Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (26): 4252-4257.doi: 10.12307/2022.831
Previous Articles Next Articles
Carvacrol: antibacterial activity, bone repair, and prevention and treatment in oral diseases
Zhang Zihan, Wang Wenli, Li Jinnuo, Li Yourui
- Binzhou Medical University Hospital, Binzhou 256600, Shandong Province, China
-
Received:2021-09-18Accepted:2021-10-25Online:2022-09-18Published:2022-03-09 -
Contact:Li Yourui, MD, Associate chief physician, Binzhou Medical University Hospital, Binzhou 256600, Shandong Province, China -
About author:Zhang Zihan, Master candidate, Binzhou Medical University Hospital, Binzhou 256600, Shandong Province, China -
Supported by:Shandong Provincial Medicine and Health Science and Technology Development Project, No. 2017WS753 (to LYR); Science and Technology Planning Project of Binzhou Medical University, No. BY2016KJ006 (to LYR)
CLC Number:
Cite this article
Zhang Zihan, Wang Wenli, Li Jinnuo, Li Yourui. Carvacrol: antibacterial activity, bone repair, and prevention and treatment in oral diseases[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(26): 4252-4257.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
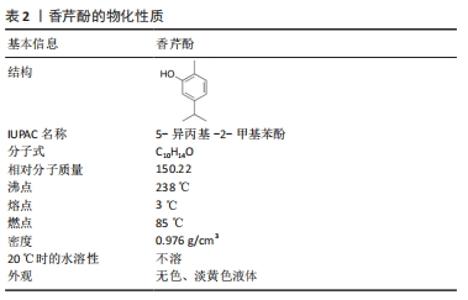
2.1.1 理化性质 香芹酚(5-异丙基-2-甲基苯酚)是一种单萜类化合物,常见于牛至、百里香和其他植物中的精油成分[7],其理化性质见表2。该化合物具有广泛的生物活性,包括抗菌、抗真菌、抗病毒、抗氧化和抗癌特性,可以对抗多种细菌、真菌和病毒,如大肠杆菌、金黄色葡萄球菌、表皮葡萄球菌、奇异变形杆菌[8];白色念珠菌[9]、铜绿假单胞菌和革兰阳性菌[10]、革兰阴性菌都有很高的抑制作用[8];黑曲霉、表皮癣菌、轮状镰刀菌等,其羟基和离域电子系统的存在对其抗菌活性起着关键作用。香芹酚不仅被添加到食品、保健品中,还早已应用于口腔疾病的防治过程中,如漱口水、牙科封闭剂,在临床诊疗中,添加了香芹酚成分的水门汀、凝胶、甘油等药物可以用来治疗牙本质敏感、牙周炎、牙髓炎等口腔常见疾病。"
2.1.2 抗菌分析 生物膜是一种组织良好的细菌结构,能够降低抗生素的疗效。抑制细菌病原体生物膜形成和毒力是控制细菌感染的关键,研究香芹酚对干扰生物膜形成和破坏生物膜的能力十分重要。香芹酚可以抑制生物膜形成初期的特定过程,阻止成熟生物膜的形成,降低抗生素耐药性的风险。研究表明,香芹酚不仅对浮游生物细胞有很强的抑菌能力,对细菌生物膜也有抗菌功效[11]。香芹酚在最小抑菌浓度(MIC)为100 mg/L时可有效抑制白色念珠菌和变形链球菌,扫描电镜显示单一生物膜和混合生物膜中的细胞膜、基质结构被破坏[12]。对于牙龈卟啉单胞菌和具核梭杆菌的最小抑制生物膜浓度分别为0.03%,0.06%,表明香芹酚在低浓度下具有抗生物膜活性[13]。目前,已对香芹酚的构效关系进行了分析,以了解其化学-生物相互作用,香芹酚的大多数抗菌活性取决于自由羟基以及芳环上取代的类型和数量[14]。 香芹酚的抗菌性能归因于它在细胞水平上是通过多个靶点协同发挥作用,并不是单一的机制。最常见的抗菌作用机制包括破坏细胞壁和细胞膜,导致细胞内容物泄漏和细菌溶解[15];其疏水性使其整合到细菌细胞膜中对其功能造成干扰;积聚在膜脂肪酸链中,导致膜脂双层的构象变化。其他已提出的机制还包括:影响ATP酶活性,似是一种质子交换器,可降低细胞膜上的pH梯度,导致质子动力的崩溃和ATP的耗尽,从而使细胞死亡;抑制已知的导致耐药性的外排泵;生物膜减量;对鞭毛的运动抑制[16]。在较低浓度下,香芹酚抑制参与能量生产的酶,在较高浓度下观察到蛋白质沉淀。通过扫描电子显微镜获得的图像表明,导致被评估细菌细胞死亡机制是细胞膜完整性的丧失[17]。另一种机制表明香芹酚使线粒体失稳,导致电子传递链的耦合效率受到干扰,进而产生活性氧中间体,导致氧化应激反应[3]。 天然产物香芹酚虽然在结构上与目前可用的抗真菌药物完全不同,但已观察到其不仅对浮游细胞具有良好的抑制活性,而且对真菌生物膜也具有良好的抑制活性。与细菌不同的是,真菌是真核生物,因此抗真菌药物需要更具针对性,以引导人类宿主无法共享的独特靶点。香芹酚杀菌性质的可能机制之一是细胞膜破坏和细胞壁生物合成抑制引起的细胞死亡。挥发性酚类物质以真菌细胞壁为靶标,是因为真菌特有的几丁质积累。