胞类型,其可通过单羧酸转运蛋白1吸收星形胶质细胞释放的乳酸以进行能量代谢和脂质合成,促进髓鞘再生。因此,星形胶质细胞为少突胶质细胞谱系细胞提供胆固醇及乳酸对于髓鞘再生能量代谢至关重要。 2.2.5 星形胶质细胞衍生神经营养因子通过干预少突胶质细胞前体细胞增殖、迁移和分化过程进而促进髓鞘再生 星形胶质细胞通过分泌血小板衍生生长因子AA、成纤维细胞生长因子2、神经生长因子促进少突胶质细胞前体细胞增殖。由星形胶质细胞产生的血小板衍生生长因子AA是少突胶质细胞前体细胞最有效的有丝分裂原和存活因子,其可与少突胶质细胞前体细胞表面的血小板衍生生长因子受体α结合,导致少突胶质细胞前体细胞数量增加。成纤维细胞生长因子2在早期增强少突胶质细胞前体细胞的增殖和存活,但在长时间刺激后可能抑制其分化为少突胶质细胞,在中枢神经系统髓鞘再生中的作用尚存在争议[38]。星形胶质细胞衍生的纤毛神经营养因子可诱导心营养因子样细胞因子1的产生,显著促进少突胶质细胞前体细胞的分化[39];星形胶质细胞来源的脑源性神经营养因子以原肌球蛋白相关激酶B受体依赖性方式刺激少突胶质细胞前体细胞分化,促进髓鞘再生[40]。星形胶质细胞还可通过分泌白血病抑制因子、胰岛素样生长因子1和金属蛋白酶组织抑制剂1等因子促进少突胶质细胞前体细胞分化[41],在髓鞘再生过程中发挥重要作用。此外,有研究发现星形胶质细胞可表达短蛋白聚糖的可溶性亚型,在体外以浓度依赖性方式促进髓鞘形成。短蛋白聚糖作为细胞外基质成分之一,不影响少突胶质细胞前体细胞的分化过程,而是通过作用于髓鞘形成的后期阶段以增强髓鞘形成[42]。因此,促进星形胶质细胞分泌神经营养因子或外源性神经营养因子的干预可能是增强髓鞘再生的有效靶点。 2.2.6 星形胶质细胞分泌髓鞘再生抑制因子限制髓鞘再生 发育过程中的少突胶质细胞前体细胞迁移是以脉管系统作为物理介质,星形胶质细胞端足的形成及信号素3a/6a分泌可将少突胶质细胞前体细胞从血管脱离,并允许随后的少突胶质细胞前体细胞分化[43]。由于信号素3a对少突胶质细胞前体细胞的血管迁移排斥作用,在脱髓鞘病变中添加信号素3a会抑制少突胶质细胞前体细胞募集,少突胶质细胞前体细胞向病灶中心迁移不足,导致髓鞘再生不良,因此,抑制信号素3a可能会促进髓鞘再生[44]。此外,星形胶质细胞来源的细胞间信号分子内皮素1通过作用于内皮素B受体诱导反应性星形胶质细胞中的Jagged 1表达,促进少突胶质细胞前体细胞中的NOTCH-1激活,延迟少突胶质细胞前体细胞成熟来限制髓鞘再生[45-46]。 星形胶质细胞还可产生硫酸软骨素蛋白聚糖、透明质酸和纤连蛋白等细胞外基质成分抑制髓鞘再生[47]:在受损的中枢神经系统中,由星形胶质细胞分泌的硫酸软骨素蛋白聚糖是创伤后轴突再生的有效抑制剂,其在微环境中的积累是阻碍神经修复的主要屏障。硫酸软骨素蛋白聚糖作为星形胶质细胞瘢痕的一部分高度上调,通过蛋白酪氨酸磷酸酶sigma受体介导,激活RhoA/ROCK通路,抑制少突胶质细胞前体细胞迁移及髓鞘再生。 研究表明,低分子质量透明质酸可抑制少突胶质细胞前体细胞的成熟过程。少突胶质细胞前体细胞表达的透明质酸酶可将高分子质量透明质酸降解为透明质酸低聚物,后者通过作用于少突胶质细胞前体细胞表面的Toll 样受体2(Toll-like receptor 2,TLR2),介导TLR2-MyD88信号传导以阻断少突胶质细胞前体细胞成熟和髓鞘再生;纤连蛋白也可激活TLR信号传导并促进免疫反应,通过核转录因子κB和p38-MAPK-2信号转导轴诱导促炎细胞因子表达,增强细胞毒性T细胞反应;此外,纤连蛋白聚集体可抑制少突胶质细胞前体细胞的分化和髓鞘再生。因此,干扰纤连蛋白的聚集及清除纤连蛋白可能是促进髓鞘再生的策略之一。 少突胶质细胞前体细胞分化为成熟髓鞘少突胶质细胞的过程,除了受细胞外基质成分组成的影响,还取决于细胞外基质蛋白的刚度,刚性基质促进少突胶质细胞前体细胞增殖和早期分化,而软基质有利于少突胶质细胞成熟和髓鞘形成。细胞外基质蛋白在胼胝体中的沉积可能有助于少突胶质细胞前体细胞的募集和早期分化,但少突胶质细胞成熟和髓鞘形成需要基质金属蛋白酶去除这些蛋白,细胞外基质成分的动态调节在一定程度上影响髓鞘再生过程。 2.3 靶向星形胶质细胞促进髓鞘再生的药物 大多数可用的治疗脱髓鞘疾病的药物都是免疫调节剂,可以降低复发、延缓进展、改善临床功能,然而,并不能消除慢性炎症、阻止神经变性。目前,促进少突胶质细胞前体细胞分化为少突胶质细胞的内源性髓鞘再生药物疗法被认为是治疗脱髓鞘疾病很有前途的方法[48]。研究表明,部分药物可通过调控星形胶质细胞功能及其衍生因子促进髓鞘再 生[49-63],具体见表3。 "

| [1] LUBETZKI C, ZALC B, WILLIAMS A, et al. Remyelination in multiple sclerosis: from basic science to clinical translation. Lancet Neurol. 2020;19(8):678-688. [2] GIL M,GAMA V. Emerging mitochondrial-mediated mechanisms involved in oligodendrocyte development. J Neurosci Res. 2023;101(3):354-366. [3] KUHN S, GRITTI L, CROOKS D, et al. Oligodendrocytes in development, myelin generation and beyond. Cells. 2019;8(11):1424. [4] LEE Y, MORRISON BM, LI Y, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487(7408):443-448. [5] NEELY SA, WILLIAMSON JM, KLINGSEISEN A, et al. New oligodendrocytes exhibit more abundant and accurate remyelination than those that survive demyelination. Nat Neurosci. 2022;25(4):415-420. [6] LUDWIN SK. The perineuronal satellite oligodendrocyte. A role in remyelination. Acta Neuropathol. 1979;47(1):49-53. [7] ARENELLA LS,HERNDON RM. Mature oligodendrocytes. Division following experimental demyelination in adult animals. Arch Neurol. 1984;41(11):1162-1165. [8] ITOYAMA Y, OHNISHI A, TATEISHI J, et al. Spinal cord multiple sclerosis lesions in Japanese patients: Schwann cell remyelination occurs in areas that lack glial fibrillary acidic protein(GFAP). Acta Neuropathol. 1985;65(3-4):217-223. [9] GARD AL,PFEIFFER SE. Oligodendrocyte progenitors isolated directly from developing telencephalon at a specific phenotypic stage: myelinogenic potential in a defined environment. Development. 