Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (2): 254-261.doi: 10.12307/2025.246
Previous Articles Next Articles
Expression of IP3R2 and RYR2 mediated Ca2+ signals in a mouse model of delayed encephalopathy after acute carbon monoxide poisoning
Zhao Jili1, Meng Tianyu1, Yue Yarong1, Zhang Xin1, Du Wenqian1, Zhang Xinyu1, Xue Hui2, Xiang Wenping3
- 1Clinical School of Medicine, Baotou Medical College, Inner Mongolia University of Science and Technology, Baotou 014040, Inner Mongolia Autonomous Region, China; 2Department of Neurology, Baotou Central Hospital, Baotou 014040, Inner Mongolia Autonomous Region, China; 3Baotou Mongolian Medicine Hospital, Baotou 014040, Inner Mongolia Autonomous Region, China
-
Received:2024-01-19Accepted:2024-04-22Online:2025-01-18Published:2024-05-24 -
Contact:Xue Hui, MD, Associate chief physician, Department of Neurology, Baotou Central Hospital, Baotou 014040, Inner Mongolia Autonomous Region, China Co-corresponding author: Xiang Wenping, MD, Associate chief physician, Baotou Mongolian Medicine Hospital, Baotou 014040, Inner Mongolia Autonomous Region, China -
About author:Zhao Jili, Master, Physician, Clinical School of Medicine, Baotou Medical College, Inner Mongolia University of Science and Technology, Baotou 014040, Inner Mongolia Autonomous Region, China -
Supported by:The Central Government Guides Local Funds for Science and Technology Development Program, No. 2021ZY0038 (to XWP)
CLC Number:
Cite this article
Zhao Jili, Meng Tianyu, Yue Yarong, Zhang Xin, Du Wenqian, Zhang Xinyu, Xue Hui, Xiang Wenping. Expression of IP3R2 and RYR2 mediated Ca2+ signals in a mouse model of delayed encephalopathy after acute carbon monoxide poisoning [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(2): 254-261.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
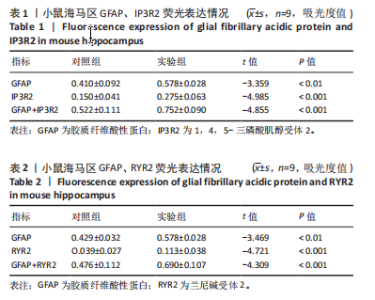
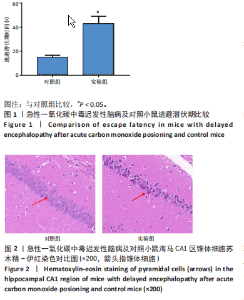
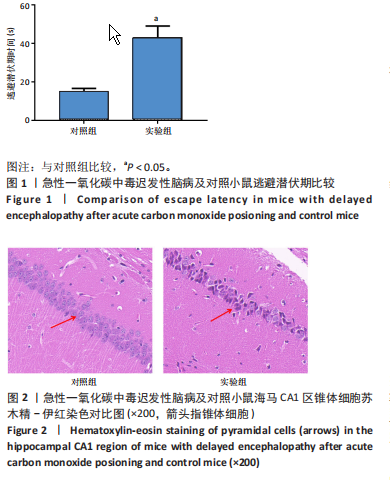
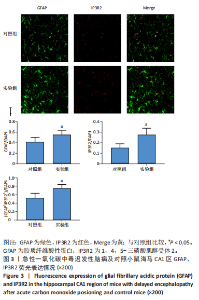
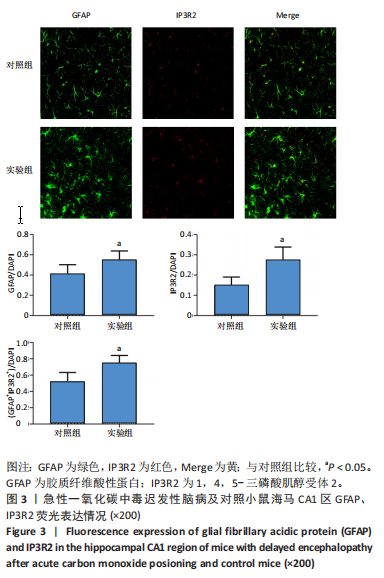
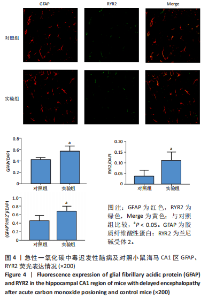
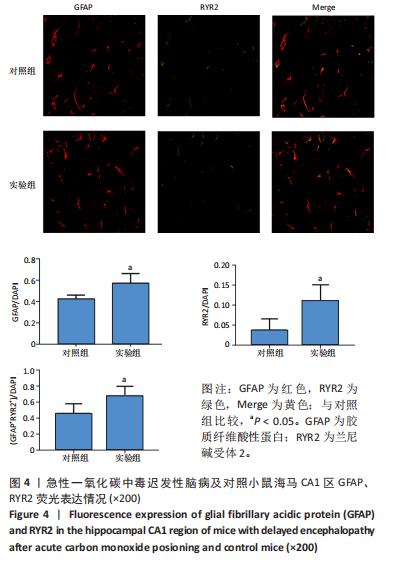
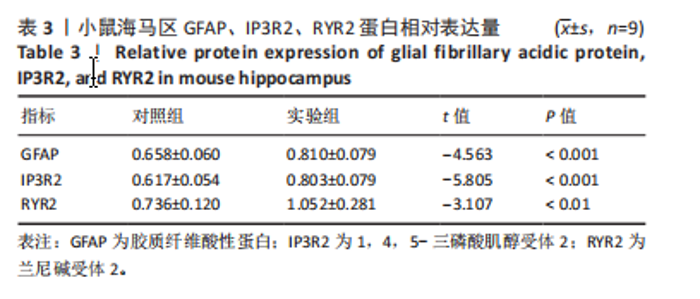
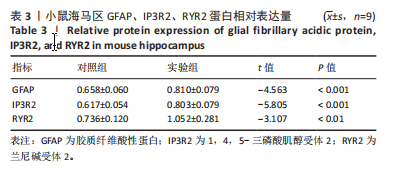
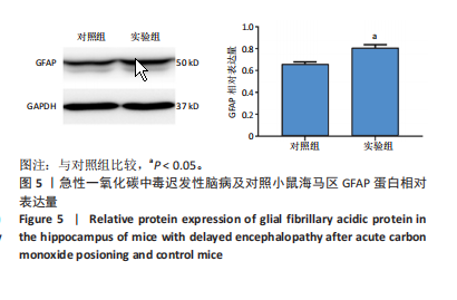
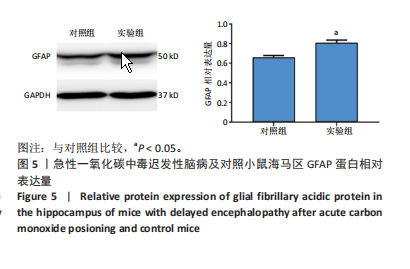
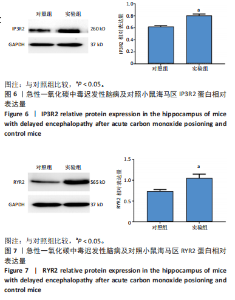
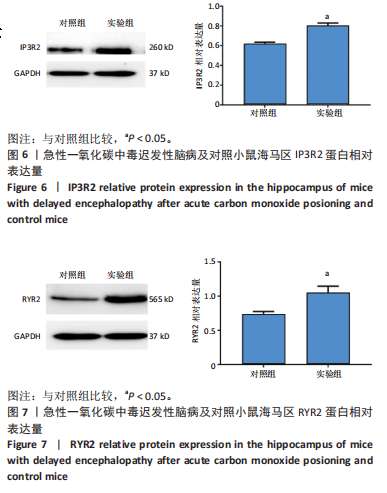