这些酚类通过干扰表面静电而影响膜的完整性,它们的抗真菌作用还伴随着广泛的膜损伤和麦角甾醇含量的降低。香芹酚和唑类药物的主要抗真菌作用模式相似,这是为了破坏麦角甾醇生物合成途径[18]。麦角甾醇生物合成的抑制以及细胞壁和膜的解体进一步导致细胞器的泄漏,最终导致细胞死亡。香芹酚杀菌作用的另一个重要机制是诱导氧化应激,这可以通过增加活性氧水平以及酶和非酶成分的抗氧化防御系统来证明[19],应激反应在疾病的形成和真菌细胞耐药性的发展中起着关键的作用。 2.1.3 安全性和毒性 美国联邦药品管理局(FDA)已批准将香芹酚用于人类食用的食品中;香芹酚被归类为GRAS(generally recognized as safe),其在食品中的用途同样获得欧洲议会和理事会的批准。 虽然香芹酚的抗菌作用在文献中有很好的记载,但其可能具有潜在毒性作用,如致突变性和遗传毒性[20-21]。但在中国仓鼠肺成纤维细胞、人肝细胞和淋巴细胞中未发现任何效应[22]。 SUNTRES等[23]回顾了香芹酚毒理学数据,报告了香芹酚的半致死剂量:在大鼠中,口服给药的剂量为810 mg/kg;在小鼠中,110-233.3 mg/kg 香芹酚导致死亡。据报道,家兔、小鼠经皮给药后的半数致死剂量为2 700,680 mg/kg。 2.2 香芹酚对口腔疾病防治的作用 "

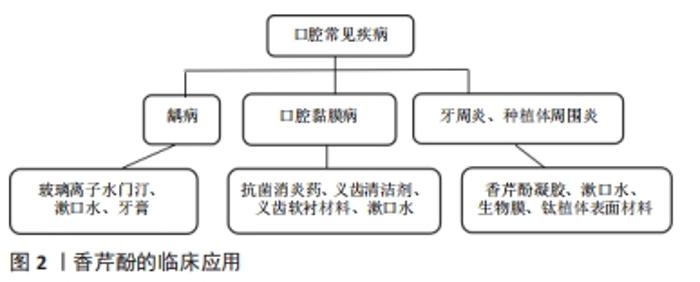
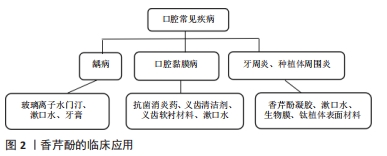
2.2.1 龋病 龋齿是世界范围内最主要的口腔疾病,牙齿表面的口腔菌群是龋病的主要病因,主要致病菌为变异链球菌、血链球菌及一些乳杆菌属、放线菌属。已有实验证明香芹酚对口腔致龋菌有较好的抗菌性和抗生物膜特性。BOTELHO等[24]研究了山梨油及其主要化合物(TH/CV),使用4株致龋菌(变形链球菌、血球链球菌、唾液链球菌和米特链球菌)和1株白色念珠菌,表现出较强的抗菌和抗真菌活性,这是第一份支持香芹酚具有抗口腔病原菌活性的报告,在所有受试微生物中,变异链球菌最为敏感。由此引发了一系列关于香芹酚可否用于龋病的临床治疗的研究。金黄色葡萄球菌是与医用植入物相关的最常见的人类病原体之一,可从牙周炎、龋病和牙龈炎中分离出来。MILADI等[25]对香芹酚(CAR)、百里酚(TYH)单独或与四环素联合使用对口腔细菌的抗菌和抗生物膜活性进行了测试,还筛选了其对聚苯乙烯和釉质表面生物膜的影响,结果表明香芹酚对受试菌(金黄色葡萄球菌、变异链球菌、粪肠球菌)的最小抑菌浓度值范围为32-256 mg/L,四环素与香芹酚具有协同作用;扫描电镜分析表明,香芹酚成分显著减少了黏附在釉质表面的细菌,证实了香芹酚对口腔致龋菌(金葡、变链、粪肠球菌)有抗菌、抗生物膜活性;植物化学物质与传统药物的结合在对抗耐药菌株方面更为有效的结论。 香芹酚可能是一种抗生素的替代品,增加临床疗效、减少耐药性。许多研究将抗菌剂与修复材料(尤其是玻璃离子水门汀)结合,试图减少生物膜的形成,防止继发性龋齿的发生,从而提高抗菌活性,同时提高临床疗效。传统玻璃离子水门汀已与抗生素(多西环素、甲硝唑、米诺环素等)联合使用,并显示出优异的抗菌性能[26-27]。NUNES等[28]在玻璃离子水门汀GIC中添加百里酚(香芹酚的同分异构体)成分,发现变异链球菌生物膜减少且对修饰后的玻璃离子水门汀高度敏感,作用持续至96 h。KHAN等[29]证实了香芹酚可诱导变异链球菌自溶、应激、生长抑制和减少生物膜的形成,使变异链球菌的活力和代谢活性降低50%以上;在扫描电子显微镜和比色分析下观察到,在100 mg/L质量浓度下,香芹酚可显著减少聚苯乙烯板表面的生物膜形成;这些结果与RT-PCR研究一致,经香芹酚处理后,观察到ymcA基因增加,gtfB基因表达水平降低。这些结果都证实了香芹酚对变异链球菌具有良好的杀菌和抗生物膜活性,又因其被归类为GRAS的食用植物,故可添加于漱口水、牙膏中,控制口腔细菌保持良好口腔卫生,预防和治疗龋病,见图2。"
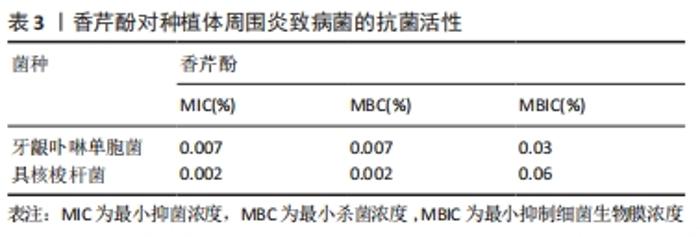
2.2.2 黏膜疾病 口腔念珠菌病是真菌-念珠菌属感染所引起的口腔黏膜疾病。近年来抗生素和免疫抑制剂在临床上的广泛应用,发生菌群失调或免疫力降低,而使内脏、皮肤、黏膜被真菌感染者日益增多,口腔黏膜念珠菌病的发生率也相应增高。其中白色念珠菌是最主要的病原菌,包括幼儿假膜性念珠菌病(鹅口疮)、增生性或萎缩性(义齿)念珠菌病、线性牙龈红斑、口角炎等,在口腔念珠菌病患者中,白色念珠菌的分离率为70%-80%[30]。一些关于萜类抗生物膜活性的研究在体外对白色念珠菌进行了测试,其中0.06%的香芹酚对白色念珠菌的抑制率大于80%[30]。AHMAD等[18]发现香芹酚对白色念珠菌具有强烈的生长抑制作用,对氟康唑耐药白色念珠菌具有高度敏感性,形成的抑菌环边缘清晰,表明其具有潜在杀菌活性;流式细胞仪检测出香芹酚可破坏真菌细胞的完整性,且其抗菌效果优于大多数抗真菌药物;另外,还总结了香芹酚对白色念珠菌(对氟康唑敏感/耐药的)麦角甾醇生物合成的影响:在25,50,75 mg/L香芹酚中测定各分离株总麦角甾醇含量,当加入香芹酚的情况下生长时,观察到麦角甾醇产量呈剂量依赖性下降;还发现香芹酚即使在非常高的浓度下对人体红细胞的影响也可忽略不计。此实验证实了香芹酚对一些口腔黏膜疾病的病原菌白色念珠菌的抑菌活性,可以作为预防和治疗白色念珠菌感染的方案。 义齿性口炎是指活动性义齿与接触的口腔腭部、口腔黏膜发生的炎症性损害,是一种影响义齿佩戴者的口腔黏膜疾病[31]。JANJIC-PAVLOVIC等[32]测定了精油对义齿性口炎病原菌金黄色葡萄球菌、变异链球菌、白色念珠菌的最小抑菌浓度,证实了香芹酚对义齿性口炎防治的重要意义。基于此或可将香芹酚成分加入义齿清洁剂、漱口水或软衬材料中,以防治义齿性口炎。