Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (2): 245-253.doi: 10.12307/2025.205
Previous Articles Next Articles
Carnosic acid inhibits osteoclast differentiation by inhibiting mitochondrial activity
Li Haishan1, 2, 3, Wu Yuheng3, 4, Liang Zixuan1, 2, 3, Zhang Shiyin1, 2, 3, Zhang Zhen1, 2, 3, Mai Bin1, 2, 3, Deng Wei1, 2, 3, Li Yongxian1, 2, Tang Yongchao1, 2, Zhang Shuncong1, 2, Yuan Kai1, 2
- 1First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; 2Guangdong Clinical Research Academy of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; 3Lingnan Medical Research Center, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; 4Seventh Clinical College of Medicine of Guangzhou University of Chinese Medicine, Shenzhen 518100, Guangdong Province, China
-
Received:2023-12-15Accepted:2024-01-25Online:2025-01-18Published:2024-05-24 -
Contact:Yuan Kai, MD, Associate chief physician, First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; Guangdong Clinical Research Academy of Chinese Medicine, Guangzhou 510405, Guangdong Province, China Co-corresponding author: Zhang Shuncong, MD, Chief physician, First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; Guangdong Clinical Research Academy of Chinese Medicine, Guangzhou 510405, Guangdong Province, China -
About author:Li Haishan, Master candidate, Physician, First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; Guangdong Clinical Research Academy of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; Lingnan Medical Research Center, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China -
Supported by:Guangzhou Science and Technology Project, No. 202201020295 (to YK); The Natural Science Foundation of Guangdong Province, No. 2021A1515012168 (to ZSC); Guangzhou Science and Technology Project, No. 202201020500 (to TYC); Guangzhou Science and Technology Project, No. 202201020533 (to ZSC); Guangzhou Science and Technology Project, No. 202102021040 (to LYX); The Young and Middle aged Key Talent Cultivation Project of The First Affiliated Hospital of Guangzhou University of Chinese Medicine, No. 2023.10 (to LYX); Youth Talent Lifting Project of Chinese Society of Traditional Chinese Medicine, No. 2022-QNRC2-B11 (to LYX)
