Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (28): 6052-6060.doi: 10.12307/2025.489
Previous Articles Next Articles
MXene nanoparticles Ti3C2Tx and photothermal effect promote wound healing in diabetic mice
Li Meiyun1, 2, Liu Sen1, Chen Kaiyuan1, Shi Ling1, Song Meichen1, Cao Jiahong1, Wu Yan1, Yu Jing3
- 1College of Life Sciences, Mudanjiang Medical University, Mudanjiang 157001, Heilongjiang Province, China; 2Department of Clinical Pathology, General Hospital of Southern Theater Command of People’s Liberation Army of China, Guangzhou 510030, Guangdong Province, China; 3Department of Endocrinology, Hongqi Hospital, Mudanjiang Medical College, Mudanjiang 157001, Heilongjiang Province, China
-
Received:2024-06-07Accepted:2024-08-14Online:2025-10-08Published:2024-12-07 -
Contact:Yu Jing, Associate chief physician, Department of Endocrinology, Hongqi Hospital, Mudanjiang Medical College, Mudanjiang 157001, Heilongjiang Province, China -
About author:Li Meiyun, MS, College of Life Sciences, Mudanjiang Medical University, Mudanjiang 157001, Heilongjiang Province, China; Department of Clinical Pathology, General Hospital of Southern Theater Command of People’s Liberation Army of China, Guangzhou 510030, Guangdong Province, China -
Supported by:Mudanjiang City Guided Science and Technology Plan Project, No. HT2022JG125 (to WY); Heilongjiang Provincial Higher Education Institutions Basic Scientific Research Business Expense Research Project, No. 2021-KYYWF-0515 (to YJ)
CLC Number:
Cite this article
Li Meiyun, Liu Sen, Chen Kaiyuan, Shi Ling, Song Meichen, Cao Jiahong, Wu Yan, Yu Jing. MXene nanoparticles Ti3C2Tx and photothermal effect promote wound healing in diabetic mice[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 6052-6060.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

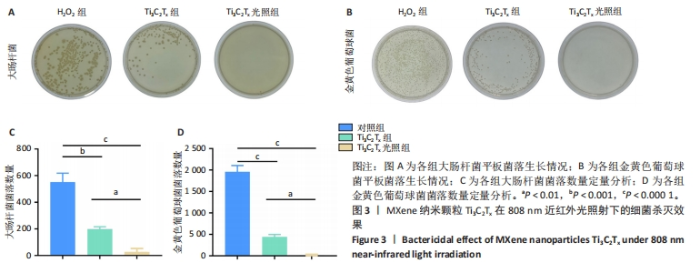
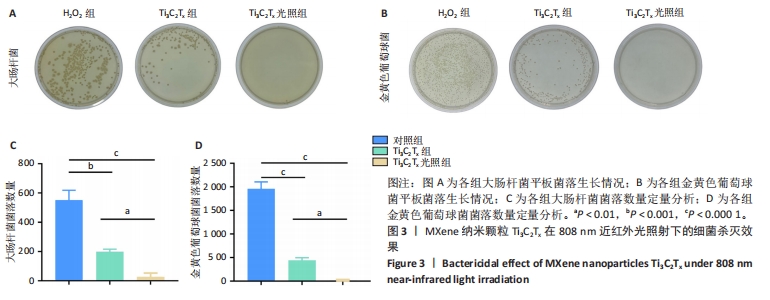
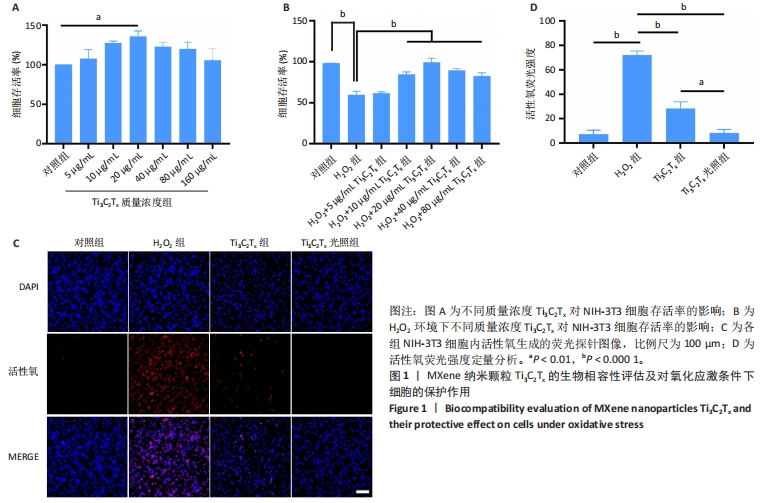