BAYGAR等[33]在实验中将香芹酚加入到义齿软衬材料中进行检测,得出结论:香芹酚对酵母菌、革兰阴性菌和革兰阳性菌均有较强的抗菌活性;香芹酚软衬对变形链球菌和白色念珠菌的抑制率最高,分别为(40.33±0.58) mm和(38.33±1.15) mm。香芹酚加入软衬后,白色念珠菌的生物被膜形成率降低。另外,MARCOS-ARIAS等[34]对香芹酚等8种萜类衍生物对白色念珠菌的抗菌活性进行测试,测出最小抑菌浓度值为0.06%-0.5%,MFC90在0.25%-0.5%之间,也肯定了其较强的抗菌力,为香芹酚治疗口腔念珠菌病和念珠菌相关的义齿性口炎的前景提供依据。 2.2.3 牙周疾病/种植体周围炎 牙周和种植体周围疾病是生物膜引起的感染,导致局部和/或系统的炎症反应,并导致牙周或种植体周围组织破坏。现有文献指出牙周病和种植体周围疾病在病因、发病机制、危险因素和临床表现方面具有相似性,并且检测出两种疾病的微生物群类似(革兰阴性厌氧菌)。在牙周炎的情况下,细菌生物膜形成于含羟基磷灰石的牙齿根表面;在种植体周围炎中,其定植于种植体的钛表面。牙周和种植体周围疾病的治疗重点是去除生物膜,除了必要的机械治疗外,通常辅以抗生素或抗菌剂等佐剂来清除细菌生物膜等[35-37]。但是,定期使用这些抗菌药会引起各种不良反应,以及可能发生细菌耐药性和细菌坏死。例如洗必泰(CHX)是一种治疗牙周病、种植体周围炎常用的抗菌剂,能抑制生物膜形成,因其广谱抗菌性和高效性被认为是口腔抗菌剂的“金标准”。根据使用时间和浓度的不同,可能会有一些局部不良反应,包括牙齿变色、味觉改变、黏膜侵蚀以及伤口愈合延迟或受到干扰[38],这限制了洗必泰的长期使用。此外,已报告临床浓度下的洗必泰对不同类型的细胞有毒性作用[39-40]。虽然洗必泰被认为是一种安全的抗菌药,但最近人们对潜在的抗生素耐药性发展的认识有所提高,开发新型抗菌药物成为当前的研究热点,其中药用植物提取物香芹酚的抗炎、抗菌特性是否对牙周病、种植体周围疾病也同样有效而被广泛研究。 MAQUERA HUACHO等[41]测定了香芹酚对人类口腔角质细胞的细胞毒性浓度,研究了无毒浓度下香芹酚对羟基磷灰石和钛表面形成的单一/多种生物膜的抗菌作用,结果见表3。"
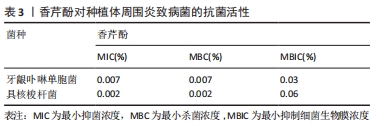

结果表明,0.06%香芹酚对口腔角质细胞无明显毒性作用。香芹酚对单种细菌生物膜(血链球菌、微炎链球菌、口腔链球菌、唾液链球菌、变异链球菌、黏性放线菌、中间普氏杆菌、牙龈卟啉单胞菌、具核梭杆菌、伴放线菌等)的形成均表现出浓度依赖性作用,浓度为0.06%时对单种生物膜的清除效果最强;在多孔羟基磷灰石和钛片上预成多种生物膜,在0.06%的香芹酚中作用24 h后分别检测两个测试表面上预成生物膜中每一种细菌的生存状况,发现无论是羟基磷灰石组还是钛片组,不同种类的相关致病菌的数量均显著减少,有明显抑制作用,且在钛片生物膜上的抗菌作用比在羟基磷灰石上更明显,说明香芹酚可以抑制和根除羟基磷灰石和钛表面上单种和多种生物膜中的牙周炎、种植体周围炎的病原体。 另一方面,BOTELHO等[42]研究了局部使用香芹酚凝胶(CAG)对大鼠实验性牙周病(EPD)的影响,对大鼠进行实验性牙周炎诱导后立即局部应用香芹酚凝胶进行治疗,3次/d,共11 d,运用常规组织病理检查牙周组织及周围牙龈组织;利用原子力显微镜(AFM)研究牙周病原体酸暴露后牙槽骨、牙骨质和胶原纤维表面的形态学变化;髓过氧化物酶活性检测中性粒细胞流入牙龈的情况;通过牙龈组织培养评估细菌区系。结果表明,局部使用质量分数0.5%香芹酚凝胶的大鼠牙周微生物生长显著减少,香芹酚凝胶明显抑制了牙槽骨吸收,减轻了组织病理学损害,保留了牙周组织,降低了牙龈组织中髓过氧化物酶活性,也防止了牙周微生物的增殖和大鼠体质量减轻;香芹酚凝胶治疗抑制了实验性牙周炎的牙槽骨吸收,具有抗炎和抗菌作用,利于牙周愈合。同样地,KUO等[43]也进行了类似的研究,实验过程为将40只大鼠随机分为结扎组、非结扎组和结扎+香芹酚组,从结扎前1 d开始灌胃给予香芹酚,通过牙片、微电脑断层扫描和组织学检查牙周标本的牙槽骨丢失和牙龈炎情况,用RT-PCR和酶谱法检测牙龈组织中肿瘤坏死因子、白细胞介素1β、白细胞介素6和诱导型一氧化氮合酶(iNOS) mRNA的表达以及基质金属蛋白酶2和基质金属蛋白酶9的水平。实验结果:牙片显示结扎组和结扎+香芹酚组的牙周骨支持率均低于非结扎组,其中结扎组最低;与非结扎组相比,结扎组和结扎+香芹酚组牙骨质-牙釉质结合部(CEJ)-骨距离明显延长,而结扎+香芹酚组无论采用何种X射线摄片方法均显示较短的距离;组织学和组织计量学显示结扎+香芹酚组牙槽骨吸收、附着丧失和炎症(ICT区域)持续显著减少,较结扎组炎症面积小、结缔组织附着强;RT-PCR分析结果表明结扎+香芹酚组细胞因子/介质mRNA的表达和基质金属蛋白酶9的水平明显降低,诱导型一氧化氮合酶的表达受到抑制。此结果提示香芹酚可能有助于减轻氧化应激反应,降低全身某些促炎细胞因子的水平,进而发挥免疫调节作用,改善了牙周炎大鼠模型的治疗结果,得出结论:香芹酚可减少实验性牙周炎引起的组织损伤和骨吸收,提示香芹酚可以通过下调促炎递质和基质金属蛋白酶的表达来减轻组织破坏。 但也有效果不尽如人意的实验结果,LAURITANO等[44]在5例慢性牙周炎患者进行机械洁治和根部平整后,让患者回家后自行使用一种新型口腔凝胶(香芹酚和百里香酚的混合物),每位患者选择了不同象限的4个不相邻的部位进行监测,在第1天和第15天进行了关于牙周炎病原菌的微生物分析,所研究的细菌(伴放线杆菌、牙龈卟啉单胞菌、福赛坦氏菌、齿密螺旋体、具核梭杆菌、直肠弯曲菌和总载菌量)虽有绝对下降,但均未达到统计学意义,提示仍需要更多的研究来检测香芹酚凝胶的疗效。CIANDRINI等[11]也证实了香芹酚对口腔常见菌变异链球菌和牙周炎主要致病菌牙龈卟啉单胞菌、具核梭杆菌的抑菌性,测得其最小抑菌浓度、最小杀菌浓度值分别为0.25%,0.5%。