CLC Number:
Cite this article
Li Haishan, Wu Yuheng, Liang Zixuan, Zhang Shiyin, Zhang Zhen, Mai Bin, Deng Wei, Li Yongxian, Tang Yongchao, , Zhang Shuncong, , Yuan Kai, . Carnosic acid inhibits osteoclast differentiation by inhibiting mitochondrial activity[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(2): 245-253.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
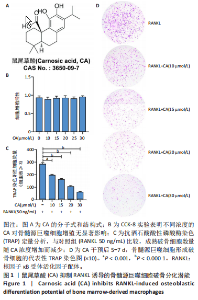
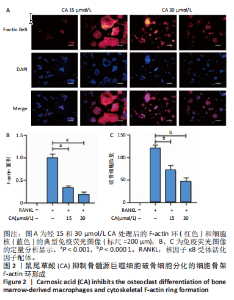
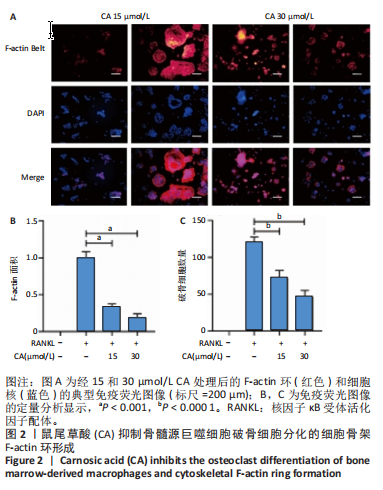
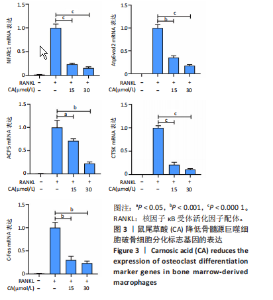
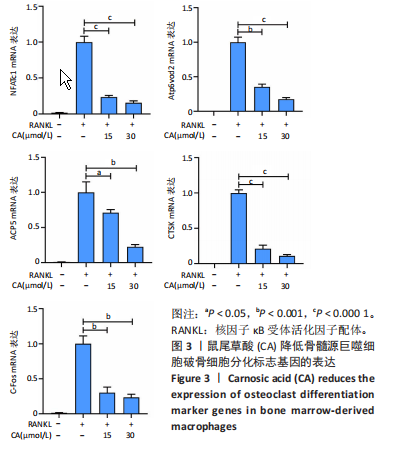
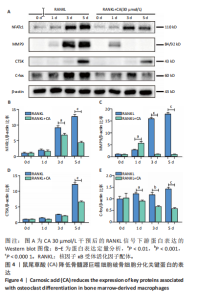
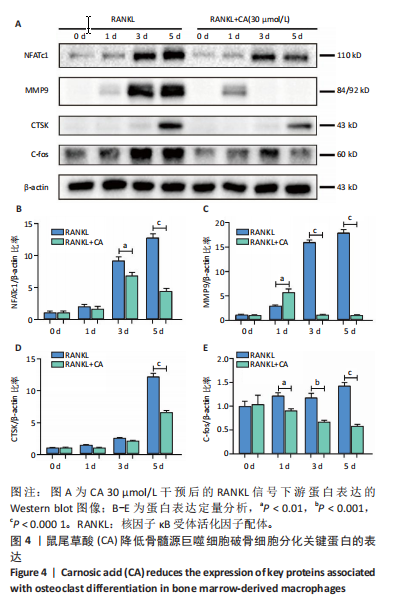
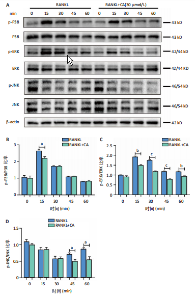
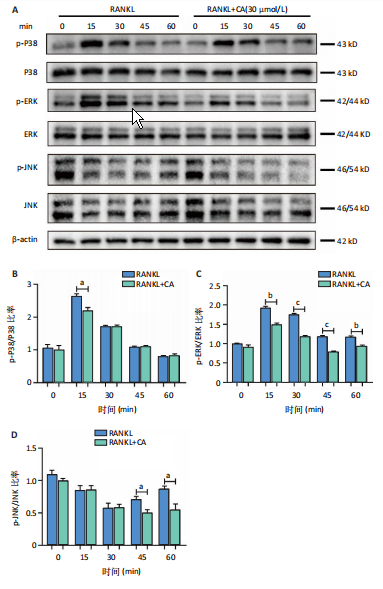
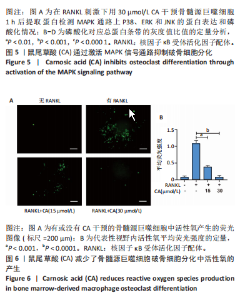
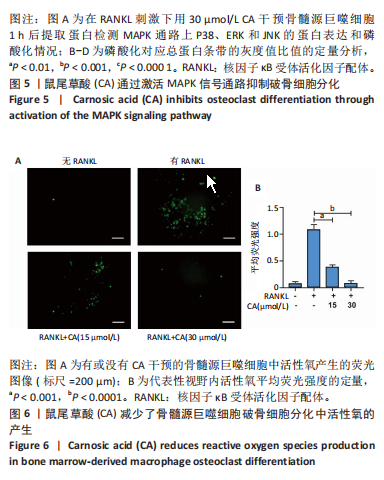
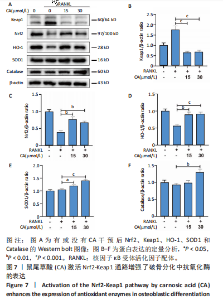
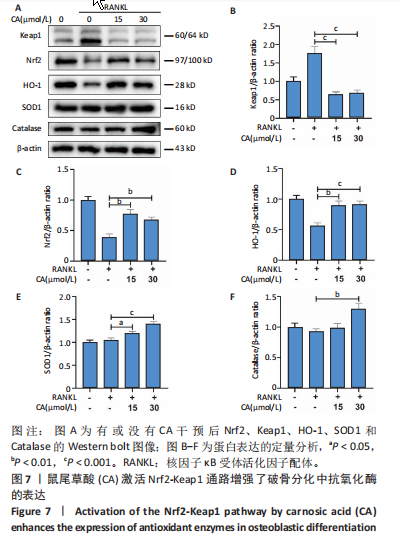
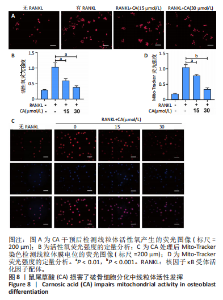
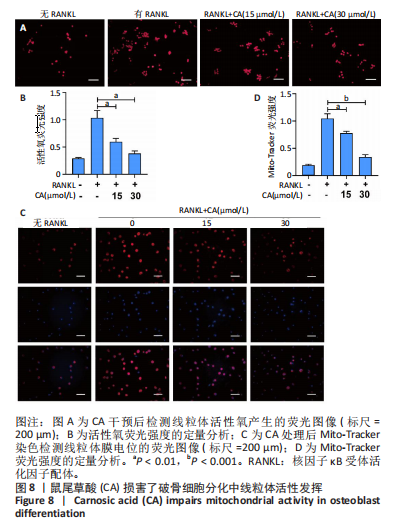
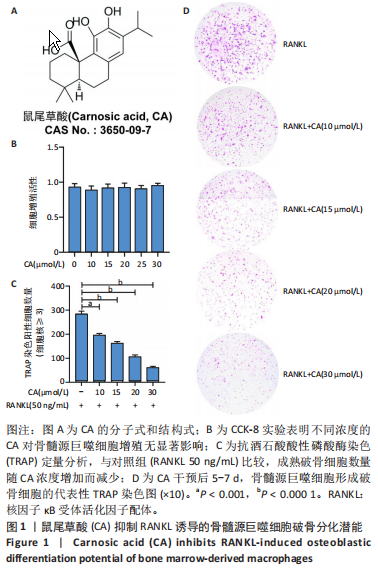
2.1 鼠尾草酸抑制RANKL诱导的骨髓源巨噬细胞破骨分化潜能 鼠尾草酸的化学结构式见图1A。为了探讨鼠尾草酸对RANKL诱导的破骨细胞生成和细胞毒性的影响,选择小鼠来源的骨髓源巨噬细胞作为破骨细胞前体细胞。将不同浓度的鼠尾草酸与骨髓源巨噬细胞共孵育以检测细胞毒性。 CCK-8实验验证鼠尾草酸对细胞毒性和细胞增殖的影响,结果表明,与单纯的完全培养基相比,鼠尾草酸10-30 μmol/L对骨髓源巨噬细胞没有潜在的细胞毒性,见图1B。为了确定鼠尾草酸对破骨细胞形成的影响,将骨髓源巨噬细胞与不同浓度的鼠尾草酸和破骨细胞诱导培养基一起孵育特定时间,使用抗酒石酸酸性磷酸酶染色试剂盒检测成熟破骨细胞时,在RANKL刺激后出现许多多核抗酒石酸酸性磷酸酶阳性破骨细胞,而增加鼠尾草酸浓度会增强对骨髓源巨噬细胞破骨细胞分化的抑制作用,见图1C,D。这些结果表明鼠尾草酸对骨髓源巨噬细胞破骨细胞分化的抑制呈浓度依赖性。 2.2 鼠尾草酸抑制骨髓源巨噬细胞破骨细胞分化的细胞骨架F-actin环形成 F-actin环的形成在成熟破骨细胞的形成中起着至关重要的作用[15]。因此,用免疫荧光染色骨髓源巨噬细胞、F-actin环和细胞核(DAPI)来观察破骨细胞的大小和核聚集,并评估鼠尾草酸干预和不干预对破骨细胞形成的影响。RANKL刺激后,成熟破骨细胞(含3个以上细胞核)数量显著增加。随着鼠尾草酸浓度的增加,F-actin环的大小和环内细胞核的数量明显减少,见图2A-C。这些结果表明,鼠尾草酸以剂量依赖的方式减弱破骨分化的细胞骨架和核聚集来抑制RANKL诱导的破骨细胞的形成。 2.3 鼠尾草酸降低骨髓源巨噬细胞破骨细胞分化标志基因的表达 通过RT-PCR检测鼠尾草酸对破骨细胞特异性基因的影响。正如预期的那样,不同浓度的鼠尾草酸抑制了NFATc1、Atp6vod2、ACP5、CTSK和C-fos在骨髓源巨噬细胞中的相对表达水平,抑制的程度与鼠尾草酸的浓度有关,见图3。