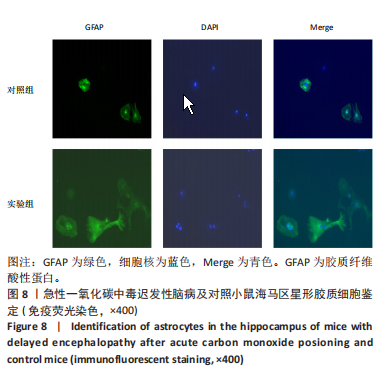
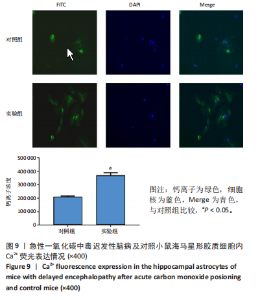
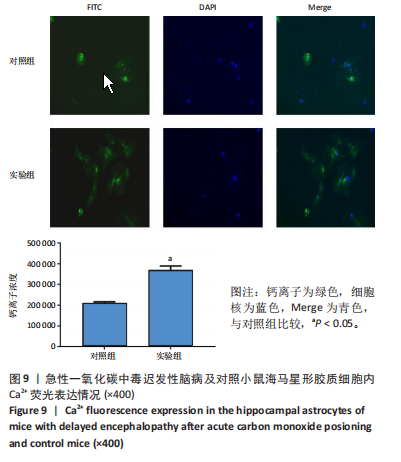
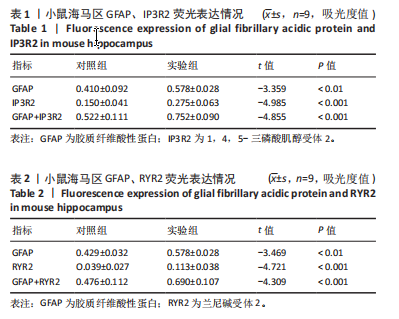
2.1 实验动物数量分析 实验选用100只小鼠进行Morris水迷宫实验后,筛选出合格小鼠91只,随机分成2组,最终以每组36只进入结果分析。 2.2 Morris水迷宫逃避潜期伏比较 与对照组相比,实验组小鼠Morris水迷宫逃避潜伏期显著延长(t=4.702,P < 0.05),见图1;说明在21 d时小鼠记忆力显著下降,出现认知功能受损,发生了行为学改变。 2.3 海马区苏木精-伊红染色 对照组海马CA1区锥体细胞多层排列,细胞排列整齐,结构清晰完整,胞质均一染色,胞核呈蓝色;实验组小鼠海马CA1区锥体细胞层数减少,结构不清,细胞皱缩明显,胞浆浓缩呈深蓝色,细胞核固缩、碎裂,甚至发生神经元变性坏死,见图2。结合水迷宫结果,证实小鼠急性一氧化碳中毒迟发性脑病造模成功。 2.4 免疫荧光检测GFAP、IP3R2、RYR2表达情况 海马CA1区存在GFAP与IP3R2及GFAP与RYR2的共表达;与对照组相比,实验组小鼠海马区 IP3R2、RYR2 和 GFAP 表达均显著增多(P < 0.05)(表1,2,图3,4)。 "
| [1] MATTIUZZI C, LIPPI G. Worldwide epidemiology of carbon monoxide poisoning. Hum Exp Toxicol. 2020;39(4):387-392. [2] ROSE JJ, WANG L, XU Q, et al. Carbon Monoxide Poisoning: Pathogenesis, Management, and Future Directions of Therapy. Am J Respir Crit Care Med. 2017;195(5):596-606. [3] YANAGIHA K, ISHII K, TAMAOKA A. Acetylcholinesterase inhibitor treatment alleviated cognitive impairment caused by delayed encephalopathy due to carbon monoxide poisoning: two case reports and a review of the literature. Medicine (Baltimore). 2017;96(8):e6125. [4] BANO D, ANKARCRONA M. Beyond the critical point: An overview of excitotoxicity, calcium overload and the downstream consequences. Neurosci Lett. 2018;663:79-85. [5] ZHAO N, LIANG P, ZHUO X, et al. After treatment with methylene blue is effective against delayed encephalopathy after acute carbon monoxide poisoning. Basic Clin Pharmacol Toxicol. 2018;122(5):470-480. [6] ZHANG W, YU J, GUO M, et al. Dexmedtomidine attenuats glutamate-induced cytotoxicity by inhibiting the mitochondrial mediated apptotic pathway. Med Sci Monit. 2020;26:922139. [7] BI MJ, SUN XN, ZOU Y, et al. N-butylphthalide improves cognitive function in rats after carbon monoxide poisoning. Front Pharmacol. 2017;8:64. [8] ESCARTIN C, GALEA E, LAKATOS A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021;24(3):312-325. [9] LIM D, RONCO V, GROLLA AA, et al. Glial calcium signaling in Alzheimer’s disease. Rev Physiol Biochem Pharmacol. 2014;167:45-65. [10] EGOROVA PA, BEZPROZVANNY IB. Inositol 1,4,5-trisphosphate receptors and neurodegenerative disorders. FEBS J. 2018;285(19):3547-3565. [11] MURALI S, ZHANG M, NURSE CA. Evidence that 5-HT stimulates intracellular Ca2+ signalling and activates pannexin-1 currents in type II cells of the rat carotid body. J Physiol. 