将流式细胞仪技术FCM和SYBR-I和PI荧光色素结合起来,评估膜的完整性,观察到3种菌株在暴露于香芹酚5 min后活细胞数均显著下降,直至0-1%;用透射电镜直接观察了细菌超微结构,在暴露于香芹酚的5 min和30 min后,在处理过的细菌中观察到了细胞膜的破裂,胞质和核酸的耗尽。另外,在钛片上预成生物膜进行体外实验,香芹酚与洗必泰相比对钛附着的单/多物种生物膜表现出更强的形成预防作用,证实香芹酚可能通过减少钛种植体表面的细菌生长而用于牙周疾病的预防和治疗[45]。 另一方面,香芹酚的抗菌性能已被证实,但其对骨组织工程的影响知之甚少。VU等[46]经实验证明在特定条件下,香芹酚在聚己内酯/聚乙二醇、羟基磷灰石涂层中均可以完全释放,且可使破骨细胞活性显著降低,说明香芹酚不仅可以防感染,还能够支持骨愈合,增加翻瓣或种植手术的成功率。DEEPAK等[47]发现香芹酚能有效抑制RANKL和细菌内毒素诱导的破骨细胞生成,还可以通过激活caspase-3来诱导成熟破骨细胞凋亡,表明香芹酚对破骨细胞有抑制作用,可能是一种潜在的治疗牙周炎和种植体周围炎的药物。"

| [1] SAKKAS H, PAPADOPOULOU C. Antimicrobial activity of basil, oregano, and thyme essential oils. J Microbiol Biotechnol. 2017;27(3):429-438. [2] PLANT RM, DINH L, ARGO S, et al. The essentials of essential oils. Adv Pediatr. 2019;66:111-122. [3] AHMAD A, ELISHA IL, VAN VUUREN S, et al. Volatile phenolics: A comprehensive review of the anti-infective properties of an important class of essential oil constituents. Phytochemistry. 2021;190:112864. [4] DE OLIVEIRA CARVALHO I, PURGATO GA, PÍCCOLO MS, et al. In vitro anticariogenic and antibiofilm activities of toothpastes formulated with essential oils. Arch Oral Biol. 2020;117:104834. [5] LAURITANO D, BIGNOZZI CA, PAZZI D, et al. Evaluation of the efficacy of a new oral gel as an adjunct to home oral hygiene in the management of chronic periodontitis. A microbiological study using PCR analysis. J Biol Regul Homeost Agents. 2016;30(2):123-28. [6] JAFRI H, ANSARI FA, AHMAD I. Prospects of essential oils in controlling pathogenic biofilm//New look to phytomedicine. Academic Press, 2019: 203-236. [7] FACHINI-QUEIROZ FC, KUMMER R, ESTEVAO-SILVA CF, et al. Effects of thymol and carvacrol, constituents of thymus vulgaris L. essential oil, on the inflammatory response. Evid Based Complement Alternat Med. 2012;2012(11):1-10 [8] BNYAN IA, ABID AT, OBIED HN. Antibacterial activity of carvacrol against different types of bacteria. J Nat Sci Res. 2014;4(9):13-17. [9] ČABARKAPA I, ŠKRINJAR M, LEVIĆ J, et al. Influence of thymol and carvacrol on initial cell attachment and biofilm of Candida albicans. Food Feed Res. 2015;42(1):23-30. [10] EL ABED S, SAAD I, LATRACHE H, et al. Carvacrol and thymol components inhibiting Pseudomonas aeruginosa adherence and biofilm formation. Afr J Microbiol Res. 2011;5:3229-3232. [11] CIANDRINI E, CAMPANA R, FEDERICI S, et al. In vitro activity of Carvacrol against titanium-adherent oral biofilms and planktonic cultures. Clin Oral Investig. 2014;18(8):2001-2013. [12] JAFRI H, KHAN MSA, AHMAD I. In vitro efficacy of eugenol in inhibiting single and mixed-biofilms of drug-resistant strains of Candida albicans and Streptococcus mutans. Phytomedicine. 