1989;106(1):119-132. [10] NAIT-OUMESMAR B, DECKER L, LACHAPELLE F, et al. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11(12):4357-4366. [11] ZAWADZKA M, RIVERS LE, FANCY SP, et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6(6):578-590. [12] TALBOTT JF, LOY DN, LIU Y, et al. Endogenous Nkx2.2+/Olig2+ oligodendrocyte precursor cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes. Exp Neurol. 2005;192(1):11-24. [13] 王明达,周亮,罗天元,等.星形胶质细胞在髓鞘形成与修复中作用的研究进展[J].神经解剖学杂志,2019,35(4):451-454. [14] SEN MK, MAHNS DA, COORSSEN JR, et al. The roles of microglia and astrocytes in phagocytosis and myelination: insights from the cuprizone model of multiple sclerosis. Glia. 2022;70(7):1215-1250. [15] CIGNARELLA F, FILIPELLO F, BOLLMAN B, et al. TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta Neuropathol. 2020;140(4):513-534. [16] AIGROT MS, BARTHELEMY C, MOYON S, et al. Genetically modified macrophages accelerate myelin repair. EMBO Mol Med. 2022;14(8):e14759. [17] KALAFATAKIS I,KARAGOGEOS D. Oligodendrocytes and microglia: key players in myelin development, damage and repair. Biomolecules. 2021;11(7):1058. [18] ZHOU T, ZHENG Y, SUN L, et al. Microvascular endothelial cells engulf myelin debris and promote macrophage recruitment and fibrosis after neural injury. Nat Neurosci. 2019;22(3):421-435. [19] DE LA FUENTE AG, LANGE S, SILVA ME, et al. Pericytes stimulate oligodendrocyte progenitor cell differentiation during CNS remyelination. Cell Rep. 2017;20(8): 1755-1764. [20] DE LA VEGA GALLARDO N, DITTMER M, DOMBROWSKI Y, et al. Regenerating CNS myelin: emerging roles of regulatory T cells and CCN proteins. Neurochem Int. 2019;130:104349. [21] DE LA VEGA GALLARDO N, PENALVA R, DITTMER M, et al. Dynamic CCN3 expression in the murine CNS does not confer essential roles in myelination or remyelination. Proc Natl Acad Sci U S A. 2020;117(30):18018-18028. [22] WANG C, ZHANG CJ, MARTIN BN, et al. IL-17 induced NOTCH1 activation in oligodendrocyte progenitor cells enhances proliferation and inflammatory gene expression. Nat Commun. 2017;8:15508. [23] DIMOVASILI C, FAIR AE, GARZA IR, et al. Aging compromises oligodendrocyte precursor cell maturation and efficient remyelination in the monkey brain. Geroscience. 2023;45(1):249-264. [24] MA XR, ZHU X, XIAO Y, et al. Restoring nuclear entry of Sirtuin 2 in oligodendrocyte progenitor cells promotes remyelination during ageing. Nat Commun. 2022;13(1):1225. [25] NEUMANN B, SEGEL M, CHALUT KJ, et al. Remyelination and ageing: reversing the ravages of time. Mult Scler. 2019;25(14):1835-1841. [26] NEUMANN B, BAROR R, ZHAO C, et al. Metformin restores cns remyelination capacity by rejuvenating aged stem cells. Cell Stem Cell. 2019;25(4):473-485.e478. [27] LI X, DING Z, LIU K, et al. Astrocytic phagocytosis of myelin debris and reactive characteristics in vivo and in vitro. Biol Cell. 2023;115(12):e202300057. [28] PONATH G, RAMANAN S, MUBARAK M, et al. Myelin phagocytosis by astrocytes after myelin damage promotes lesion pathology. Brain. 2017;140(2):399-413. [29] WAN T, ZHU W, ZHAO Y, et al. Astrocytic phagocytosis contributes to demyelination after focal cortical ischemia in mice. Nat Commun. 