这些结果表明,鼠尾草酸在体外抑制破骨细胞的形成,并抑制破骨细胞特异性标志基因的表达。 2.4 鼠尾草酸降低骨髓源巨噬细胞破骨细胞分化关键蛋白的表达 C-Fos与NFATc1的转录激活密切相关,在RANKL诱导的信号转导中发挥重要作用[14,16]。选取30 μmol/L浓度观察鼠尾草酸对破骨细胞分化关键蛋白表达的影响。Western blot结果表明,RANKL的加入增加了CTSK、C-fos、MMP9和NFATc1的表达,而鼠尾草酸则逆转这些作用,在第3天和第5天抑制了CTSK、C-fos、MMP9和NFATc1的表达,见图4A-E。 2.5 鼠尾草酸通过激活MAPK信号通路抑制破骨细胞分化 MAPK信号通路包括P38、JNK和ERK三个主要家族成员,位于调节破骨细胞分化的RANKL信号通路下游[17]。因此,研究了这些信号通路是否参与了鼠尾草酸对RANKL诱导的破骨细胞生成的抑制。Western blot结果显示,鼠尾草酸处理15 min时,P38的磷酸化受到抑制,随后得到恢复,见图5A,B)。JNK的磷酸化水平在鼠尾草酸干预的45-60 min受到抑制,而ERK在鼠尾草酸处理的15-60 min内磷酸化水平持续被抑制,可能是抑制破骨细胞分化主要激活的信号通路,见图5A-D。 2.6 鼠尾草酸减少了骨髓源巨噬细胞破骨细胞分化中活性氧的产生 为了检测鼠尾草酸对RANKL诱导的破骨细胞分化中活性氧的影响,使用敏感的活性氧探针H2DCFDA和荧光显微镜检测细胞内活性氧的产生。当骨髓源巨噬细胞被RANKL刺激时,经鼠尾草酸15 μmol/L和30 μmol/L处理后,DCFs的荧光强度呈剂量依赖性地下降,鼠尾草酸显著减少了骨髓源巨噬细胞中活性氧的产生,见图6。相同操作下的流式检测结果也表明鼠尾草酸以浓度依赖性抑制细胞活性氧的产生,结果表明,鼠尾草酸通过降低活性氧活性而抑制RANK诱导的破骨细胞形成。 2.7 鼠尾草酸激活Nrf2-Keap1通路增强了破骨分化中抗氧化酶的表达 在正常的生理状态下,活性氧的清除依赖于几种抗氧化酶[18- 19]。因此,研究了关键抗氧化酶Nrf2、Keap1、HO-1、SOD1和Catalase的表达。Western bolt分析表明,鼠尾草酸激活了Nrf2-Keap1通路,增加了RANKL诱导后HO-1、SOD1和Catalase的蛋白表达,见图7A-F。综上所述,鼠尾草酸通过增加活性氧清除酶的表达减少活性氧的产生,有效地抑制了RANKL诱导的破骨细胞分化。 2.8 鼠尾草酸损害了破骨细胞分化中线粒体活性发挥 由于线粒体呼吸链是活性氧产生的主要来源[19-20],进一步检测了线粒体中的活性氧水平,见图8A,B。RANKL刺激促进线粒体活性氧的积累,而鼠尾草酸干预减弱了RANKL诱导的线粒体活性氧产生。在呼吸和氧化磷酸化过程中,线粒体产生的能量以线粒体膜电位的方式表现出来[21-22]。由于破骨细胞分化中的能量密集性,见图8C,D所示,成熟破骨细胞的线粒体膜电位相对增加,而线粒体膜电位在鼠尾草酸处理下以浓度依赖性方式降低。总体而言,鼠尾草酸通过损害线粒体活性发挥起到抑制破骨细胞分化的作用。"
| [1] AYERS C, KANSAGARA D, LAZUR B, et al. Effectiveness and Safety of Treatments to Prevent Fractures in People With Low Bone Mass or Primary Osteoporosis: A Living Systematic Review and Network Meta-analysis for the American College of Physicians. Ann Intern Med. 2023; 176(2):182-195. [2] LORENZO J. From the gut to bone: connecting the gut microbiota with Th17 T lymphocytes and postmenopausal osteoporosis. J Clin Invest. 2021;131(5):e146619. [3] SONG S, GUO Y, YANG Y, et al. Advances in pathogenesis and therapeutic strategies for osteoporosis, Pharmacol Ther. 2022;237:108168. [4] LEE S, KIM GJ, KWON H, et al. Estrogenic Effects of Extracts and Isolated Compounds from Belowground and Aerial Parts of Spartina anglica. Mar Drugs. 