Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (27): 4403-4408.doi: 10.3969/j.issn.2095-4344.2017.27.024
Previous Articles Next Articles
Application of autologous platelet-rich plasma in spinal surgeries
Guo Ying1, 2, Jia Lian-shun1, Huang Zhi2
- 1Department of Spinal Surgery, Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; 2Department of Orthopedics, No.187 Hospital of Chinese PLA, Haikou 571159, Hainan Province, China
-
Online:2017-09-28Published:2017-10-24 -
Contact:Jia Lian-shun, Chief physician, Department of Spinal Surgery, Changzheng Hospital, Second Military Medical University, Shanghai 200003, China -
About author:Guo Ying, Studying for doctorate, Attending physician, Department of Spinal Surgery, Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Orthopedics, No.187 Hospital of Chinese PLA, Haikou 571159, Hainan Province, China -
Supported by:the Natural Science Foundation of Hainan Province, No. 20168345; the Medical and Health Science and Technology Project of Hainan Province, No. 1440320.27A2004
CLC Number:
Cite this article
Guo Ying, Jia Lian-shun, Huang Zhi. Application of autologous platelet-rich plasma in spinal surgeries[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(27): 4403-4408.
share this article
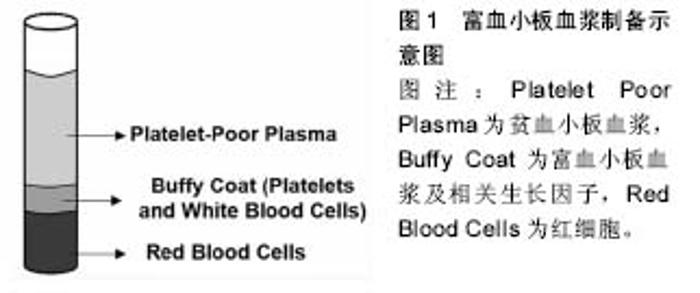
2.1 富血小板血浆的制备方法及成品性能 富血小板血浆最经典的制备方法是二次离心法,随着临床广泛应用及研究深入,出现了专门制备富血小板血浆的设备,据统计目前用近40种商用富血小板血浆制备系统[3],因为制备工艺不同,最终形成的富血小板血浆成品也多种多样,这些成品中富含生长因子及其他生物活性因子的差异也是文献报道富血小板血浆应用效果不一的重要原因。有学者通过动物实验发现商用富血小板血浆制备能比传统的离心法获得更高的血小板和白细胞浓度,也能获得更多的生长因子[4],在临床应用中富血小板血浆成品中相关的生长因子及其他内容物也受患者年龄、循环系统及合并疾病等因素的影响[5]。另外一个影响富血小板血浆作用的重要原因为富血小板血浆的最终物理形态。目前临床使用的富血小板血浆由从液态到固态凝胶的多种形态,以满足不同手术方式需要。新鲜制备的富血小板血浆最终作用形态主要为凝胶(图1)。富血小板血浆凝胶的形成受其激活方式的影响,同时与血小板计数与血小板源性生长因子释放动力相关。在富血小板血浆成品中血小板的浓度与制备时全血的量,血小板悬浮的血浆容量等有关[6],据估计其与初始全血量的容量比在10%-16%之间[7]。