2022;13(1): 1134. [30] XU T, LIU C, DENG S, et al. The roles of microglia and astrocytes in myelin phagocytosis in the central nervous system. J Cereb Blood Flow Metab. 2023; 43(3):325-340. [31] LINNERBAUER M, WHEELER MA,QUINTANA FJ. Astrocyte Crosstalk in CNS Inflammation. Neuron. 2020;108(4):608-622. [32] LIDDELOW SA, GUTTENPLAN KA, CLARKE LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481-487. [33] LIU X, LI C, LI J, et al. EGF signaling promotes the lineage conversion of astrocytes into oligodendrocytes. Mol Med. 2022;28(1):50. [34] DING Z, DAI C, ZHONG L, et al. Neuregulin-1 converts reactive astrocytes toward oligodendrocyte lineage cells via upregulating the PI3K-AKT-mTOR pathway to repair spinal cord injury. Biomed Pharmacother. 2021;134:111168. [35] MOLINA-GONZALEZ I, HOLLOWAY RK, JIWAJI Z, et al. Astrocyte-oligodendrocyte interaction regulates central nervous system regeneration. Nat Commun. 2023; 14(1):3372. [36] WERKMAN IL, KöVILEIN J, DE JONGE JC, et al. Impairing committed cholesterol biosynthesis in white matter astrocytes, but not grey matter astrocytes, enhances in vitro myelination. J Neurochem. 2021;156(5):624-641. [37] BERGHOFF SA, GERNDT N, WINCHENBACH J, et al. Dietary cholesterol promotes repair of demyelinated lesions in the adult brain. Nat Commun. 2017;8:14241. [38] ZHANG Q, CHEN Z, ZHANG K, et al. FGF/FGFR system in the central nervous system demyelinating disease: recent progress and implications for multiple sclerosis. CNS Neurosci Ther. 2023;29(6):1497-1511. [39] JI-WEI S, ZI-YING L, XIANG T, et al. CNTF induces Clcf1 in astrocytes to promote the differentiation of oligodendrocyte precursor cells. Biochem Biophys Res Commun. 2022;636(Pt 1):170-177. [40] FLETCHER JL, WOOD RJ, NGUYEN J, et al. Targeting TrkB with a brain-derived neurotrophic factor mimetic promotes myelin repair in the brain. J Neurosci. 2018;38(32):7088-7099. [41] RAWJI KS, GONZALEZ MARTINEZ GA, SHARMA A, et al. The role of astrocytes in remyelination. Trends Neurosci. 2020;43(8):596-607. [42] SEILER S, RUDOLF F, GOMES FR, et al. Astrocyte-derived factors regulate CNS myelination. Glia. 2024. doi: 10.1002/glia.24596. [43] SU Y, WANG X, YANG Y, et al. Astrocyte endfoot formation controls the termination of oligodendrocyte precursor cell perivascular migration during development. Neuron. 2023;111(2):190-201.e198. [44] BINAMé F, PHAM-VAN LD, SPENLé C, et al. Disruption of Sema3A/Plexin-A1 inhibitory signalling in oligodendrocytes as a therapeutic strategy to promote remyelination. EMBO Mol Med. 2019;11(11):e10378. [45] HAMMOND TR, GADEA A, DUPREE J, et al. Astrocyte-derived endothelin-1 inhibits remyelination through notch activation. Neuron. 2014;81(3):588-602. [46] HAMMOND TR, MCELLIN B, MORTON PD, et al. Endothelin-B receptor activation in astrocytes regulates the rate of oligodendrocyte regeneration during remyelination. Cell Rep. 2015;13(10):2090-2097. [47] GHORBANI S,YONG VW. The extracellular matrix as modifier of neuroinflammation and remyelination in multiple sclerosis. Brain. 2021;144(7):1958-1973. [48] NAJM FJ, MADHAVAN M, ZAREMBA A, et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature. 2015; 522(7555):216-220. [49] HE Y, AN J, YIN JJ, et al. Ethyl pyruvate-derived transdifferentiation of astrocytes to oligodendrogenesis in cuprizone-induced demyelinating model. Neurotherapeutics. 