2.1 Ti3C2Tx的细胞毒性作用 采用NIH-3T3细胞作为模型细胞,系统评估了Ti3C2Tx的生物相容性。MTT检测结果显示,在0-160 μg/mL质量浓度范围内,Ti3C2Tx对NIH-3T3细胞无毒性作用,并且20 μg/mL Ti3C2Tx可显著提升NIH-3T3细胞存活率,见图1A。 2.2 Ti3C2Tx对氧化应激条件下NIH-3T3细胞的保护作用 实验探索了Ti3C2Tx在氧化应激环境下对细胞的保护作用。MTT检测结果显示,H2O2组NIH-3T3细胞存活率明显低于对照组(P < 0.000 1);相较于H2O2组,10,20,40,80 μg/mL Ti3C2Tx可显著提升NIH-3T3细胞存活率(P < 0.000 1),并且20 μg/mL Ti3C2Tx 的提升作用最显著,见图1B。 采用二氢乙锭作为荧光探针,用于精确检测细胞内的活性氧水平,结果见图1C,D所示。经过H2O2处理后,NIH-3T3细胞内产生了高强度的活性氧荧光信号,这与细胞内活性氧水平显著增高的趋势紧密相关,这一实验结果提供了关于氧化应激状态下细胞内活性氧变化情况的直观证据。与H2O2组相比,Ti3C2Tx组、Ti3C2Tx光照组活性氧荧光信号明显减弱,并且Ti3C2Tx光照组活性氧荧光信号减弱更显著,表明Ti3C2Tx结合808 nm近红外光可有效降低H2O2诱导的细胞内活性氧生成。"
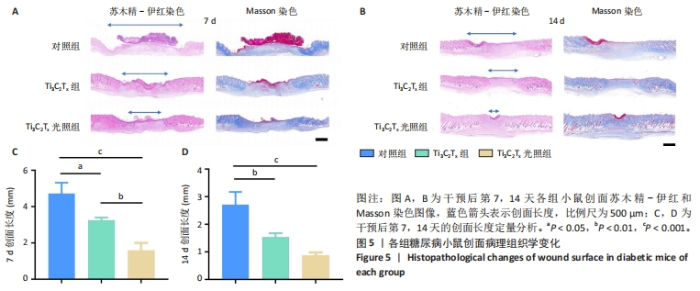
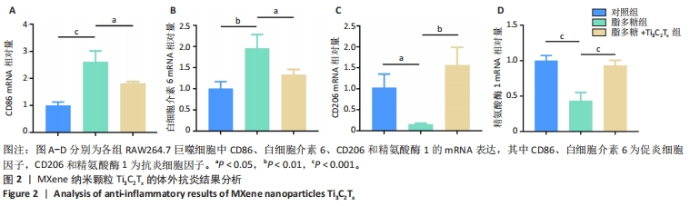
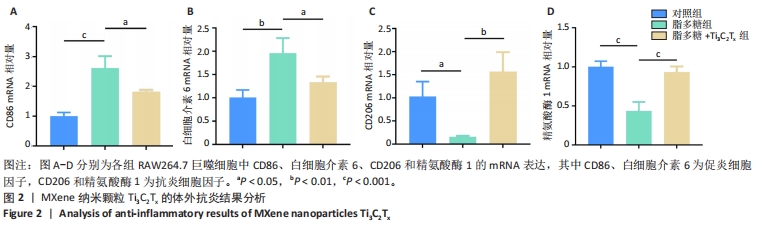
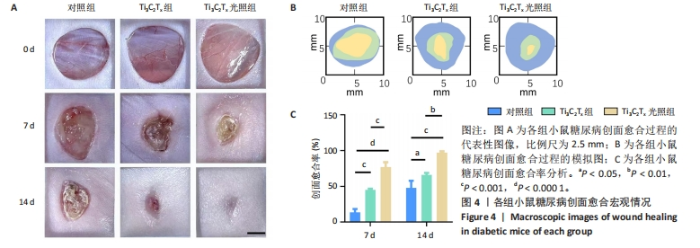
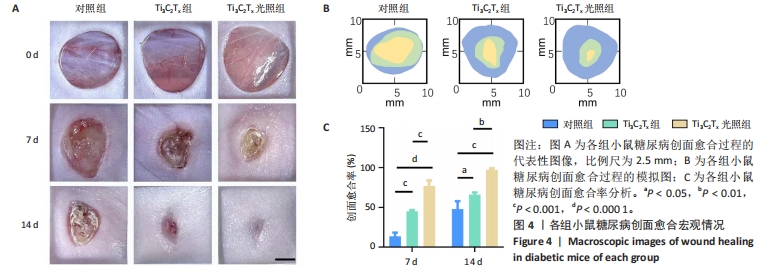
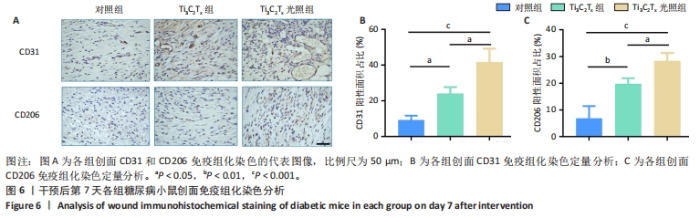
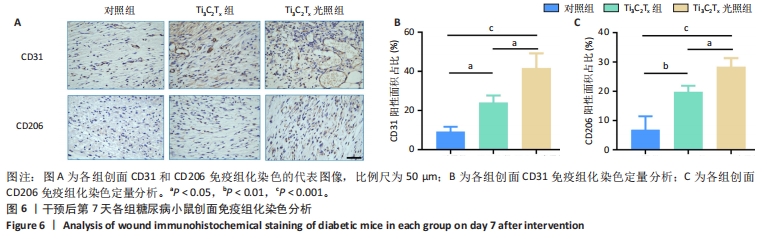
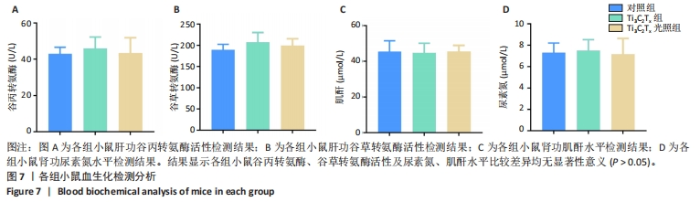
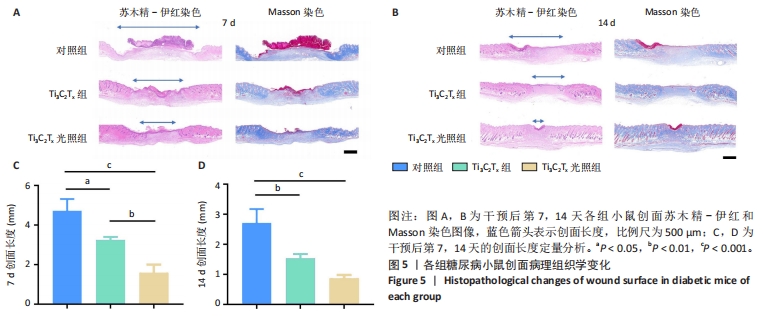
2.5.3 各组糖尿病小鼠创面组织学染色分析 通过组织学染色分析来评价各组创面愈合效果,并测量创面长度,结果见图5。苏木精-伊红染色结果显示,干预后第7天,各组创面长度有显著差异,其中对照组创面长度最长,Ti3C2Tx光照组创面长度最短;干预后第14天,各组创面长度进一步缩短,与对照组相比较,Ti3C2Tx组和Ti3C2Tx光照组创面愈合更快,其中愈合效果最好的是Ti3C2Tx光照组,并且在Ti3C2Tx光照组组织样本中观察到了较多的皮肤附属器,而相比之下,其他组别中几乎未见或仅见少量皮肤附属器。此外,通过Masson染色进一步分析发现,干预后第7天,各组创面均产生了大量的胶原纤维,其中以Ti3C2Tx光照组胶原纤维最为丰富。结果提示,Ti3C2Tx的光照处理不仅促进了创面愈合,还刺激了皮肤附属器的生成,为创面的快速修复提供了有力支持。"
| [1] IM SH, KIM CY, JUNG Y, et al. Biodegradable vascular stents with high tensile and compressive strength: a novel strategy for applying monofilaments via solid-state drawing and shaped-annealing processes. Biomater Sci. 2017;5(3):422-431. [2] OLSSON M, JÄRBRINK K, DIVAKAR U, et al. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019;27(1):114-125. [3] HOLL J, KOWALEWSKI C, ZIMEK Z, et al. Chronic Diabetic Wounds and Their Treatment with Skin Substitutes. Cells. 2021;10(3):655. [4] GUILLAMAT-PRATS R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells. 2021; 10(7):1729. [5] ALVEN S, ADERIBIGBE BA. Hyaluronic Acid-Based Scaffolds as Potential Bioactive Wound Dressings. Polymers (Basel). 2021;13(13):2102. [6] DENG L, DU C, SONG P, et al. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid Med Cell Longev. 