2019;54:206-213. [13] MAQUERA-HUACHO PM, TONON CC, CORREIA MF, et al. In vitro antibacterial and cytotoxic activities of carvacrol and terpinen-4-ol against biofilm formation on titanium implant surfaces. Biofouling. 2018;34(6):699-709. [14] NAZZARO F, FRATIANNI F, DE MARTINO L, et al. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6(12):1451-1474. [15] HYLDGAARD M, MYGIND T, PIOTROWSKA R, et al. Isoeugenol has a non-disruptive detergent-like mechanism of action. Front Microbiol. 2015;6:754. [16] KACHUR K, SUNTRES Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit Rev Food Sci Nutr. 2020;60(18):3042-3053. [17] GUIMARÃES AC, MEIRELES LM, LEMOS MF, et al. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules. 2019; 24(13):2471. [18] AHMAD A, KHAN A, MANZOOR N, et al. Evolution of ergosterol biosynthesis inhibitors as fungicidal against Candida. Microb Pathog. 2010;48(1):35-41. [19] KHAN A, AHMAD A, AKHTAR F, et al. Induction of oxidative stress as a possible mechanism of the antifungal action of three phenylpropanoids. FEMS Yeast Res. 2011;11(1):114-122. [20] LLANA-RUIZ-CABELLO M, PICHARDO S, MAISANABA S, et al. In vitro toxicological evaluation of essential oils and their main compounds used in active food packaging: A review. Food Chem Toxicol. 2015;81: 9-27. [21] LLANA-RUIZ-CABELLO M, MAISANABA S, PUERTO M, et al. Evaluation of the mutagenicity and genotoxic potential of carvacrol and thymol using the Ames Salmonella test and alkaline, Endo III-and FPG-modified comet assays with the human cell line Caco-2. Food Chem Toxicol. 2014;72:122-128. [22] MAISANABA S, PRIETO AI, PUERTO M, et al. In vitro genotoxicity testing of carvacrol and thymol using the micronucleus and mouse lymphoma assays. Mutat Res. 2015;784:37-44. [23] SUNTRES Z E, COCCIMIGLIO J, ALIPOUR M. The bioactivity and toxicological actions of carvacrol. Crit Rev Food Sci Nutr. 2015;55(3): 304-318. [24] BOTELHO MA, NOGUEIRA NAP, BASTOS GM, et al. Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Braz J Med Biol Res. 2007;40: 349-356. [25] MILADI H, ZMANTAR T, KOUIDHI B, et al. Synergistic effect of eugenol, carvacrol, thymol, p-cymene and γ-terpinene on inhibition of drug resistance and biofilm formation of oral bacteria. Microb Pathog. 