Cavallo等[8]研究发现,混合氯化钙后富血小板血浆形成凝块的时间是30 min,而混合凝血酶或凝血酶/氯化钙后凝块时间为15 min,而混合有Ⅰ型胶原蛋白在24 h内均无凝块形成。但Fufa等[9]则发现混合胶原蛋白的富血小板血浆也能形成凝块,虽然远比混有凝血酶的凝块疏松。这可能与实验条件,比如胶原蛋白类型以及富血小板血浆制备方法等不同有关。除此之外可能影响富血小板血浆生物活性的关键步骤是血小板的激活。在制备富血小板血浆的过程当中血小板的激活有两个过程组成,一是血小板α-颗粒脱颗粒以释放生长因子,二是纤维蛋白原分裂开始形成基质,即允许血小板凝胶形成的过程,从而将分子的分泌限制于所选择的位点[10]。 大量基础研究证实富血小板血浆的质量跟其释放生长因子的数量与速度,以及使用时的物理形态有明确相关。而临床使用还与其对治疗区域的亲和力以及使用的剂量、使用的时效性有关。富血小板血浆添加抗凝剂后能保持稳定达8 h甚至更长[11]。Moore等[12]发现虽然放置后血小板的完整性及血小板源性生长因子BB浓度下降,但通过不透氧保存并间歇性摇动容器可以重新获得血小板的完整性及功能,以及血小板源性生长因子BB的释放,通过此种保存可供临床5-8 h内使用。为了延长富血小板血浆的最佳作用时间,有些学者开始将富血小板血浆制成冻干粉并对其性能进行相关研究。Nakatani等[13]观察发现冻干富血小板血浆与蒸馏水按1∶1再水化后(x1FD-富血小板血浆)释放的生长因子与新鲜富血小板血浆相当,而与1/3的蒸馏水再水化后(x3FD-富血小板血浆)生长因子的浓度比新鲜制备的富血小板血浆高3倍,体内研究也证实x3FD-富血小板血浆 能诱导更多的骨生成,Shiga等[14]通过体内研究发现冻干富血小板血浆能以与新鲜富血小板血浆及骨形态发生蛋白相同的速度加速植骨融合,同时通过塑形使融合骨更厚、更坚实。"
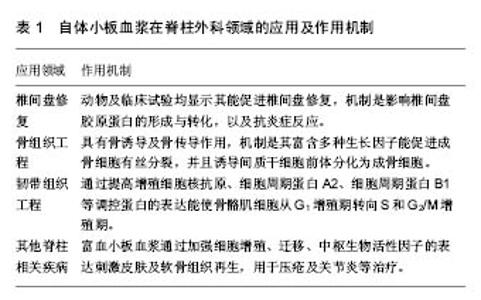
2.2 富血小板血浆在修复椎间盘退变中的作用研究 目前富血小板血浆在脊柱外科领域研究最为广泛的是富血小板血浆对退变椎间盘的作用研究,见表1。椎间盘退变引起的盘源性腰痛是腰背部疼痛的主要原因,跟年龄增大、腰椎负荷大、基因以及日常活动等相关。椎间盘退变后椎间盘内流体静力气压(Hydrostatic pressure)降低,诱导蛋白聚糖和和胶原蛋白发生改变,从而降低椎间盘载荷并加速退变[15]。有学者研究指出富血小板血浆通过促进聚集蛋白聚糖(aggrecan)和胶原蛋白(collagen)的合成,以及直接刺激骨髓基质细胞分化为成熟的椎间盘细胞,聚集蛋白聚糖和胶原蛋白是椎间盘细胞外基质的两种主要成分,同时作为主要的蛋白聚糖,聚集蛋白聚糖能促进水分的吸收和椎间盘的水合作用,从而延缓椎间盘脱水等退变表现;胶原蛋白则是将软组织锚定于骨组织之上,起抗牵张的作用[16]。"


富血小板血浆中的生长因子因促进其合成而保持椎间盘的功能,其中转化生长因子β1能促进人椎间盘纤维环细胞增殖,胰岛素样生长因子1能显著抑制人椎间盘纤维环细胞凋亡[17]。Cho等通过使用从猪退变的椎间盘分离纤维环细胞,经过肿瘤坏死因子处理后放入富有生长因子的富血小板血浆中生长,能促进蛋白聚糖和Ⅱ型胶原蛋白的形成并降低抑制性胶原酶,基质金属蛋白酶1的形成[18]。Khalaf等[19]用逆有限元方法(inverse finite element approach)通过对完整、变性和经过富血小板血浆处理的椎间盘进行材料性能对比分析后显示富血小板血浆能使变性的椎间盘生物力学性能得到恢复。 已有一些动物实验证实通过单纯进行富血小板血浆椎间盘内注射对修复椎间盘退变有效,其作用机制是影响椎间盘胶原蛋白的形成与转化。Obata等[20]与Cui等[21]先后通过建立兔的盘源性腰痛模型,证实经富血小板血浆注射后恢复了椎间盘的高度和髓核信号强度,同时蛋白聚糖和Ⅱ型胶原蛋白表达增加。组织学观察椎间盘组织接近正常。Gullung等[22]将富血小板血浆注入大鼠腰椎退变性椎间盘内,通过设置不同时间点观察,发现富血小板血浆对于椎间盘早期与晚期严重退变均有很好的效果,但愈早使用,效果愈好。 另一个富血小板血浆对于退变椎间盘有修复作用的机制是其抗炎症反应。退变的椎间盘组织中有多种炎症调节因子,包括一氧化氮、白细胞介素1、基质金属蛋白酶类、前列腺素E2、肿瘤坏死因子α等。