2021; 2021:1-11. [7] SHI Z, GE Y, YUN Q, et al. Two-Dimensional Nanomaterial-Templated Composites. Acc Chem Res. 2022;55(24):3581-3593. [8] NAGUIB M, GOGOTSI Y. Synthesis of Two-Dimensional Materials by Selective Extraction. Acc Chem Res. 2015;48(1):128-135. [9] SANG X, XIE Y, LIN MW, et al. Atomic Defects in Monolayer Titanium Carbide (Ti3C2Tx) MXene. ACS Nano. 2016;10(10):9193-9200. [10] AKUZUM B, MALESKI K, ANASORI B, et al. Rheological Characteristics of 2D Titanium Carbide (MXene) Dispersions: A Guide for Processing MXene. ACS Nano. 2018;12(3):2685-2694. [11] JU X, KONG J, QI G, et al. A wearable electrostimulation-augmented ionic-gel photothermal patch doped with MXene for skin tumor treatment. Nat Commun. 2024;15(1):762. [12] GAO F, XUE C, ZHANG T, et al. MXene‐Based Functional Platforms for Tumor Therapy. Adv Mater. 2023;35(51):2302559. [13] AHAMED M, AKHTAR MJ, KHAN MAM, et al. Antibacterial, antifungal, and anticancer potential of two-dimensional Ti3C2Tx MXene. Mater Lett. 2022;327:133020. [14] LIU X, XIE H, ZHUO S, et al. Ru(II) Complex Grafted Ti3C2Tx MXene Nano Sheet with Photothermal/Photodynamic Synergistic Antibacterial Activity. Nanomaterials (Basel). 2023;13(6):958. [15] LIU Y, CHEN X, SUN J, et al. Large-scale production of MXene as nanoknives for antibacterial application. Nanoscale Adv. 2023;5(23): 6572-6581. [16] LI T, MA J, WANG W, et al. Bioactive MXene Promoting Angiogenesis and Skeletal Muscle Regeneration through Regulating M2 Polarization and Oxidation Stress. Adv Healthc Mater. 2023;12(4):2201862. [17] ZHU Y, MA X, LI L, et al. Surface Functional Modification by Ti3C2Tx MXene on PLLA Nanofibers for Optimizing Neural Stem Cell Engineering. Adv Healthc Mater. 2023;12(25):2300731. [18] REN X, XIAO M, XU Y, et al. Injectable MXene conductive hydrogel improves myocardial infarction through scavenging ROS and repairing myocardium electrical integrity. Chem Eng J. 2024;481:148791. [19] 张津,崔新刚,朱彦兆,等.姜黄素对糖尿病小鼠皮肤创面愈合的影响[J].