2017; 112:156-163. [26] CASTILHO ARF, DUQUE C, KRELING PF, et al. Doxycycline-containing glass ionomer cement for arresting residual caries: an in vitro study and a pilot trial. J Appl Oral Sci. 2018;26. doi: 10.1590/1678-7757-2017-0116 [27] MITTAL S, SONI H, SHARMA DK, et al. Comparative evaluation of the antibacterial and physical properties of conventional glass ionomer cement containing chlorhexidine and antibiotics. J Int Soc Prev Community Dent. 2015;5:268-275. [28] NUNES JMFF, FARIAS IAP, VIEIRA CA, et al. Antimicrobial activity and toxicity of glass ionomer cement containing an essential oil. Braz J Med Biol Res. 2020;53(12):e9468. [29] KHAN ST, KHAN M, AHMAD J, et al. Thymol and carvacrol induce autolysis, stress, growth inhibition and reduce the biofilm formation by Streptococcus mutans. AMB Express. 2017;7(1):49. [30] BAUMGARDNER DJ. Oral fungal microbiota: to thrush and beyond. J Patient Cent Res Rev. 2019;6(4):252. [31] AOUN G, CASSIA A. Evaluation of denture-related factors predisposing to denture stomatitis in a Lebanese population. Mater Sociomed. 2016;28(5):392. [32] JANJIĆ-PAVLOVIĆ O, STANČIĆ I, CICMIL S, et al. The use of essential oils based antiseptic solution in the treatment of denture stomatitis. Stomatološki Glasnik Srbije. 2017;64(1):7-13. [33] BAYGAR T, UGUR A, SARAC N, et al. Functional denture soft liner with antimicrobial and antibiofilm properties. J Dent Sci. 2018;13(3):213-219. [34] MARCOS-ARIAS C, ERASO E, MADARIAGA L, et al.In vitro activities of natural products against oral Candida isolates from denture wearers. BMC Complement Altern Med. 2011;11(1):1-7. [35] SMEETS R, HENNINGSEN A, JUNG O, et al.Definition, etiology, prevention and treatment of peri-implantitis–a review. Head Face Med. 2014;10(1):1-13. [36] FELLMAN M. Pharmacology and periodontal disease: implications and future options. CDHA J. 2010;25(2): 9-11. [37] MAHATO N, WU X, WANG L. Management of peri-implantitis: a systematic review, 2010–2015. Springerplus. 2016;5(1):1-9. [38] SAJJAN P, LAXMINARAYAN N, KAR PP, et al.Chlorhexidine as an antimicrobial agent in dentistry–a review. Oral Health Dent Manag. 2016;15(2):93-100. [39] HERRERO ER, BOON N, PAUWELS M, et al. Necrotrophic growth of periodontopathogens is a novel virulence factor in oral biofilms. Sci Rep. 2017;7(1):1-10. [40] WAND ME, BOCK LJ, BONNEY LC, et al. Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob Agents Chemother. 2017;61(1): e01162-16. [41] MAQUERA HUACHO PM, RODRIGUEZ HERRERO E, VERSPECHT T, et al. Terpinen-4-ol and carvacrol affect multi-species biofilm composition. Biofouling. 