Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (25): 4082-4088.doi: 10.3969/j.issn.2095-4344.1775
Previous Articles Next Articles
Stem cells in the treatment of skin defects: a focus of future research
- Department of Cardiovascular Surgery, Affiliated Yan’an Hospital of Kunming Medical University, Kunming 650051, Yunnan Province, China
-
Revised:2019-03-11Online:2019-09-08Published:2019-09-08 -
Contact:Li Yaxiong, Professor, Department of Cardiovascular Surgery, Affiliated Yan’an Hospital of Kunming Medical University, Kunming 650051, Yunnan Province, China -
About author:Liu Boyu, Master, Department of Cardiovascular Surgery, Affiliated Yan’an Hospital of Kunming Medical University, Kunming 650051, Yunnan Province, China -
Supported by:the Science and Technology Talents and Platform Program of Yunnan Province, No. 2018DG008 (to LYX)
CLC Number:
Cite this article
Liu Boyu, Pu Lei, Zhang Yingjie, Yang Yingnan, Li Yaxiong. Stem cells in the treatment of skin defects: a focus of future research[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(25): 4082-4088.
share this article
2.1 细胞来源 2.1.1 皮肤来源干细胞 表皮包括3部分:毛囊间表皮、皮脂腺和毛囊。毛囊间表皮及皮脂腺不断进行自我更新,而毛囊则是依次经历休眠、生长和退化的周期性变化。在生理条件下,这些表皮成分通过自我更新保持活力。在病理损伤条件下,这些表皮成分又能够相互再生[2]。对于累及皮肤全层的损伤,因毛囊丢失,主要依靠肉芽组织的形成和角化细胞迁移进行修复,从伤口边缘缓慢向中心进行愈合;而对于未累及皮肤全层的损伤,因毛囊尚存,来自毛囊和皮脂腺的细胞迁移使得伤口愈合及再上皮化明显加快[3]。Jiménez等[4]运用自体头皮毛囊移植治疗腿部慢性溃疡,研究显示经过18周治疗后,对照组的溃疡面积较治疗前缩小了6.5%,实验组的溃疡面积较治疗前缩小了27.1%,显著加快了溃疡愈合,并且实验组的溃疡愈合在上皮化、新生血管和修复后的真皮结构等方面均优于对照组,这提示对于慢性皮肤缺损,毛囊移植可作为一种有前景的治疗方式。Navsaria等[5]联合应用毛囊移植及组织工程皮肤治疗全层头皮烧伤的临床研究显示,毛囊移植能够促进皮肤缺损部位再上皮化及创伤愈合,经治疗后愈合处皮肤呈现出正常皮肤的多层结构。毛囊及结缔组织鞘因其易于获得性和含丰富干细胞的特性,成为了再生医学的热点。Ackermann等[6]静脉注射内皮祖细胞至皮肤缺损小鼠,皮肤缺损部位再上皮化更快,新生血管更多并且组织强度更高。很多研究表明内皮祖细胞具有促血管生成的能力,已有一些针对于糖尿病及心脑血管疾病的研究,但关于应用内皮祖细胞治疗皮肤缺损修复的研究较少,仍需进一步探索。此外,对于内皮祖细胞的分离和分类仍然存在争议[7]。De Rosa等[8]运用基因编辑的表皮干细胞治疗大疱性表皮松解症的临床试验显示,表皮干细胞移植不仅能够治疗该疾病,并且治疗的长期稳定性和安全性均较为理想。Wang等[9]联合移植表皮干细胞和内皮祖细胞治疗皮肤缺损小鼠,发现表皮干细胞和内皮祖细胞具有重建毛囊和皮脂腺的作用,并促进皮肤缺损愈合。一些临床研究也显示出表皮干细胞对于多种皮肤疾病均有治疗效果,如烧伤、创伤修复及白癜风等[10]。 2.1.2 胚胎干细胞 胚胎干细胞本质上是存在于囊胚内的多能性细胞。这些细胞有潜力分化成内胚层、中胚层或外胚层。在含有特定生长因子的培养基中,胚胎干细胞可分化为角化上皮细胞,这些角化上皮细胞经过培养能够形成多层表皮结构[11]。因此,该细胞可作为组织工程皮肤构建的种子细胞。Suh等[12]研究发现,小鼠胚胎干细胞能够促进上皮迁移及血管生成进而加速皮肤缺损修复,并且Sonic hedgehog蛋白能加强小鼠胚胎干细胞的这一作用。Uluer等[13]应用诱导胚胎干细胞分化产生的角化上皮细胞治疗皮肤缺损,结果显示接受细胞移植治疗的小鼠皮肤缺损愈合加速,进一步研究表明移植的细胞通过增加白细胞介素8、成纤维细胞生长因子、单核细胞趋化蛋白、Ⅰ型胶原和表皮生长因子的表达在皮肤愈合的炎症期、增殖期和重塑期发挥作用。也有研究发现,胚胎干细胞来源的纳米囊泡能够促进皮肤成纤维细胞增殖,并且促进细胞外基质蛋白以及生长因子的合成和分泌[14]。然而,由于该细胞需从活体胚胎中获取,使用胚胎干细胞在伦理学上存在很大争议。此外,胚胎干细胞还有潜在免疫排斥和致畸胎瘤的可能性。