Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (12): 2528-2535.doi: 10.12307/2025.362
Previous Articles Next Articles
Extraction and characterization of three types of primary cells from rat intervertebral disc and their matrix expression in monolayer and micromass culture
Hu Siyuan, Chen Jianquan
- Orthopedic Institute, Suzhou Medical College, Soochow University, Suzhou 215031, Jiangsu Province, China
-
Received:2024-03-26Accepted:2024-04-24Online:2025-04-28Published:2024-09-10 -
Contact:Chen Jianquan, Professor, Doctoral supervisor, Orthopedic Institute, Suzhou Medical College, Soochow University, Suzhou 215031, Jiangsu Province, China -
About author:Hu Siyuan, Master candidate, Orthopedic Institute, Suzhou Medical College, Soochow University, Suzhou 215031, Jiangsu Province, China -
Supported by:the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD; to CJQ [project participant])
CLC Number:
Cite this article
Hu Siyuan, Chen Jianquan. Extraction and characterization of three types of primary cells from rat intervertebral disc and their matrix expression in monolayer and micromass culture[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(12): 2528-2535.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
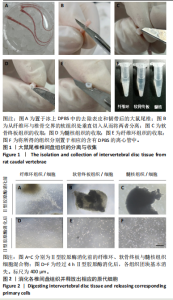
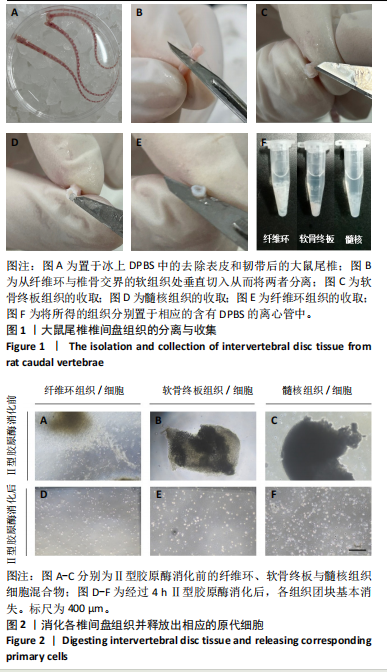
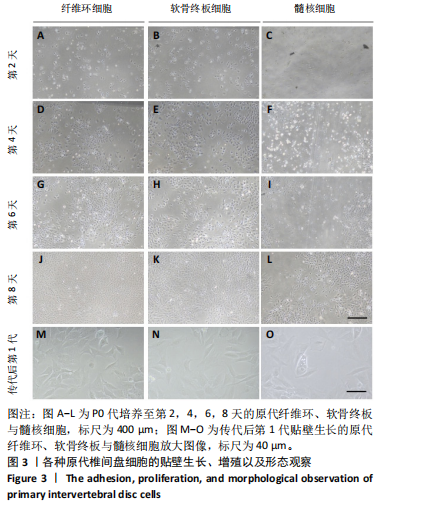
2.1 尾椎椎间盘组织消化 在倒置显微镜下,可以观察到在Ⅱ型胶原酶消化前,各组织均有明显的组织团块。在纤维环组织/细胞混合物中,可以观察到丝状细胞束(图2A);在软骨终板组织/细胞混合物中,可以观察到致密透光的板状结构(图2B);在髓核组织/细胞混合物中,可以观察到近圆形的组织团块(图2C)。经过4 h Ⅱ型胶原酶消化后,较大的组织团块基本消失,释放出其中的细胞,细胞大小不一,主要呈圆形悬浮于培养基中(图2D-F)。 2.2 尾椎椎间盘细胞贴壁、增殖与形态记录 原代纤维环与软骨终板细胞在培养第2天后细胞开始逐渐贴壁生长(图 3A,B),于4 d换液后可见贴壁细胞集落产生(图 3D,E);原代髓核细胞在培养第2天可见少量细胞贴壁生"
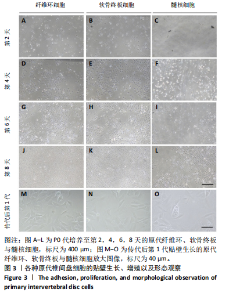
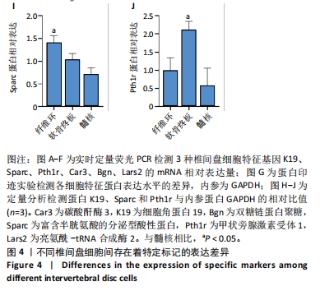
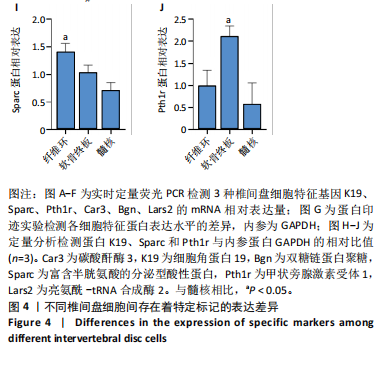
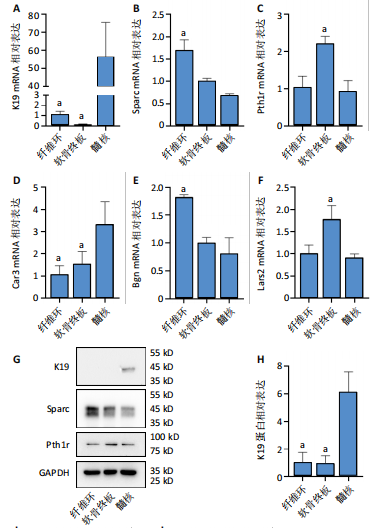
长(图 3C),并于培养第4天时取培养基,离心后取新鲜含1%青霉素-链霉素溶液和10%胎牛血清的DMEM/F-12培养基重悬沉淀并继续返回培养基中培养,此时可以观察到贴壁细胞数量增加(图 3F)。随着培养天数的延长,3种椎间盘细胞不断增殖,其集落逐渐扩大(图 3G-I),至第8天细胞连接成片,集落形成密集处成“铺路石”样,少数悬浮细胞夹杂生长(图 3J-L)。传代后可见原代纤维环、软骨终板与髓核细胞均为短梭形、三角形或不规则多角形等多样态分布,胞质丰富、边缘清晰,呈现成纤维细胞样(图 3M-O),其中原代髓核细胞培养过程中可见多囊泡结构的脊索样细胞(图3O)。 2.3 三种原代椎间盘细胞的分子生物学鉴定 为了验证所提取的原代椎间盘细胞,通过实时定量荧光PCR检测原代纤维环、软骨终板与髓核细胞中标记基因K19、Sparc、Pth1r、Car3、Bgn与Lars2在转录水平的表达差异;通过蛋白免疫印迹检测3种原代细胞中K19、Sparc、Pth1r在"
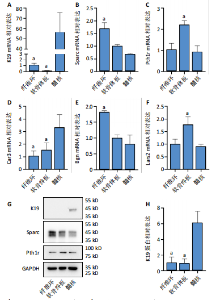