2019;35(5):561-572. [42] BOTELHO MA, MARTINS JG, RUELA RS, et al. Protective effect of locally applied carvacrol gel on ligature-induced periodontitis in rats: a tapping mode AFM study. Phytother Res. 2009;23(10):1439-1448. [43] KUO PJ, HUNG TF, LIN CY, et al. Carvacrol ameliorates ligation‐induced periodontitis in rats. J Periodontol. 2017;88(7):e120-e128. [44] LAURITANO D, PAZZI D, IAPICHINO A, et al. Evaluation of the efficacy of a new oral gel containing carvacrol and thymol for home oral care in the management of chronic periodontitis using PCR analysis: a microbiological pilot study. J Biol Regul Homeost Agents. 2016;30(2 Suppl 1):129-134. [45] VERARDI G, CENCI MS, MASKE TT, et al. Antiseptics and microcosm biofilm formation on titanium surfaces. Braz Oral Res. 2016;30:S1806-83242016000100225. [46] VU AA, BOSE S. Natural Antibiotic Oregano in Hydroxyapatite-Coated Titanium Reduces Osteoclastic Bone Resorption for Orthopedic and Dental Applications. ACS Appl Mater Interfaces. 2020;12(47):52383-52392. [47] DEEPAK V, KASONGA A, KRUGER MC, et al. Carvacrol inhibits osteoclastogenesis and negatively regulates the survival of mature osteoclasts. Biol Pharm Bull. 2016;39(7):1150-1158. [48] RASSU G, NIEDDU M, BOSI P, et al. Encapsulation and modified-release of thymol from oral microparticles as adjuvant or substitute to current medications. Phytomedicine. 2014;21(12):1627-1632. [49] CHEN H, ZHANG Y, ZHONG Q. Physical and antimicrobial properties of spray-dried zein–casein nanocapsules with co-encapsulated eugenol and thymol. J Food Eng. 2015;144:93-102. |
| [1] | Tan Xinfang, Guo Yanxing, Qin Xiaofei, Zhang Binqing, Zhao Dongliang, Pan Kunkun, Li Yuzhuo, Chen Haoyu. Effect of uniaxial fatigue exercise on patellofemoral cartilage injury in a rabbit [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(在线): 1-6. |
| [2] | Xu Xinzhong, Wu Zhonghan, Yu Shuisheng, Zhao Yao, Xu Chungui, Zhang Xin, Zheng Meige, Jing Juehua. Biomechanical analysis of different ways of inserting Steinmann Pins into the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1313-1317. |
| [3] | Li Huo, Wang Peng, Gao Jianming, Jiang Haoran, Lu Xiaobo, Peng Jiang. Relationship between revascularization and internal microstructure changes in osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1323-1328. |
| [4] | Yuan Jiabin, Zhu Zongdong, Tang Xiaoming, Wei Dan, Tan Bo, Xiao Chengwei, Zhao Ganlinwei, Liao Feng. Classification and reduction strategies for irreducible intertrochanteric femoral fracture based on anatomy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1341-1345. |