因此,研究重点已经转向成体干细胞,作为胚胎干细胞的替代细胞,成体干细胞有望应用于各种疾病的治疗。 2.1.3 诱导多能干细胞 诱导多能干细胞是被重新编辑到胚胎状态的成熟细胞。这些细胞是通过一组特定的转录因子诱导而产生的,并在胚胎干细胞条件下培养而获得。与胚胎干细胞相比,诱导多能干细胞不仅不涉及伦理问题,而且发生免疫排斥的概率更低[15]。有研究表明,人皮肤成纤维细胞诱导而来的诱导多能干细胞存在可忽略不计的免疫反应[16]。诱导多能干细胞技术具有许多潜在的临床应用价值。细胞可以从患者体内分离出,重新编程为多能状态,分化成所需的组织类型,然后移植到自体以治疗特定疾病。这种干细胞应用方式可以有效地解决应用胚胎干细胞所带来的免疫排斥和伦理问题。 近年来,对于诱导多能干细胞的研究取得了巨大的进展,包括鼠和人的诱导多能干细胞能分化为皮肤干细胞和毛囊细胞系,以及能够诱导形成真皮乳头、成纤维细胞、黑素细胞、角化上皮细胞等[16-21]。诱导多能干细胞的多向分化能力和低免疫反应性使其成为治疗慢性皮肤疾病和难愈性伤口的理想细胞[22]。一些研究已经表明,来自患者自身的诱导多能干细胞可以被修饰,并仍然有潜力能够应用于治疗。Itoh等[23-24]成功从大疱性表皮松解症患者的表皮获取得到诱导多能干细胞,并进一步将细胞诱导分化为角化上皮细胞。Sebastiano等[24]将大疱性表皮松解症患者来源的诱导多能干细胞进行基因编辑,纠正COL7A1基因突变后的诱导多能干细胞再诱导分化为角化上皮细胞,得到的角化上皮细胞能够分泌Ⅳ型胶原,克服了患者固有角化上皮细胞不能分泌Ⅳ型胶原的缺点,这种来源于患者自身、具有功能性的角化上皮细胞有望成为大疱性表皮松解症的全新治疗手段。诱导多能干细胞在皮肤缺损修复和再生医学领域有着广阔的应用前景。然而,诱导多能干细胞存在致畸胎瘤以及突变等可能[25],应用其治疗人类疾病需要进一步研究以确保该技术的安全和可靠。 2.1.4 间充质干细胞 间充质干细胞是具有自我更新和多向分化潜能的成体干细胞[26]。间充质干细胞可以从骨髓、脂肪、脐血、羊水等组织中获取,并能够分化为骨、软骨、肌肉、脂肪等组织[27-29]。不同于胚胎干细胞,间充质干细胞的应用不涉及伦理问题。此外,同种异体间充质干细胞移植产生的免疫排斥反应很小[30]。这些特性使得间充质干细胞在伤口修复中的应用得到广泛关注。间充质干细胞已被用于各种急、慢性皮肤缺损的修复和再生,如急性皮肤创伤、糖尿病皮肤溃疡、辐射损伤和烧伤等[31-32]。伤口愈合过程中产生的炎症反应和氧化应激不仅在伤口局部募集骨髓间充质干细胞[33],更通过促进细胞分化及血管生成来促进伤口愈合。有研究表明,间充质干细胞通过促进血管生成、再上皮化和肉芽组织形成来促进伤口愈合[34-35]。Badiavas等[36]针对1年以上未愈合的慢性皮肤缺损患者外用骨髓间充质干细胞治疗,伤口在短期内出现改善,创口面积明显减小,真皮血管分布增加,创口处真皮厚度增加,并最终促进皮肤缺损愈合。Dash等[37]对难愈合性下肢溃疡外用骨髓间充质干细胞治疗12周后,治疗组相比对照组无论是溃疡面积还是患者症状都有明显改善,并且组织活检提示治疗组患者溃疡愈合更佳。Sarasúa等[38]运用自体骨髓间充质干细胞治疗压疮,接受治疗的22例患者中最终19例患者痊愈。这些研究结果均表明间充质干细胞能够促进伤口修复,并可作为再生医学的理想资源。 2.2 干细胞促进皮肤缺损修复机制 2.2.1 免疫调节 炎症反应失调导致皮肤缺损难以愈合,持续存在的炎症反应导致慢性皮肤缺损的产生以及纤维化瘢痕的形成。在慢性皮肤缺损中,炎症反应导致蛋白酶活性增加和成纤维细胞活性失调,进而导致胶原沉积和细胞外基质形成减少。特别对于糖尿病皮肤缺损来说,治疗重点是调节炎症反应。近年来,很多研究发现了脐血、羊水和骨髓等来源的间充质干细胞具有免疫调节功能,有利于皮肤缺损的修复。 大量研究表明,间充质干细胞在治疗皮肤缺损愈合和其他炎症反应中具有显著的免疫调节作用,间充质干细胞可通过介导伤口局部的免疫调节来促进皮肤缺损修复,并能够减少瘢痕形成[39-40],见图2。移植人脐带间充质干细胞治疗小鼠烧伤皮肤缺损的实验表明,脐带间充质干细胞能够减少促炎细胞因子如白细胞介素1和肿瘤坏死因子α等分泌并显著降低炎症细胞数量以促进伤口愈合[41]。Smith等[42-43]将小鼠骨髓间充质干细胞与人成纤维细胞共培养,发现成纤维细胞增殖及迁移能力增强,并且细胞黏附分子1及血管细胞黏附分子1的mRNA水平下降,提示骨髓间充质干细胞通过免疫调节作用调控成纤维细胞进而发挥促伤口愈合作用。这些研究均表明了间充质干细胞在炎症期能起到减轻炎症反应并促进愈合的能力,这种对宿主的免疫调节作用不仅使得间充质干细胞成为同种异体移植的理想细胞,更使其成为治疗慢性皮肤缺损的理想资源。"
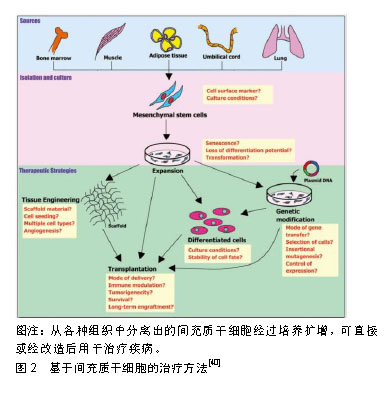
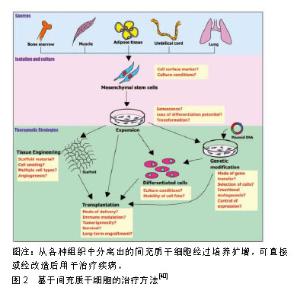