2017;595(13):4261-4277. [12] ZHANG T, GUO T, MISHRA S, et al. Phospholamban ablation rescues sarcoplasmic reticulum Ca(2+) handling but exacerbates cardiac dysfunction in CaMKIIdelta(C) transgenic mice. Circ Res. 2010;106(2):354-362. [13] DEMYDENKO K, EKHTERAEI-TOUSI S, RODERICK HL. Inositol 1, 4, 5-trisphosphate receptors in cardiomyocyte physiology and disease. Philos Trans R Soc Lond B Biol Sci. 2022;377(1864):20210319. [14] ROSA N, IVANOVA H, WAGNER LE 2ND, et al. Bcl-xL acts as an inhibitor of IP3R channels, thereby antagonizing Ca2+-driven apoptosis. Cell Death Differ. 2022;29(4):788-805. [15] HAMADA K, MIKOSHIBA K. IP3 Receptor Plasticity Underlying Diverse Functions. Annu Rev Physiol. 2020;82:151-176. [16] SMITH HA, TAYLOR CW. Dissociation of inositol 1,4,5-trisphosphate from IP3 receptors contributes to termination of Ca2+ puffs. J Biol Chem. 2023; 299(2):102871. [17] KOFUJI P, ARAQUE A. G-Protein-Coupled Receptors in Astrocyte-Neuron Communication. Neuroscience. 2021;456:71-84. [18] OKUBO Y, KANEMARU K, SUZUKI J, et al. Inositol 1,4,5-trisphosphate receptor type 2-independent Ca2+ release from the endoplasmic reticulum in astrocytes. Glia. 2019;67(1):113-124. [19] REICHENBACH N, DELEKATE A, BREITHAUSEN B, et al. P2Y1 receptor blockade normalizes network dysfunction and cognition in an Alzheimer’s disease model. J Exp Med. 2018;215(6):1649-1663. [20] ADASME T, HAEGER P, PAULA-LIMA AC, et al. Involvement of ryanodine receptors in neurotrophin-induced hippocampal synaptic plasticity and spatial memory formation. Proc Natl Acad Sci U S A. 2011;108(7):3029-3034. [21] MORE JY, BRUNA BA, LOBOS PE, et al. Calcium Release Mediated by Redox-Sensitive RyR2 Channels Has a Central Role in Hippocampal Structural Plasticity and Spatial Memory. Antioxid Redox Signal. 2018;29(12):1125-1146. [22] DATTA D, LESLIE SN, WANG M, et al. Age-related calcium dysregulation linked with tau pathology and impaired cognition in non-human primates. Alzheimers Dement. 2021;17(6):920-932. [23] LACAMPAGNE A, LIU X, REIKEN S, et al. Post-translational remodeling of ryanodine receptor induces calcium leak leading to Alzheimer’s disease-like pathologies and cognitive deficits. Acta Neuropathol. 2017;134(5):749-767. [24] 牛翻燕,项文平,薛慧,等.星形胶质细胞及其炎症因子在CO中毒所致迟发性脑损伤中的作用[J].脑与神经疾病杂志,2022,30(11):665-670. [25] 王雅明,项文平,牛翻燕,等.P2Y1受体介导星形胶质细胞的激活在急性一氧化碳中毒迟发性脑病中的作用[J].