2021;19(4):210. [5] RODRÍGUEZ V, RIVOIRA M, PICOTTO G, et al. Analysis of the Molecular Mechanisms by Flavonoids with Potential Use for Osteoporosis Prevention or Therapy. Curr Med Chem. 2022;29(16):2913-2936. [6] MCDONALD MM, KHOO WH, NG PY, et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell. 2021; 184(5):1330-1347.e13. [7] MENG J, ZHANG X, GUO X, et al. Briarane-type diterpenoids suppress osteoclastogenisis by regulation of Nrf2 and MAPK/NF-kB signaling pathway. Bioorg Chem. 2021;112:104976. [8] ALIPRANTIS AO, UEKI Y, SULYANTO R, et al. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008; 118(11):3775-3789. [9] MA JD, JING J, WANG JW, et al. Activation of the Peroxisome Proliferator-Activated Receptor gamma Coactivator 1beta/NFATc1 Pathway in Circulating Osteoclast Precursors Associated With Bone Destruction in Rheumatoid Arthritis. Arthritis Rheumatol. 2019;71(8): 1252-1264. [10] DENG W, DING Z, WANG Y, et al. Dendrobine attenuates osteoclast differentiation through modulating ROS/NFATc1/ MMP9 pathway and prevents inflammatory bone destruction. Phytomedicine. 2022; 96:153838. [11] ZHAO Y, GAO J, ZHANG Y, et al. Cyclosporine A Promotes Bone Remodeling in LPS-Related Inflammation via Inhibiting ROS/ERK Signaling: Studies In Vivo and In Vitro. Oxid Med Cell Longev. 2021; 2021:8836599. [12] BIRTIĆ S, DUSSORT P, PIERRE FX, et al. Carnosic acid. Phytochemistry. 2015;115:9-19. [13] ZHENG ZG, CHENG HM, ZHOU YP, et al. Dual targeting of SREBP2 and ERRalpha by carnosic acid suppresses RANKL-mediated osteoclastogenesis and prevents ovariectomy-induced bone loss.Cell Death Differ. 2020;27(7):2048-2065. [14] LI H, DENG W, QIN Q, et al. Isoimperatorin attenuates bone loss by inhibiting the binding of RANKL to RANK. Biochem Pharmacol. 2023;211:115502. [15] HONG G, CHEN Z, HAN X, et al. A novel RANKL-targeted flavonoid glycoside prevents osteoporosis through inhibiting NFATc1 and reactive oxygen species. Clin Transl Med. 2021;11(5):e392. [16] LI Y, ZHUANG Q, TAO L, et al. Urolithin B suppressed osteoclast activation and reduced bone loss of osteoporosis via inhibiting ERK/NF-kappaB pathway. Cell Prolif. 