抗菌活性对于避免皮肤缺损处感染十分关键。有研究表明,干细胞具有抗菌活性,这也可能有助于慢性皮肤缺损的愈合。Krasnodembskaya等[43]研究发现,骨髓间充质干细胞可直接分泌抗菌肽hCAP-18/LL-37并显著抑制细菌生长。Mei等[44]运用间充质干细胞治疗菌血症小鼠的研究显示,间充质干细胞可通过上调抗原提呈、吞噬和杀伤相关基因来提高机体免疫系统对细菌的吞噬和杀伤能力,以此提高存活率。间充质干细胞发挥抗菌活性的具体机制仍有待进一步探索。组织局部和外周血中的巨噬细胞在皮肤损伤后的炎症启动和损伤愈合过程中起着重要作用。巨噬细胞在促炎性M1型和抗炎性M2型细胞之间转化。有研究表明,骨髓间充质干细胞与巨噬细胞接触后可能促使巨噬细胞由M1型向M2型转化[45]。在小鼠伤口愈合模型中,人牙龈来源间充质干细胞能够与巨噬细胞紧密结合并促进巨噬细胞向M2型极化,进而发挥抗炎特性并显著加速伤口的愈合[46]。M2型巨噬细胞有消炎和清除伤口坏死细胞的作用,间充质干细胞具有的促进M1型向M2型极化的特性对于治疗慢性难愈性伤口亦十分重要。此外,细胞外基质过度沉积可引起纤维化或瘢痕化失调。炎症反应也在很大程度上能够调节纤维化过程。间充质干细胞所具备的免疫调节能力可通过调节纤维化过程,进而减少瘢痕形成。在小鼠皮肤损伤模型中,移植骨髓间充质干细胞可有效减轻伤口愈合过程中的纤维化,并有利于细胞外基质的重塑[47]。 2.2.2 分化 在伤口愈合过程中,皮肤来源干细胞在各种刺激下进行分化[48-50]。Sasaki等[48]将骨髓间充质干细胞静脉注射到皮肤缺损小鼠体内,发现骨髓间充质干细胞可以分化成角化上皮细胞、内皮细胞和周细胞。Altman等[51]发现脂肪间充质干细胞可分化为角化上皮细胞、内皮细胞和成纤维细胞。 2.2.3 血管生成 功能性血管生成是伤口愈合的关键因素之一。一些研究已经证明,移植的成体干细胞通过直接分化成所需要的细胞类型,或以旁分泌方式作用于内源性介质或细胞来促进血管生成[34-35]。有研究发现,间充质干细胞通过旁分泌作用以及释放可溶性细胞因子(如血管内皮生长因子等)促进皮肤损伤后的血管生 成[52]。诱导多能干细胞来源的细胞外囊泡具有促进血管生成作用[53]。Crisan等[54]猜测间充质干细胞可能作为周细胞以支持血管的构建。Kwon等[55]运用骨髓间充质干细胞治疗糖尿病小鼠创伤愈合模型,结果显示骨髓间充质干细胞可促进创伤部位新生血管的形成,并且小鼠高表达血管内皮生长因子等细胞因子。尽管干细胞促血管生成的具体机制有待进一步研究,但众多研究均已证实干细胞具有促血管生成作用。 2.3 细胞选择 选择合适的细胞种类是应用干细胞治疗皮肤缺损的关键,不同来源的干细胞具有不同的优势与不足,见表1。"
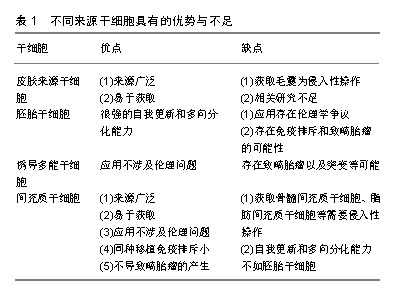
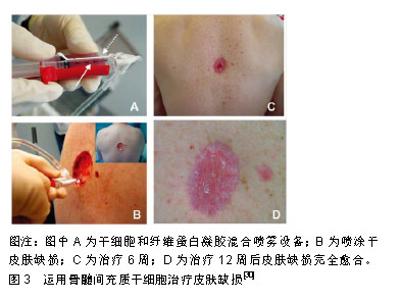
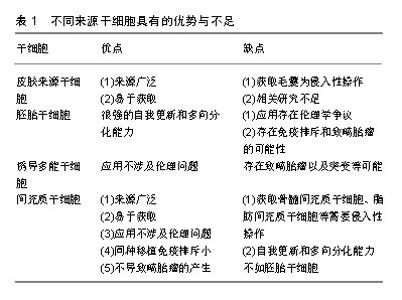
对于皮肤来源干细胞,因毛囊易获得性,毛囊移植成为了有前景的治疗方式,但获取毛囊仍然是有创性操作,会给供者带来一定伤害。有关应用内皮祖细胞和表皮干细胞治疗皮肤缺损的研究较少,仍需进一步研究验证其功能及机制。对于胚胎干细胞,虽然其具有很强的自我更新和多向分化能力,但应用该细胞存在很大的伦理学争议,并存在免疫排斥和致畸胎瘤的可能性。诱导多能干细胞虽然能够避免伦理问题,但仍然存在致畸胎瘤以及突变等可能,安全性有待更多研究来验证。相比其他种类的干细胞,间充质干细胞具有来源广泛、易于获取、应用不涉及伦理问题、同种移植免疫排斥很小及不会致畸胎瘤等优势。相比起脂肪、骨髓、外周血等来源的间充质干细胞,脐带间充质干细胞具有更佳的增殖分化能力、低免疫原性、获取无需侵入性操作等优点[56],因此作者认为脐带间充质干细胞可作为皮肤缺损修复的良好细胞来源。但不同来源的干细胞在治疗不同种类皮肤缺损上有各自优势,可联合多种细胞治疗以形成优势互补。例如治疗烧伤皮肤缺损,可联合应用毛囊移植和间充质干细胞移植,以获得更佳的再上皮化和血管生成;治疗大疱性表皮松解症,可联合应用基因编辑的诱导多能干细胞及间充质干细胞,诱导多能干细胞克服了患者固有角化上皮细胞缺陷的同时,间充质干细胞发挥免疫调节及促血管生成作用促进皮肤缺损修复。 2.4 应用途径 干细胞移植治疗皮肤缺损的途径有很多,大部分以注射方式为主[38,48,55,57]。还有外用涂抹等方式,如Dash等[37]在皮肤创面区域涂抹活细胞并加以包扎;Edwards等[58]将脂肪间充质干细胞种植到胶原纤维基质上,再将胶原纤维基质覆盖于创面。还有临床试验运用混合骨髓间充质干细胞和纤维蛋白凝胶制作而成的喷雾剂,局部喷涂于皮肤缺损部位[30],见图3。尽管众多研究结果均提示运用上述途径能够发挥干细胞促进皮肤缺损的作用,然而也有一些研究表明其疗效并不十分理想,因为无论是局部注射、静脉注射或是外用涂抹干细胞,最终存活的干细胞数量有限[59],导致治疗效果不理想。在干细胞治疗皮肤缺损修复中,主要通过旁分泌发挥作用[60],有研究表明,应用干细胞条件性培养基治疗也能发挥干细胞的治疗效果[61-62]。干细胞条件性培养基富含干细胞分泌的各种细胞因子,可大量生产,易于储存,可采用滤过的方式来灭菌,治疗前无需复苏及扩增细胞,这些优势使其应用更广阔[63]。相对于直接应用干细胞,外用干细胞条件性培养基是更好的选择。"

| [1]Hanson SE, Bentz ML, Hematti P. Mesenchymal stem cell therapy for nonhealing cutaneous wounds. Plast Reconstr Surg. 2010;125(2):510-516.[2]Levy V, Lindon C, Zheng Y, et al. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21(7):1358-1366.[3]Langton AK, Herrick SE, Headon DJ. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol. 2008;128(5):1311-1318.[4]Jiménez F, Garde C, Poblet E, et al. A pilot clinical study of hair grafting in chronic leg ulcers. Wound Repair Regen. 2012;20(6):806-814.