中风与神经疾病杂志,2023, 40(2):103-108. [26] OCHI S, ABE M, LI C, et al. The nicotinic cholinergic system is affected in rats with delayed carbon monoxide encephalopathy. Neurosci Lett. 2014;569:33-37. [27] LANANNA BV, MCKEE CA, KING MW, et al. Chi3l1/YKL-40 is controlled by the astrocyte circadian clock and regulates neuroinflammation and Alzheimer’s disease pathogenesis. Sci Transl Med. 2020;12(574):eaax3519. [28] XUE M, YONG VW. Neuroinflammation in intracerebral haemorrhage: immunotherapies with potential for translation. Lancet Neurol. 2020; 19(12):1023-1032. [29] KUSHNIR A, WAJSBERG B, MARKS AR. Ryanodine receptor dysfunction in human disorders. Biochim Biophys Acta Mol Cell Res. 2018;1865(11 Pt B): 1687-1697. [30] LI R, DANG S, YAO M, et al.Osthole alleviates neuropathic pain in mice by inhibiting the P2Y1-receptor-dependent JNK signaling pathway. Aging (Albany NY). 2020;12(9):7945-7962. [31] BADIA-SOTERAS A, HEISTEK TS, KATER MSJ, et al. Retraction of Astrocyte Leaflets From the Synapse Enhances Fear Memory. Biol Psychiatry. 2023;94(3):226-238. [32] KOL A, GOSHEN I. The memory orchestra: the role of astrocytes and oligodendrocytes in parallel to neurons. Curr Opin Neurobiol. 2021;67:131-137. [33] LYON KA, ALLEN NJ. From Synapses to Circuits, Astrocytes Regulate Behavior. Front Neural Circuits. 2022;15:786293. [34] KATER MSJ, BADIA-SOTERAS A, VAN WEERING JRT, et al. Electron microscopy analysis of astrocyte-synapse interactions shows altered dynamics in an Alzheimer’s disease mouse model. Front Cell Neurosci. 2023;17:1085690. [35] PADMASHRI R, SURESH A, BOSKA MD, et al. Motor-Skill Learning Is Dependent on Astrocytic Activity. Neural Plast. 2015;2015:938023. [36] PINTO-DUARTE A, ROBERTS AJ, OUYANG K, et al. Impairments in remote memory caused by the lack of Type 2 IP3 receptors. Glia. 2019;67(10):1976-1989. [37] GOENAGA J, ARAQUE A, KOFUJI P, et al. Calcium signaling in astrocytes and gliotransmitter release. Front Synaptic Neurosci. 2023;15:1138577. [38] NAVARRETE M, PEREA G, FERNANDEZ DE SEVILLA D, et al. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 2012;10(2):e1001259. [39] BERTAN F, WISCHHOF L, SOSULINA L, et al. Loss of Ryanodine Receptor 2 impairs neuronal activity-dependent remodeling of dendritic spines and triggers compensatory neuronal hyperexcitability. Cell Death Differ. 