2021;18(1):488-502. [50] ZHENG J, LU J, MEI S, et al. Ceria nanoparticles ameliorate white matter injury after intracerebral hemorrhage: microglia-astrocyte involvement in remyelination. J Neuroinflammation. 2021;18(1):43. [51] BRÜCK W, PFÖRTNER R, PHAM T, et al. Reduced astrocytic NF-κB activation by laquinimod protects from cuprizone-induced demyelination. Acta Neuropathol. 2012;124(3):411-424. [52] LI T, NIU J, YU G, et al. Connexin 43 deletion in astrocytes promotes CNS remyelination by modulating local inflammation. Glia. 2020;68(6):1201-1212. [53] ZAMORA NN, CHELI VT, SANTIAGO GONZÁLEZ DA, et al. Deletion of voltage-gated calcium channels in astrocytes during demyelination reduces brain inflammation and promotes myelin regeneration in mice. J Neurosci. 2020;40(17):3332-3347. [54] MENG-RU Z, RUO-XUAN S, MING-YANG Y, et al. Antagonizing astrocytic platelet activating factor receptor-neuroinflammation for total flavone of epimedium in response to cuprizone demyelination. Int Immunopharmacol. 2021;101(Pt A):108181. [55] WANG TJ, WU ZY, YANG CH, et al. Multiple mechanistic models reveal the neuroprotective effects of diterpene ginkgolides against astrocyte-mediated demyelination via the PAF-PAFR pathway. Am J Chin Med. 2022;50(6):1565-1597. [56] LI QY, MIAO Q, SUI RX, et al. Ginkgolide K supports remyelination via induction of astrocytic IGF/PI3K/Nrf2 axis. Int Immunopharmacol. 2019;75:105819. [57] FARHANGI S, DEHGHAN S, TOTONCHI M, et al. In vivo conversion of astrocytes to oligodendrocyte lineage cells in adult mice demyelinated brains by Sox2. Mult Scler Relat Disord. 2019;28:263-272. [58] ZARE L, BAHARVAND H,JAVAN M. In vivo conversion of astrocytes to oligodendrocyte lineage cells using chemicals: targeting gliosis for myelin repair. Regen Med. 2018;13(7):803-819. [59] GHASEMI-KASMAN M, ZARE L, BAHARVAND H, et al. In vivo conversion of astrocytes to myelinating cells by miR-302/367 and valproate to enhance myelin repair. J Tissue Eng Regen Med. 2018;12(1):e462-e472. [60] SILVA OLIVEIRA JUNIOR M, SCHIRA-HEINEN J, REICHE L, et al. Myelin repair is fostered by the corticosteroid medrysone specifically acting on astroglial subpopulations. EBioMedicine. 2022;83:104204. [61] KEOUGH MB, ROGERS JA, ZHANG P, et al. An inhibitor of chondroitin sulfate proteoglycan synthesis promotes central nervous system remyelination. Nat Commun. 2016;7:11312. [62] ROSENZWEIG ES, SALEGIO EA, LIANG JJ, et al. Chondroitinase improves anatomical and functional outcomes after primate spinal cord injury. Nat Neurosci. 2019;22(8):1269-1275. [63] FELIU A, MESTRE L, CARRILLO-SALINAS FJ, et al. 2-arachidonoylglycerol reduces chondroitin sulphate proteoglycan production by astrocytes and enhances oligodendrocyte differentiation under inhibitory conditions. Glia. 2020;68(6):1255-1273. [64] KERKERING J, MUINJONOV B, ROSIEWICZ KS, et al. iPSC-derived reactive astrocytes from patients with multiple sclerosis protect cocultured neurons in inflammatory conditions. J Clin Invest. 2023;133(13):e164637. [65] CARNERO CONTENTTI E,CORREALE J. Neuromyelitis optica spectrum disorders: from pathophysiology to therapeutic strategies. J Neuroinflammation. 2021;18(1):208. [66] ISHIKURA T, KINOSHITA M, SHIMIZU M, et al. Anti-AQP4 autoantibodies promote ATP release from astrocytes and induce mechanical pain in rats. J Neuroinflammation. 2021;18(1):181. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Zhou Panpan, Cui Yinglin, Zhang Wentao, Wang Shurui, Chen Jiahui, Yang Tong . Role of cellular autophagy in cerebral ischemic injury and the regulatory mechanism of traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1650-1658. |