[5]Navsaria HA, Ojeh NO, Moiemen N, et al. Reepithelialization of a full-thickness burn from stem cells of hair follicles micrografted into a tissue-engineered dermal template (Integra). Plast Reconstr Surg. 2004;113(3):978-981.[6]Ackermann M, Pabst AM, Houdek JP, et al. Priming with proangiogenic growth factors and endothelial progenitor cells improves revascularization in linear diabetic wounds. Int J Mol Med. 2014;33(4):833-839.[7]Chopra H, Hung MK, Kwong DL, et al. Insights into Endothelial Progenitor Cells: Origin, Classification, Potentials, and Prospects. Stem Cells Int. 2018;2018:9847015.[8]De Rosa L, Carulli S, Cocchiarella F, et al. Long-term stability and safety of transgenic cultured epidermal stem cells in gene therapy of junctional epidermolysis bullosa. Stem Cell Reports. 2013;2(1):1-8.[9]Wang X, Wang X, Liu J, et al. Hair Follicle and Sebaceous Gland De Novo Regeneration With Cultured Epidermal Stem Cells and Skin-Derived Precursors. Stem Cells Transl Med. 2016;5(12):1695-1706.[10]Jackson CJ, Tønseth KA, Utheim TP. Cultured epidermal stem cells in regenerative medicine. Stem Cell Res Ther. 2017;8(1):155.[11]Aberdam D. Derivation of keratinocyte progenitor cells and skin formation from embryonic stem cells. Int J Dev Biol. 2004;48(2-3):203-206.[12]Suh HN, Han HJ. Sonic hedgehog increases the skin wound-healing ability of mouse embryonic stem cells through the microRNA 200 family. Br J Pharmacol. 2015;172(3):815-828.[13]Uluer ET, Vatansever HS, Aydede H, et al. Keratinocytes derived from embryonic stem cells induce wound healing in mice. Biotech Histochem. 2018:1-10.[14]Jeong D, Jo W, Yoon J, et al. Nanovesicles engineered from ES cells for enhanced cell proliferation. Biomaterials. 2014; 35(34):9302-9310.[15]Guha P, Morgan JW, Mostoslavsky G, et al. Lack of Immune Response to Differentiated Cells Derived from Syngeneic Induced Pluripotent Stem Cells. Cell Stem Cell. 2017;21(1): 144-148.[16]Lu Q, Yu M, Shen C, et al. Negligible immunogenicity of induced pluripotent stem cells derived from human skin fibroblasts. PLoS One. 2014;9(12):e114949.[17]Sugiyama-Nakagiri Y, Fujimura T, Moriwaki S. Induction of Skin-Derived Precursor Cells from Human Induced Pluripotent Stem Cells. PLoS One. 2016;11(12):e0168451.[18]Veraitch O, Mabuchi Y, Matsuzaki Y, et al. Induction of hair follicle dermal papilla cell properties in human induced pluripotent stem cell-derived multipotent LNGFR(+)THY-1(+) mesenchymal cells. Sci Rep. 2017;7:42777. [19]Hewitt KJ, Shamis Y, Hayman RB, et al. Epigenetic and phenotypic profile of fibroblasts derived from induced pluripotent stem cells. PLoS One. 2011;6(2):e17128.