医药导报,2024,43(2):167-174. [20] MARTIN P, NUNAN R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370-378. [21] BURANASIN P, MIZUTANI K, IWASAKI K, et al. High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS One. 2018;13(8):e0201855. [22] GONG F, ZHANG Y, CHENG S, et al. Inhibition of TGFβ1/Smad pathway by NF-κB induces inflammation leading to poor wound healing in high glucose. Cells Dev. 2022;172:203814. [23] LIN X, SONG D, SHAO T, et al. A Multifunctional Biosensor via MXene Assisted by Conductive Metal–Organic Framework for Healthcare Monitoring. Adv Funct Mater. 2023:2311637. [24] FENG J, LIU R, YUAN X, et al. MXene-enhanced ePatch with antibacterial activity for wound healing. Front Chem. 2023;11:1280040. [25] HUANG Y, HE S, YU S, et al. MXene‐Decorated Nanofibrous Membrane with Programmed Antibacterial and Anti-Inflammatory Effects via Steering NF-κB Pathway for Infectious Cutaneous Regeneration. Small. 2024;20(4):2304119. [26] SAHOO BM, BANIK BK, BORAH P, et al. Reactive Oxygen Species (ROS): Key Components in Cancer Therapies. Anticancer Agents Med Chem. 2022;22(2):215-222. [27] LIU H, CHEN R, WANG P, et al. Electrospun polyvinyl alcohol-chitosan dressing stimulates infected diabetic wound healing with combined reactive oxygen species scavenging and antibacterial abilities. Carbohydr Polym. 2023;316:121050. [28] LIU J, LU W, LU X, et al. Versatile Ti3C2Tx MXene for free-radical scavenging. Nano Res. 2022;15(3):2558-2566. [29] CAI F, WANG P, CHEN W, et al. The physiological phenomenon and regulation of macrophage polarization in diabetic wound. Mol Biol Rep. 2023;50(11):9469-9477. [30] GENG K, MA X, JIANG Z, et al. High glucose-induced STING activation inhibits diabetic wound healing through promoting M1 polarization of macrophages. Cell Death Discov. 2023;9(1):136. [31] LI T, MA J, WANG W, et al. Bioactive MXene Promoting Angiogenesis and Skeletal Muscle Regeneration through Regulating M2 Polarization and Oxidation Stress. Adv Healthc Mater. 2023;12(4): 2201862. [32] CHANG M, NGUYEN TT. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc Chem Res. 2021;54(5):1080-1093. [33] HE X, KOO S, OBENG E, et al. Emerging 2D MXene for antibacterial applications: Current status, challenges, and prospects. Coord Chem Rev. 2023;492:215275. [34] SEIDI F, ARABI SHAMSABADI A, DADASHI FIROUZJAEI M, et al. MXene Antibacterial Properties and Applications: A Review and Perspective. Small. 2023;19(14):2206716. [35] FENG H, WANG W, WANG T, et al. Interfacial regulation of BiOI@Bi2S3/MXene heterostructures for enhanced photothermal and photodynamic therapy in antibacterial applications. Acta Biomater. 2023;171:506-518. [36] HAN Z, DONG L, LI A, et al. Efficient angiogenesis-based wound healing through hydrogel dressing with extracellular vesicles release. Mater Today Bio. 2022;16:100427. |
| [1] | Wang Zilin, Mu Qiuju, Liu Hongjie, Shen Yuxue, Zhu Lili. Protective effects of platelet-rich plasma hydrogel on oxidative damage in L929 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 771-779. |
| [2] | Dong Meilin, Du Haiyu, Liu Yuan. Quercetin-loaded carboxymethyl chitosan hydrogel promotes wound healing in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 692-699. |
| [3] | Li Ruotong, Wu Yuening, Deng Yunyi, Chen Shichao, Lan Xiaorong, Li Shiting, Li Guangwen. Preparation and antibacterial evaluation of nanosilver-reduced graphene oxide/polydopamine/methacrylated gelatin@Gap19 hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7333-7343. |
| [4] | Shi Chunrong, He Jiaxu, Deng Lishan, Wang Hailan, Zhao Aimin, Yu Yiling, Geng Haixia, Song Weijun. Application of graphene oxide in field of oral implant restoration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 6118-6126. |
| [5] | Chen Jingyu, Hong Ge, Guo Ning, Liu Tianjun. Hernia repair patch: recent advances in material design and application [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3494-3502. |
| [6] | Guan Zhenju, Xie Yonglin, Xiang Shougang, Zhang Chengdong, Li Xiaolong, Li Xingping, Pu Chao, Zhang Bo, Luo Xuwei, Xiao Dongqin. Preparation of polyphenol-mediated copper ion coating on titanium surface and antibacterial and antioxidant properties [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 1997-2005. |