2020;27(12):3354-3373. [40] YAO J, SUN B, INSTITORIS A, et al. Limiting RyR2 Open Time Prevents Alzheimer’s Disease-Related Neuronal Hyperactivity and Memory Loss but Not β-Amyloid Accumulation. Cell Rep. 2020;32(12):108169. [41] Dridi H, Liu Y, Reiken S, et al. Heart failure-induced cognitive dysfunction is mediated by intracellular Ca2+ leak through ryanodine receptor type 2. Nat Neurosci. 2023;26(8):1365-1378. [42] Huffels CFM, Osborn LM, Cappaert NLM, et al. Calcium signaling in individual APP/PS1 mouse dentate gyrus astrocytes increases ex vivo with Aβ pathology and age without affecting astrocyte network activity. J Neurosci Res. 2022;100(6):1281-1295. [43] SHAH D, GSELL W, WAHIS J, et al. Astrocyte calcium dysfunction causes early network hyperactivity in Alzheimer’s disease. Cell Rep. 2022;40(8):111280. [44] BAKKER A, KRAUSS GL, ALBERT MS, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74(3):467-474. [45] INSTITORIS A, MURPHY-ROYAL C, TARANTINI S, et al. Whole brain irradiation in mice causes long-term impairment in astrocytic calcium signaling but preserves astrocyte-astrocyte coupling. Geroscience. 2021;43(1):197-212. |
| [1] | Shui Jing, He Yu, Jiang Nan, Xu Kun, Song Lijuan, Ding Zhibin, Ma Cungen, Li Xinyi. Astrocytes regulate remyelination in central nervous system [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7889-7897. |
| [2] | Yu Hui, Yang Yang, Wei Ting, Li Wenli, Luo Wenqian, Liu Bin. Gadd45b alleviates white matter damage in chronic ischemic rats by modulating astrocyte phenotype [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7797-7803. |
| [3] | Deng Qing, Wang Qingjun, Zhang Yeting. Visual analysis of dynamic evolution of research topics in the field of physical activity and hippocampal tissue [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6997-7003. |
| [4] | Zhang Zihan¹, Wang Jiaxin¹, Yang Wenyi², Zhu Lei¹. Regulatory mechanism of exercise promoting mitochondrial biogenesis in skeletal muscle [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6499-6508. |
| [5] | Xu Zhenhua, Li Yanjie, Qin Hewei, Liu Haoyuan, Zhu Bochao, Wang Yupu. Traditional Chinese medicine monomer in treatment of neuroinflammation after spinal cord injury: effects of nuclear transcription factor kappa B signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 590-598. |