[20]Ohta S, Imaizumi Y, Akamatsu W, et al. Generation of human melanocytes from induced pluripotent stem cells. Methods Mol Biol. 2013;989:193-215.[21]Bilousova G, Chen J, Roop DR. Differentiation of mouse induced pluripotent stem cells into a multipotent keratinocyte lineage. J Invest Dermatol. 2011;131(4):857-864.[22]Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49.[23]Itoh M, Kiuru M, Cairo MS, et al. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2011;108(21):8797-8802.[24]Sebastiano V, Zhen HH, Haddad B, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6(264):264ra163.[25]Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313-317.[26]Nakagawa H, Akita S, Fukui M, et al. Human mesenchymal stem cells successfully improve skin-substitute wound healing. Br J Dermatol. 2005;153(1):29-36.[27]Hoogduijn MJ, Crop MJ, Peeters AM, et al. Human heart, spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacities. Stem Cells Dev. 2007;16(4):597-604.[28]Yen BL, Huang HI, Chien CC, et al. Isolation of multipotent cells from human term placenta. Stem Cells. 2005;23(1):3-9.[29]Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22(7):1330-1337.[30]Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13(6):1299-1312.[31]Dai Y, Li J, Li J, et al. Skin epithelial cells in mice from umbilical cord blood mesenchymal stem cells. Burns. 2007;33(4):418-428.[32]Lataillade JJ, Doucet C, Bey E, et al. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Med. 2007;2(5):785-794.[33]Grayson WL, Zhao F, Bunnell B, et al. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358(3): 948-953.[34]Kanji S, Das M, Aggarwal R, et al. Nanofiber-expanded human umbilical cord blood-derived CD34(+) cell therapy accelerates cutaneous wound closure in NOD/SCID mice. J Cell Mol Med. 2014;18(4):685-697.[35]Kanji S, Das M, Aggarwal R, et al. Nanofiber-expanded human umbilical cord blood-derived CD34+ cell therapy accelerates murine cutaneous wound closure by attenuating pro-inflammatory factors and secreting IL-10. Stem Cell Res. 2014;12(1):275-288.[36]Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139(4): 510-516.[37]Dash NR, Dash SN, Routray P, et al. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009;12(5):359-366.