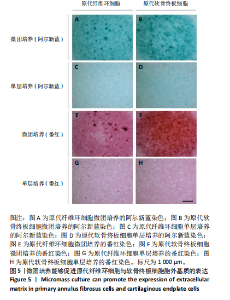
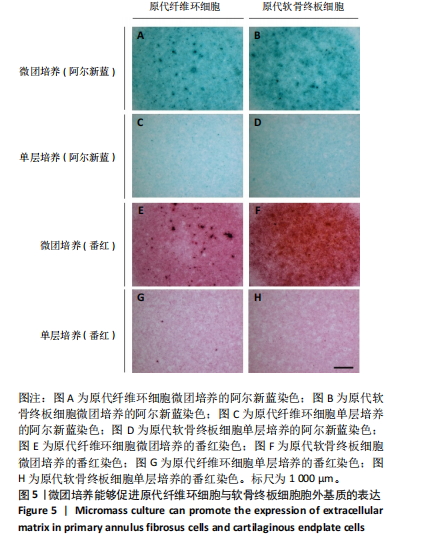
蛋白水平表达量的差异。结果显示,相比于其他两种细胞,在原代髓核细胞中,K19与Car3在转录水平有较高的表达(图 4A,D);在原代纤维环细胞中,Sparc与Bgn在转录水平有较高的表达(图 4B,E);在原代软骨终板细胞中,Pth1r与Lars2在转录水平有较高的表达(图 4C,F)。在蛋白水平上,进一步验证了各椎间盘细胞的特异性标记物K19、Sparc与Pth1r的特异性高表达(图 4G-J)。 2.4 原代纤维环细胞与软骨终板细胞微团培养与染色 实验成功构建了一种体外三维细胞培养模型,该模型能够精准地再现体内细胞的堆叠状态,使细胞得以展现出更多类似体内的特征[27]。阿尔新蓝和番红是常用的亲酸性染色剂,基于其与软骨基质中胶原蛋白和酸性多糖之间的亲和性,两种染料主要用于直观展示样品的软骨特性,能够使得具有软骨特性的组织分别呈现出蓝色和红色[26,28]。 通过阿尔新蓝与番红染色,微团培养的原代纤维环与软骨终板细胞均着明显的深蓝色(图 5A,B)或深红色(图 5E,F),而平铺培养的对应细胞则呈现浅蓝色(图 5C,D)或浅红色(图 5G,H)。因此可以通过显色程度的差异[29],确定微团培养能够促进纤维环与软骨终板细胞表现出更多的软骨特性,进而说明相较于传统的平铺式细胞培养,在培养纤维环与软骨终板原代细胞的过程中这种微团培养方式在模拟软骨细胞特性方面展现出了显著的优势。"
| [1] JIN Z, WANG D, ZHANG H, et al. Incidence trend of five common musculoskeletal disorders from 1990 to 2017 at the global, regional and national level: results from the global burden of disease study 2017. Ann Rheum Dis. 2020;79(8):1014-1022. [2] CHEN S, WU X, LAI Y, et al. Kindlin-2 inhibits Nlrp3 inflammasome activation in nucleus pulposus to maintain homeostasis of the intervertebral disc. Bone Res. 2022;10(1):5. [3] GENEDY H H, HUMBERT P, LAOULAOU B, et al. MicroRNA-targeting nanomedicines for the treatment of intervertebral disc degeneration. Adv Drug Deliv Rev. 2024;207:115214. [4] CHEN F, LEI L, CHEN S, et al. Serglycin secreted by late-stage nucleus pulposus cells is a biomarker of intervertebral disc degeneration. Nat Commun. 2024;15(1):47. [5] ZHANG L, HU S, XIU C, et al. Intervertebral disc-intrinsic Hedgehog signaling maintains disc cell phenotypes and prevents disc degeneration through both cell autonomous and non-autonomous mechanisms. Cell Mol Life Sci. 2024;81(1):74. [6] FRANCISCO V, PINO J, GONZÁLEZ-GAY M, et al. A new immunometabolic perspective of intervertebral disc degeneration. Nat Rev Rheumatol. 2022; 18(1):47-60. [7] FRANCISCO V, AIT ELDJOUDI D, GONZáLEZ-RODRíGUEZ M, et al. Metabolomic signature and molecular profile of normal and degenerated human intervertebral disc cells. Spine J. 2023;23(10):1549-1562. [8] BINCH ALA, FITZGERALD JC, GROWNEY EA, et al. Cell-based strategies for IVD repair: clinical progress and translational obstacles. Nat Rev Rheumatol. 