| [6] | Du Juan, Zhang Yi, Hao Quanshui. Effects of exercise on activation of microglia and astrocytes and neuronal apoptosis in depressed rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6243-6248. |
| [7] | Liu Kexin, Song Lijuan, Wu Yige, Han Guangyuan, Miao Zhuyue, Wei Ruheng, Xiao Baoguo, Ma Cungen, Huang Jianjun. Hydroxysafflor yellow A intervenes astrocyte lipocalin 2 expression after cerebral ischemia/reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1063-1069. |
| [8] | Yu Jingwen, Guo Minfang, Zhang Bingxin, Mu Bingtao, Meng Tao, Zhang Huiyu, Ma Cungen, Yin Jinzhu, Song Lijuan, Yu Jiezhong. Astragaloside inhibits astrocyte activation and inflammatory response induced by inflammation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 5022-5028. |
| [9] | Li Zhen, Sun Xiao, Xie Yongpeng, Rong Wang, Sun Haitao. Neuron-derived extracellular vesicles promote neurogenesis of neural stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(25): 3994-3999. |
| [10] | Ye Jiapeng, Wang Jianwei, Wu Mao, Li Shaoshuo, Wang Guopeng, Wang Haotian, Tang Zhi, Shao Yang. miR-146a-3p regulates astrocyte proliferation, migration and apoptosis by inhibiting insulin-like growth factor 1 expression [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(25): 4048-4053. |
| [11] | Chen Na, Wang Yanlin, Sun Huifang, Fan Feiyan, Li Donghong, Zhang Yunke. Shexiang Huangqi compound dripping pills-containing serum promotes proliferation and differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(19): 2960-2966. |
| [12] | Liu Xinxin, Geng Zhizhong, Chen Jian. Effects of high-intensity interval training with different intervention durations on cognitive function in older adults: a Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(14): 2282-2289. |
| [13] | Yang Ting, Ding Zhibin, Jiang Nan, Han Hongxia, Hou Miaomiao, Ma Cungen, Song Lijuan, Li Xinyi. Astrocytes regulate glial scar formation in cerebral ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 131-138. |
| [14] | Long Qingxi, Zhang Pingshu, Liu Qing, Ou Ya, Zhang Lili, Yuan Xiaodong. Single-cell RNA sequencing reveals the heterogeneity of astrocytes [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 139-146. |
| [15] | Gao Yu, Han Jiahui, Ge Xin. Immunoinflammatory microenvironment after spinal cord ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1300-1305. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||