2022;55(10):e13291. [17] WEI L, CHEN W, HUANG L, et al. Alpinetin ameliorates bone loss in LPS-induced inflammation osteolysis via ROS mediated P38/PI3K signaling pathway. Pharmacol Res. 2022;184:106400. [18] SU X, SHEN Z, YANG Q, et al. Vitamin C kills thyroid cancer cells through ROS-dependent inhibition of MAPK/ERK and PI3K/AKT pathways via distinct mechanisms. Theranostics. 2019;9(15):4461-4473. [19] SHADEL GS, HORVATH TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163(3):560-569. [20] NI S, QIAN Z, YUAN Y, et al. Schisandrin A restrains osteoclastogenesis by inhibiting reactive oxygen species and activating Nrf2 signalling.Cell Prolif. 2020;53(10):e12882. [21] LI B, LEE WC, SONG C, et al. Both aerobic glycolysis and mitochondrial respiration are required for osteoclast differentiation. FASEB J. 2020; 34(8):11058-11067. [22] YANG B, SU Y, HAN S, et al. Aminooxyacetic acid hemihydrochloride inhibits osteoclast differentiation and bone resorption by attenuating oxidative phosphorylation. Front Pharmacol. 2022;13:980678. [23] SIMS NA, MARTIN TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit.Bonekey Rep. 2014;3:481. [24] YUAN F, PENG W, YANG C, et al. Teriparatide versus bisphosphonates for treatment of postmenopausal osteoporosis: A meta-analysis. Int J Surg. 2019;66:1-11. [25] ROZENBERG S, AL-DAGHRI N, AUBERTIN-LEHEUDRE M, et al. Is there a role for menopausal hormone therapy in the management of postmenopausal osteoporosis? Osteoporos Int. 2020;31(12): 2271-2286. [26] KHOSLA S, HOFBAUER LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5(11):898-907. [27] LIU Y, WANG C, WANG G, et al. Loureirin B suppresses RANKL-induced osteoclastogenesis and ovariectomized osteoporosis via attenuating NFATc1 and ROS activities. Theranostics. 