2021;17(3):158-175. [9] VAN DEN AKKER GGH, CREMERS A, SURTEL DAM, et al. Isolation of Nucleus Pulposus and Annulus Fibrosus Cells from the Intervertebral Disc. Methods Mol Biol. 2021;2221:41-52. [10] CHEN Y, ZHANG L, SHI X, et al. Characterization of the Nucleus Pulposus Progenitor Cells via Spatial Transcriptomics. Adv Sci (Weinh). 2024;e2303752. [11] CHERIF H, MANNARINO M, PACIS AS, et al. Single-Cell RNA-Seq Analysis of Cells from Degenerating and Non-Degenerating Intervertebral Discs from the Same Individual Reveals New Biomarkers for Intervertebral Disc Degeneration. Int J Mol Sci. 2022;23(7):3993. [12] REGAN SL, WILLIAMS MT, VORHEES CV. Review of rodent models of attention deficit hyperactivity disorder. Neurosci Biobehav Rev. 2022;132: 621-637. [13] MANNING WK, BONNER WM JR. Isolation and culture of chondrocytes from human adult articular cartilage. Arthritis Rheum. 1967;10(3):235-239. [14] 王效, 徐宏光, 肖良, 等. 大鼠腰椎终板软骨干细胞的分离及分化[J]. 中国组织工程研究,2018,22(13):2093-2097. [15] 金中行, 徐宏光, 张玙, 等. 大鼠纤维环干细胞的分离与鉴定[J]. 皖南医学院学报,2018,37(4):307-309. [16] 马东, 陈祁青, 赵继荣, 等. 贴壁法联合纤维连接蛋白差别黏附法分离纯化鉴定大鼠纤维环源干细胞[J]. 中国组织工程研究,2024,28(31): 4980-4986. [17] HE R, WANG Z, CUI M, et al. HIF1A Alleviates compression-induced apoptosis of nucleus pulposus derived stem cells via upregulating autophagy. Autophagy. 2021;17(11):3338-3360. [18] HU X, WANG Z, ZHANG H, et al. Single-cell sequencing: New insights for intervertebral disc degeneration. Biomed Pharmacother. 2023;165:115224. [19] WILLIAMS RJ, LAAGLAND LT, BACH FC, et al. Recommendations for intervertebral disc notochordal cell investigation: From isolation to characterization. JOR spine. 2023;6(3):e1272. [20] DAI Z, XIA C, ZHAO T, et al. Platelet-derived extracellular vesicles ameliorate intervertebral disc degeneration by alleviating mitochondrial dysfunction. Mater Today Bio. 2023;18:100512. [21] BASATVAT S, BACH FC, BARCELLONA MN, et al. Harmonization and standardization of nucleus pulposus cell extraction and culture methods. JOR spine. 2023;6(1):e1238. [22] GONG Y, QIU J, JIANG T, et al. Maltol ameliorates intervertebral disc degeneration through inhibiting PI3K/AKT/NF-κB pathway and regulating NLRP3 inflammasome-mediated pyroptosis. Inflammopharmacology. 2023; 31(1):369-384. [23] TENG Y, HUANG Y, YU H, et al. Nimbolide targeting SIRT1 mitigates intervertebral disc degeneration by reprogramming cholesterol metabolism and inhibiting inflammatory signaling. Acta Pharm Sin B. 2023;13(5): 2269-2280. [24] MA L, ZHANG L, LIAO Z, et al. Pharmacological inhibition of protein S-palmitoylation suppresses osteoclastogenesis and ameliorates ovariectomy-induced bone loss. J Orthop Translat. 