| [3] | Lyu Liting, Yu Xia, Zhang Jinmei, Gao Qiaojing, Liu Renfan, Li Meng, Wang Lu. Bibliometric analysis of research process and current situation of brain aging and exosomes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1457-1465. |
| [4] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [5] | Chen Yuning, Jiang Ying, Liao Xiangyu, Chen Qiongjun, Xiong Liang, Liu Yue, Liu Tong. Buqi Huoxue Compounds intervene with the expression of related factors and autophagy related proteins in a rat model of cerebral ischemia/reperfusion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1152-1158. |
| [6] | Zhao Xiaoxuan, Liu Shuaiyi, Li Qi, Xing Zheng, Li Qingwen, Chu Xiaolei. Different exercise modalities promote functional recovery after peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1248-1256. |
| [7] | Zhang Wenhua, Li Xun, Zhang Weichao, Li Xinying, Ma Guoao, Wang Xiaoqiang . Promoting myogenesis based on the SphK1/S1P/S1PR2 signaling pathway: a new perspective on improving skeletal muscle health through exercise [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1265-1275. |
| [8] | Liu Zan, An Ran, Li Baocheng. Effect of pravastatin on functional recovery from sciatic nerve crush injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 942-950. |
| [9] | Wu Guangtao, Qin Gang, He Kaiyi, Fan Yidong, Li Weicai, Zhu Baogang, Cao Ying . Causal relationship between immune cells and knee osteoarthritis: a two-sample bi-directional Mendelian randomization analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1081-1090. |
| [10] | Yu Hui, Yang Yang, Wei Ting, Li Wenli, Luo Wenqian, Liu Bin. Gadd45b alleviates white matter damage in chronic ischemic rats by modulating astrocyte phenotype [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7797-7803. |
| [11] | Guo Zhao, Zhuang Haoyan, Shi Xuewen. Role of exosomes derived from mesenchymal stem cells in treatment of colorectal cancer [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7872-7879. |
| [12] | Zhang Xiaoyu, Wei Shanwen, Fang Jiawei, Ni Li. Prussian blue nanoparticles restore mitochondrial function in nucleus pulposus cells through antioxidation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7318-7325. |
| [13] | Wang Zhaoyan, Wang Qian, Liu Weipeng, Yang Hui, Luan Zuo, Qu Suqing. Effect of fibronectin on differentiation of human neural stem cells into oligodendrocyte precursor cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6661-6666. |
| [14] | Guo Haizhen, Cong Zidong, Zhao Yuke, Li Xiaofeng, Yu Lu, Qian Shule, Wang Runying, Du Wuxun. Development of patch clamp technology in the past 10 years: visual analysis based on CiteSpace and VOSviewer [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6717-6726. |
| [15] | Yuan Xiao, Liang Songlin, Xie Yanan, Guan Dongmei, Fan Longyu, Yin Xiaoxuan. Mesenchymal stem cells from different sources in treatment of inflammatory bowel disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6811-6820. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||