[38]Sarasúa JG, López SP, Viejo MA, et al. Treatment of pressure ulcers with autologous bone marrow nuclear cells in patients with spinal cord injury. J Spinal Cord Med. 2011;34(3): 301-307.[39]Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis. 2014;10(1):29-37.[40]Satija NK, Singh VK, Verma YK, et al. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med. 2009;13(11-12):4385-4402.[41]Liu L, Yu Y, Hou Y, et al. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS One. 2014;9(2):e88348.[42]Smith AN, Willis E, Chan VT, et al. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res. 2010;316(1):48-54.[43]Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28(12):2229-2238.[44]Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182(8):1047-1057.[45]Cho DI, Kim MR, Jeong HY, et al. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70.[46]Zhang QZ, Su WR, Shi SH, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28(10):1856-1868.[47]Wu Y, Huang S, Enhe J, et al. Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice. Int Wound J. 2014;11(6):701-710.[48]Sasaki M, Abe R, Fujita Y, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180(4):2581-2587.[49]Kumamoto T, Shalhevet D, Matsue H, et al. Hair follicles serve as local reservoirs of skin mast cell precursors. Blood. 2003;102(5):1654-1660.[50]Lako M, Armstrong L, Cairns PM, et al. Hair follicle dermal cells repopulate the mouse haematopoietic system. J Cell Sci. 2002;115(Pt 20):3967-3974.[51]Altman AM, Matthias N, Yan Y, et al. Dermal matrix as a carrier for in vivo delivery of human adipose-derived stem cells. Biomaterials. 2008;29(10):1431-1442.[52]Gnecchi M, Zhang Z, Ni A, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11): 1204-1219.[53]Dougherty JA, Kumar N, Noor M, et al. Extracellular Vesicles Released by Human Induced-Pluripotent Stem Cell-Derived Cardiomyocytes Promote Angiogenesis. Front Physiol. 2018;9:1794.[54]Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301-313.[55]Kwon DS, Gao X, Liu YB, et al. Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. Int Wound J. 2008;5(3):453-463.[56]Wang Q, Yang Q, Wang Z, et al. Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton's jelly as sources of cell immunomodulatory therapy. Hum Vaccin Immunother. 2016;12(1):85-96.[57]Wu Y, Chen L, Scott PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648-2659.[58]Edwards N, Feliers D, Zhao Q, et al. An electrochemically deposited collagen wound matrix combined with adipose-derived stem cells improves cutaneous wound healing in a mouse model of type 2 diabetes. J Biomater Appl. 2018;33(4):553-565.