2023;42:1-14. [25] LUO N, ZHANG L, XIU C, et al. Piperlongumine, a Piper longum-derived amide alkaloid, protects mice from ovariectomy-induced osteoporosis by inhibiting osteoclastogenesis via suppression of p38 and JNK signaling. Food Funct. 2024;15(4):2154-2169. [26] JIANG M, FU X, YANG H, et al. mTORC1 Signaling Promotes Limb Bud Cell Growth and Chondrogenesis. J Cell Biochem.2017;118(4):748-753. [27] KIM MJ, CHI BH, YOO JJ, et al. Structure establishment of three-dimensional (3D) cell culture printing model for bladder cancer. PloS One. 2019;14(10): e0223689. [28] HE Q, YANG J, PAN Z, et al. Biochanin A protects against iron overload associated knee osteoarthritis via regulating iron levels and NRF2/System xc-/GPX4 axis. Biomed Pharmacother.2023;157:113915. [29] 康陈萍, 肖倩倩, 刘青云, 等. 利用体外替代模型评价栀子黄色素的发育毒性[J]. 中国生育健康杂志,2022,33(3):247-253. [30] CHEN J, YANG X, FENG Y, et al. Targeting Ferroptosis Holds Potential for Intervertebral Disc Degeneration Therapy. Cells. 2022;11(21):3508. [31] SAMANTA A, LUFKIN T, KRAUS P. Intervertebral disc degeneration-Current therapeutic options and challenges. Front Public Health. 2023;11:1156749. [32] WANG Y, KANG J, GUO X, et al. Intervertebral Disc Degeneration Models for Pathophysiology and Regenerative Therapy -Benefits and Limitations. J Invest Surg. 2022;35(4):935-952. [33] LING Z, CRANE J, HU H, et al. Parathyroid hormone treatment partially reverses endplate remodeling and attenuates low back pain in animal models of spine degeneration. Sci Transl Med. 2023;15(722):eadg8982. [34] LIU L, ZHANG Y, FU J, et al. Gli1 depletion induces oxidative stress and apoptosis of nucleus pulposus cells via Fos in intervertebral disc degeneration. J Orthop Translat. 2023;40:116-131. [35] LU K, WANG Q, JIANG H, et al. Upregulation of β-catenin signaling represents a single common pathway leading to the various phenotypes of spinal degeneration and pain. Bone Res. 2023;11(1):18. [36] 汪尊冬, 李蔚, 曾臻, 等. 新生SD大鼠心房肌细胞的原代培养及改良[J]. 国际心血管病杂志,2022,49(6):359-362. [37] HAN F, TU Z, ZHU Z, et al. Targeting Endogenous Reactive Oxygen Species Removal and Regulating Regenerative Microenvironment at Annulus Fibrosus Defects Promote Tissue Repair. ACS Nano. 2023;17(8):7645-7661. [38] 师雷, 金振晓, 谭延振, 等. 