| [5] | Zhuang Zhikun, Wu Rongkai, Lin Hanghui, Gong Zhibing, Zhang Qianjin, Wei Qiushi, Zhang Qingwen, Wu Zhaoke. Application of stable and enhanced lined hip joint system in total hip arthroplasty in elderly patients with femoral neck fractures complicated with hemiplegia [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1429-1433. |
| [6] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [7] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [8] | Wang Xinmin, Liu Fei, Xu Jie, Bai Yuxi, Lü Jian. Core decompression combined with dental pulp stem cells in the treatment of steroid-associated femoral head necrosis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1074-1079. |
| [9] | Li Jie, Zhang Haitao, Chen Jinlun, Ye Pengcheng, Zhang Hua, Zhou Bengen, Zhao Changqing, Sun Youqiang, Chen Jianfa, Xiang Xiaobing, Zeng Yirong. Anterior cruciate ligament rupture and patellofemoral joint stability before sagittal and axial measurement using MRI [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 969-972. |
| [10] | Zhang Xinlong, Ci Wentao, Luo Kaiwen, Yan shi. Internal fixation failure after proximal femoral nail antirotation: causes and reoperation strategies [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 973-979. |
| [11] | Wang Hailong, Li Long, Maihemuti·Yakufu, Chen Hongtao, Liu Xu, Yilihamu·Tuoheti. Finite element analysis of stress distribution of acetabular prosthesis in the Lewinnek safety zone [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 843-847. |
| [12] | Huang Hao, Hong Song, Wa Qingde. Finite element analysis of the effect of femoral component rotation on patellofemoral joint contact pressure in total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 848-852. |
| [13] | Zheng Yongze, Zheng Liqin, He Xingpeng, Chen Xinmin, Li Musheng, Li Pengfei, Lin Ziling. Extended finite element modeling analysis of femoral neck fracture based on ABAQUS software [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 853-857. |
| [14] | He Shiping, Jia Dazhou, Li Xiaolei, Wang Qiang. Establishment of prediction model of blood transfusion after proximal femoral nail anti-rotation fixation of femoral intertrochanteric fracture in elderly adults [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 929-933. |
| [15] | Li Jiajun, Xia Tian, Liu Jiamin, Chen Feng, Chen Haote, Zhuo Yinghong, Wu Weifeng. Molecular mechanism by which icariin regulates osteogenic signaling pathways in the treatment of steroid-induced avascular necrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 780-785. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