Bendinelli等[23]认为肝细胞生长因子的表达使富血小板血浆具有抗炎作用。Kim等[24]也通过体外实验发现富血小板血浆通过抑制两种炎症前体细胞因子,白细胞介素1和肿瘤坏死因子α的释放,从而维持髓核细胞微环境的动态平衡,从而延缓椎间盘的退变。 富血小板血浆椎间盘内注射修复退变椎间盘已进入临床研究阶段。Tuakli-Wosornu等[25]做了一项前瞻性随机双盲对照临床研究,他们选取了47例经过至少6个月治疗无效的盘源性腰痛患者,在椎间盘造影下疼痛激发试验阳性后,随机对29例患者进行单纯富血小板血浆注射并与只行造影剂的另一组18例患者对比,结果发现随访至第8周时,治疗组的功能及疼痛评分、患者满意度3项指标均有显著改善,而随访到1年时,治疗组的功能评分仍较对照组显著提高。但作者在文章中并未进行影像及其他客观指标评价,且样本较小,只有1年的随访期。Levi等[26]1篇临床报道中对22例盘源性腰痛患者行椎间盘富血小板血浆注射随访6个月得出类似结论。最近,Bhatia等[27]更是将富血小板血浆用于治疗腰椎退变引起的根性痛,他们对10例根性症状的腰椎疾病患者在内镜辅助下经腰后椎板间向受压神经根节段硬膜外注射5 mL富血小板血浆,结果所有患者在3个月内疼痛均有不同程度减轻,且无并发症发生。 同时也有学者开始将富血小板血浆用于与其他生物材料进行复合。Nagae等[28]将富血小板血浆浸渍于明胶水凝胶微球中,然后注入退变椎间盘的髓核中,它们发现明胶水凝胶微球理化上能固定富血小板血浆中的生长因子,通过微球的降解而达到缓慢稳定的释放。通过设置胶水凝胶微球与PBS复合物组、纯富血小板血浆组及假手术组进行对照研究,8周时组织学观察对照组与空白组间盘退变明显进展,相反实验组这种进展得到了显著的抑制。免疫组织化学发现在施用经富血小板血浆浸渍的微球体8周后观察到髓核和纤维环内层中蛋白多糖的强烈免疫染色。几乎所有的微球在注射后8周均已模糊,同时没有副反应,证实了该复合物在治疗椎间盘退变的可行性。随后该团队在兔子活体实验中再次得出相同结论,富血小板血浆-明胶水凝胶微球组蛋白聚糖核心蛋白和Ⅱ型胶原mRNA表达比其他组显著增高,同时髓核和纤维环中的调亡细胞也比其化组低很多,虽然增殖细胞各组间无差异[29]。骨形态发生蛋白2可促进骨髓间充质干细胞分化、软骨形成,Hou等[30]通过体内外实验发现。经过骨形态发生蛋白2转导骨髓间充质干细胞(术后12周出现)处理的椎间盘能更好的保护髓核结构,同时骨形态发生蛋白2转导的骨髓间充质干细胞在富血小板血浆凝胶中能保持软骨细胞样表型,骨形态发生蛋白2与富血小板血浆复合使用能显著促进椎间盘的修复。 即使大部分实验及临床研究均肯定了富血小板修复椎间盘价值,仍有部分研究存在不同意见。Mietsch等[31]通过体外实验认为富血小板血浆作为生长因子混合物,只促进细胞增殖而不能促进细胞成软骨分化,部分临床研究疗效标准为疼痛症状和局部功能改善,而没有充分证实其形态学和病理组织学的改变。 2.3 富血小板血浆在骨组织工程方面的研究 骨性组织形成始于纤维蛋白凝块形成开始、血小板聚集及脱颗粒化这个过程。血小板颗粒包含一系列的生物活性物质,像儿茶酚类、血清素、纤维蛋白原、骨结合素、骨钙素、三磷酸腺苷,以及凝血因子、生长因子等。在脊柱退行性变疾病的外科手术中,关节融合术是脊柱固定的最终目标。融合是指在移植物与其所接触的骨之间没有进行性的相对运动,即达到骨整合。骨整合是骨诱导及骨传导两个阶段完成的结果。骨诱导是指诱导骨形成的过程,骨传导是指在介质表面的骨生长。骨诱导是正常骨愈合过程的一部分,是新生骨的主要原因,骨传导发生在骨的塑形过程中,不但受生物学的影像,也对外来材料产生发应。融合的成功率与移植物的选择密切相关。自体髂骨移植是骨移植的金标准,经过长时间期的临床应用能获得满意的融合率,但同时也带来了受限于取材、植骨不充分;以及取骨处疼痛、增加手术时间和术中出血量,延长住院时间等负面结果。目的为代替血凝块的富血小板产品一旦被激活,分泌出大量的蛋白及生长因子,包括血小板源性生长因子、表皮生长因子、转化生长因子β、血管内皮生长因子、胰岛素样生长因子1等,这些生长因子能促进成骨细胞(osteoblasts)有丝分裂,并且诱导间质干细胞前体分化为成骨细胞。同时,富血小板血浆能促进白细胞趋化从而具有有抗菌作用,同时促进组织修复与塑形。