简易灌注装置同时提取成年小鼠心肌细胞和心脏成纤维细胞[J]. 中国体外循环杂志,2023,21(3):172-178. [39] KIANI AK, PHEBY D, HENEHAN G, et al. Ethical considerations regarding animal experimentation. J Prev Med Hyg. 2022;63(2 Suppl 3):E255-E266. [40] JIA Z, LIU D, LI X, et al. Cartilage Endplate-Derived Stem Cells for Regeneration of Intervertebral Disc Degeneration: An Analytic Study. J Inflamm Res. 2023;16:5791-5806. [41] 张磊, 修春美, 倪莉, 等. 小鼠髓核特异性标志物的鉴定和表达分析[J]. 中国组织工程研究,2022,26(11):1669-1674. [42] DU J, QIAN T, LU Y, et al. SPARC-YAP/TAZ inhibition prevents the fibroblasts-myofibroblasts transformation. Exp Cell Res. 2023;429(1):113649. [43] MARTIN TJ. PTH1R Actions on Bone Using the cAMP/Protein Kinase A Pathway. Front Endocrinol (Lausanne). 2022;12:833221. [44] XU H, WANG W, LIU X, et al. Targeting strategies for bone diseases: signaling pathways and clinical studies. Signal Transduct Target Ther. 2023;8(1):202. [45] 鲁燕妮, 龙雯, 常晓峰, 等. 改良全骨髓贴壁法分离及培养大鼠骨髓间充质干细胞[J]. 山西医科大学学报,2023,54(3):364-369. [46] LI M, YU Y, XUE K, et al. Genistein mitigates senescence of bone marrow mesenchymal stem cells via ERRα-mediated mitochondrial biogenesis and mitophagy in ovariectomized rats. Redox Biol. 2023;61:102649. [47] LA MANNO G, GYLLBORG D, CODELUPPI S, et al. Molecular Diversity of Midbrain Development in Mouse, Human, and Stem Cells. Cell. 2016; 167(2):566-580. [48] ZHENG Y, FU X, LIU Q, et al. Characterization of Cre recombinase mouse lines enabling cell type‐specific targeting of postnatal intervertebral discs. J Cell Physiol. 2019;234(9):14422-14431. [49] CHAN CKF, GULATI GS, SINHA R, et al. Identification of the Human Skeletal Stem Cell. Cell. 2018;175(1):43-56. [50] 杨春丽, 陆金芝, 刘贝贝, 等. 大鼠骨髓间充质干细胞的原代培养及鉴定[J]. 现代生物医学进展,2023,23(18):3425-3430. [51] MAZOR M, LESPESSAILLES E, BEST TM, et al. Gene Expression and Chondrogenic Potential of Cartilage Cells: Osteoarthritis Grade Differences. Int J Mol Sci. 2022;23(18):10610. |
| [1] | He Longcai, Song Wenxue, Ming Jiang, Chen Guangtang, Wang Junhao, Liao Yidong, Cui Junshuan, Xu Kaya. An experimental method for simultaneous extraction and culture of primary cortical neurons and microglial cells from SD rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1395-1400. |