[59]Samsonraj RM, Raghunath M, Nurcombe V, et al. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl Med. 2017;6(12):2173-2185.[60]Shohara R, Yamamoto A, Takikawa S, et al. Mesenchymal stromal cells of human umbilical cord Wharton's jelly accelerate wound healing by paracrine mechanisms. Cytotherapy. 2012;14(10):1171-1181.[61]Arno AI, Amini-Nik S, Blit PH, et al. Human Wharton's jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res Ther. 2014;5(1):28.[62]Chen L, Xu Y, Zhao J, et al. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One. 2014;9(4): e96161.[63]Kordelas L, Rebmann V, Ludwig AK, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970-973. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [4] | Liu Cong, Liu Su. Molecular mechanism of miR-17-5p regulation of hypoxia inducible factor-1α mediated adipocyte differentiation and angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1069-1074. |
| [5] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| [6] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [7] | Liao Chengcheng, An Jiaxing, Tan Zhangxue, Wang Qian, Liu Jianguo. Therapeutic target and application prospects of oral squamous cell carcinoma stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1096-1103. |
| [8] | Xie Wenjia, Xia Tianjiao, Zhou Qingyun, Liu Yujia, Gu Xiaoping. Role of microglia-mediated neuronal injury in neurodegenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1109-1115. |
| [9] | Li Shanshan, Guo Xiaoxiao, You Ran, Yang Xiufen, Zhao Lu, Chen Xi, Wang Yanling. Photoreceptor cell replacement therapy for retinal degeneration diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1116-1121. |
| [10] | Jiao Hui, Zhang Yining, Song Yuqing, Lin Yu, Wang Xiuli. Advances in research and application of breast cancer organoids [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1122-1128. |
| [11] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [12] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [13] | Kong Desheng, He Jingjing, Feng Baofeng, Guo Ruiyun, Asiamah Ernest Amponsah, Lü Fei, Zhang Shuhan, Zhang Xiaolin, Ma Jun, Cui Huixian. Efficacy of mesenchymal stem cells in the spinal cord injury of large animal models: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1142-1148. |
| [14] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [15] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||