2019;9(16):4648-4662. [28] LEE DK, JANG HD. Carnosic Acid Attenuates an Early Increase in ROS Levels during Adipocyte Differentiation by Suppressing Translation of Nox4 and Inducing Translation of Antioxidant Enzymes. Int J Mol Sci. 2021;22(11):6096. [29] HU S, LIU B, YANG M, et al. Carnosic acid protects against doxorubicin-induced cardiotoxicity through enhancing the Nrf2/HO-1 pathway. Food Funct. 2023;14(8):3849-3862. [30] ZUO RM, JIAO JY, CHEN N, et al. Carnosic acid suppressed the formation of NETs in alcoholic hepatosteatosis based on P2X7R-NLRP3 axis.Phytomedicine. 2023;110:154599. [31] FISCHER V, HAFFNER-LUNTZER M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin Cell Dev Biol. 2022;123:14-21. [32] LIU M, LIU S, ZHANG Q, et al. Curculigoside attenuates oxidative stress and osteoclastogenesis via modulating Nrf2/NF-kappaB signaling pathway in RAW264.7 cells. J Ethnopharmacol. 2021; 275:114129. [33] KIM HJ, PARK C, KIM GY, et al. Sargassum serratifolium attenuates RANKL-induced osteoclast differentiation and oxidative stress through inhibition of NF-kappaB and activation of the Nrf2/HO-1 signaling pathway. Biosci Trends. 2018;12(3):257-265. [34] YAN C, SHI Y, YUAN L, et al. Mitochondrial quality control and its role in osteoporosis. Front Endocrinol (Lausanne). 2023;14:1077058. [35] GAO J, FENG Z, WANG X, et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress.Cell Death Differ. 2018;25(2):229-240. [36] KIM HN, PONTE F, NOOKAEW I, et al. Estrogens decrease osteoclast number by attenuating mitochondria oxidative phosphorylation and ATP production in early osteoclast precursors. Sci Rep. 2020; 10(1):11933. |
| [1] | Chi Wenxin, Zhang Cunxin, Gao Kai, Lyu Chaoliang, Zhang Kefeng. Mechanism by which nobiletin inhibits inflammatory response of BV2 microglia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1321-1327. |
| [2] | Li Yueyao, Zhang Min, Yang Jiaju. Cistanoside A mediates p38/MAPK pathway to inhibit osteoclast activity [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1144-1151. |
| [3] |
Huang Xiaobin, Ge Jirong, Li Shengqiang, Xie Lihua, Huang Jingwen, He Yanyan, Xue Lipeng.