| [2] | Li Shidan, Xing Wei, Xie Xiaoyu, Li Youbin, Wang Shaochuan, Fei Jun. A new method for extracting human bone marrow mesenchymal stem cells and the comparison with traditional methods [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3814-3820. |
| [3] | Zhong Ziling, Qu Shenhong, Han Xing, Wu Di. Isolation, culture and identification of rabbit auricular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(23): 3633-3637. |
| [4] | Zhou Fanqi, Li Baoying, Wang Kaitao, Tan Quanquan, Yun Chenxia, Leng Jing. Isolation and identification of fibroblast-like synoviocytes from tree shrews and establishment of TLR8 pathway related molecular detection methods [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(23): 3721-3727. |
| [5] | Liu Yan, Chen Qingyu, Gao Xiang, Gao Junwu. Effect of collagen scaffold on proliferation and osteogenic differentiation of human periodontal ligament stem cells treated by Eucommia ulmoides Oliver leaf extract [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(16): 2537-2543. |
| [6] | Wang Jing, Cai Xia, Wang Zhiguo, Xu Quanchen, Li Kun, Hua Cheng. Isolation and identification of exosomes from human adipose-derived mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(17): 2651-2658. |
| [7] | Ning Bin, Ding Yuan-jing, Gong Wei-ming, Liu Hai-fei, Liu Yong, Wang De-chun, Hu You-gu. Adult degenerative nucleus pulpous cells cultured by a microcarrier-based stirred culture system [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(7): 1168-1173. |
| [8] | Hong Jing-xin, Liu Jian, Li Lin-fang, Han Jun-ling. Osteogenic differentiation of human umbilical cord mesenchymal stem cells induced with different concentrations of dexamethasone in vitro [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(23): 4204-4211. |
| [9] | Wei Xiao-yan, Zhang Li, Lin Ming, Chen Huan, Peng Bo, Peng Yuan-yuan, Li Fu-yun, Hong Pei-xin, Fan Yi-fan. Isolation, culture and identification of rat bone marrow-derived endothelial progenitor cells [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(14): 2570-2577. |
| [10] | Bielikezi·kadeer, Liu Yi-shan, Wang Xuan, Li Bo-qi, Ma Yan, Bi Xiao-juan, Nusilaiti·halike. Isolation and culture of dental pulp stem cells from human deciduous teeth by modified enzyme digestion [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(10): 1793-1800. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||