| [7] | Wang Weiqing, Zhou Yue. Chronic inflammation regulates adipose tissue fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1307-1312. |
| [8] | Zhang Ya, Mu Qiuju, Wang Zilin, Liu Hongjie, Zhu Lili. Hydrogel loaded with platelet-rich plasma promotes wound healing in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 690-696. |
| [9] | Tan Junjie, Du Jiaheng, Wen Zhenyu, Yan Jiyuan, He Kui, Duan Ke, Yin Yiran, Li Zhong. Antibacterial magnesium oxide-calcium phosphate composite coating prepared by combining electrodeposition and sol-gel impregnation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(29): 4663-4670. |
| [10] | Dong Bo, Li Xiaoyu, Li Birong, Li Zhen, Wang Zixuan, Yin Zhaoyi, Meng Weiyan. 3D-printed scaffolds repair infected bone defects [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(29): 4685-4690. |
| [11] | Zheng Heishu, Zhang Yingjuan, Wei Yanhua, Huang Hui, Ma Xiangyu, Liao Hongbing. Antibacterial performance of cerium oxide nanoenzyme against Escherichia coli [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(22): 3496-3501. |
| [12] | Liu Yan, Zheng Xuexin. Performance of 3D-printed polylactic acid-nano-hydroxyapatite/chitosan/doxycycline antibacterial scaffold [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(22): 3532-3538. |
| [13] | Bian Zhihong, Zhang Yuntao, Li Zeming, Hou Yudong. Potential of shikonin and its derivatives in oral soft and hard tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(17): 2747-2752. |
| [14] | Wang Jinlei, Li Ke, Zhao Liang. Platelet-camouflaged silver nanoparticle hydrogel accelerates wound healing in type 1 diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(17): 2659-2666. |
| [15] | Tang Ziyan, Gu Shunqiang, Chen Xiaoling, Wang Lei, Ma Chengbang, Zhou Mei, Chen Tianbao, Du Lina, Jin Yiguang. Recombinant expression and in vitro activity identification of a bioactive peptide QUB2984 from skin secretion of Agalychnis callidryas [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(17): 2675-2681. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||