Mechanisms of different yin nourishing and kidney tonifying methods on osteoclastysis pathway in ovariectomized rats #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1214-1219.
|
| [4] | Wang Chen, Zhang Weinan, Shen Jining, Liu Fan, Yuan Jishan, Liu Yake. Inhibitory effect of ferroptosis inhibitor toxicity induced by cobalt nanoparticles through reactive oxygen species [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7310-7317. |
| [5] | Wu Qingyun, Su Qiang. Antioxidant nanomedicine-mediated targeted therapy for myocardial ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7431-7438. |
| [6] | Lu Xiuli, Xu Huazhen, Chen Yuxing, Yao Nan, Hu Zixuan, Huang Dane. Mechanism of Jiangu Formula in treating osteoporosis based on osteoclast-osteoblast coupling [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6828-6835. |
| [7] | Xu Biao, Dong Yuzhen, Lu Tan. Effect of dihydroquercetin on the expression of inflammatory response markers in rats with spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6843-6850. |
| [8] | Wu Wangxiang, Ran Dongcheng, Xu Jiamu, Xu Jiafu, Chen Jingjing, Wang Chunqing. Long noncoding RNAs related to osteoporosis: current research status and developmental trends [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6360-6368. |
| [9] | Wu Diyou, Huang Jiajun, Tao Guangyi, Zhao Yu, Huang Junqing, Yang Bin, Xue Yun. Pueraria lobata decoction intervenes in neuroinflammatory response and apoptosis in rats with cervical spondylotic myelopathy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4249-4257. |
| [10] | Wu Haiyang, Duan Mian, Li Chenglong, Zhang Junyu, Ji Haisheng, Wang Haitao, Mao Wei, Wang Ying. Neuroprotective mechanism of electroacupuncture in cerebral ischemia-reperfusion model rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3811-3818. |
| [11] | Zhu Weiwei, Fan Zhengchao, Xu Ying, Xia Jizhu, Zhao Xiangzhi. Dual-modality imaging bionic nanoparticles for sonodynamic therapy on medullary thyroid carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3410-3419. |
| [12] | hang Zining, Deng Ronghui, Yu Jiakuo. Alleviation of oxidative stress damage in chondrocytes by a new Mn-containing bioceramic powder [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3335-3342. |
| [13] | Feng Nan, Li Yunfeng. Antibacterial piezoelectric materials: no selective killing of bacteria and no bacterial resistance [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2105-2112. |
| [14] | Zhang Mianyu, Han Jie, Zeng Hao, Chen Xiangshan, Gao Zhengang. Regulating ferroptosis of osteoblasts by traditional Chinese medicine in treatment of steroid-induced avascular necrosis of femoral head [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 185-192. |
| [15] | Huang Haoran, Fan Yinuo, Wei-Yang Wenxiang, Jiang Mengyu, Fang Hanjun, Wang Haibin, Chen Zhenqiu, Liu Yuhao, Zhou Chi. Urolithin A mediates p38/MAPK pathway to inhibit osteoclast activity [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1149-1154. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||