血小板数量与生长因子浓度之间的关系目前尚不明确。同时有学者认为血小板生长因子种类很多,因人而异,与血小板计数不成比例。 关于富血小板血浆的骨诱导及骨传导研究首先且主要集中在口腔和额面外科领域,关于其在脊柱融合方面的基础及临床研究较少且效果不一,见表1。Tarantino等[32]通过回顾性研究发现富血小板血浆混合人工松质骨可以增加腰椎术后的融合率和融合处的骨密度,且骨密度与全血血小板计数没有相关。Sys等[33]对40例行单节段腰椎后路椎体间融合术的患者植骨材料使用富血小板血浆复合自体松质骨,随访2年并未发现富血小板血浆对提高融合率有明显作用。Hee等[34]在腰后路经椎间孔椎体间融合术中得出类似结论。Li等[35]通过动物实验比较了自体骨、β-磷酸三钙、β-磷酸三钙+富血小板血浆3种材料填充椎间融合器进行腰椎前路融合手术,通过3个月观察发现自体骨组融合率最高,富血小板血浆在骨的形成及β-磷酸三钙并没有任何作用。Kamoda等[36]通过对大鼠L4/L5行后外侧关节融合术发现,使用富血小板血浆能缩短骨融合时间,在术后第4周与第8周观察使用富血小板血浆组较常规组融合的骨量明显增多。同时该团队建立大鼠椎间融合模型,3组共21只大鼠,发现富血小板血浆复合羟基磷灰石3个月后全部发生融合,而贫血小板血浆复合羟基磷灰石组仅有1只发生融全,而单纯使用羟基磷灰石组均未发生融合[37]。Cinotti等[38]使用钮西兰兔作实验动物进行L4/L5后外侧关节融合术,实验组右侧单独使用富血小板血浆,左侧使用未培养的自体骨髓,实验组右侧单独使用陶瓷制品,左侧同实验组,结果2组观察6个月后发现融合率差异并无显著性意义,并且2组均无完全的“骨桥”形成。Okamoto等[39]同样使用大鼠建立腰椎后外侧融合模型,使用富血小板血浆复合β-磷酸三钙作为融合材料,通过对照研究发现其融合的坚强度与骨量与自体骨无明显差异。 2.4 富血小板血浆在肌腱韧带组织工程的研究 体外研究发现将肌腱放入100%富血小板血浆中可增加Ⅰ、Ⅲ型胶原的基因表达。同时有学者通过实验证实富血小板血浆通过提高增殖细胞核抗原、细胞周期蛋白A2、细胞周期蛋白B1等调控蛋白的表达能使骨骼肌细胞从G1增殖期转向S和G2/M增殖期。这些基础研究为富血小板血浆治疗肌腱病提供了理论依据,目前应用于肱骨髁上炎、跟腱炎等疾病中有大量成功的临床及实验研究,见表1。由于硬脑膜由类似于肌腱的致密结缔组织构成,自从2010年开始有学者报道利用富血小板血浆治疗脑脊液患者,Boddu等[40]报道了用富血小板血浆治疗1例因结缔组织疾病而产生的自发性脑脊液漏导致严重头痛的白人女性,通过局部穿刺定位将总共80 mL的富血小板血浆与凝血酶混合注入T1水平硬膜外腔,患者术后第3天开始头痛明显减轻,症状明显好转直至术后4个月因基础疾病再次出现自发性脑脊液漏。作者在文中提到富血小板血浆对于医源性脑脊液也应该具有相同的作用,但是相关的体内、体外研究仍需进一步予以证实。 2.5 富血小板血浆在其他脊柱疾病相关方面的研究 脊髓损伤患者的生存率与生活质量与伤后继发的并发症有明确关联。压疮是主要的并发症之一,由于创面通常是不能自然愈合的,导致炎症向深层侵袭,是不能自主活动的患者致病或者致死的主要病因之一,给医疗和护理带来困难。有研究观察25例压疮患者,通过每周2次换药清除坏死组织,外敷富血小板血浆凝胶并用至少10层纱布包扎,与常规换药组对比研究发现,换药后第5周,治疗组56%的疮面坏死组织与脓液明显减少,60%的创面有很好的新鲜肉芽组织及新生血管形成。6个月后治疗组96%的疮面得到改善,明显高于控制组的68%。同时治疗平均愈合面积为57.94%,控制组只有2.36%。使用富血小板血浆处理压疮能明显改善创面,其他文献也得出类似结果,但目前的研究仅为单中心小样本的临床观察,但其有效性仍需大样本的研究予以证实。Anitua等[41]通过提取正常人的表皮成纤维细胞进行体外实验发现富血小板血浆通过加强细胞增殖、迁移、中枢生物活性因子的表达刺激皮肤再生,见表1。 另外Singla等[42]选取40例因骶髂关节退变引起腰痛的患者对激素及富血小板血浆注射进行对比研究,结果富血小板血浆治疗组在6周及3个月时疼痛明显缓解,3个月时激素治疗组仍有效果的只有25%,而富血小板血浆治疗组高达90%。"

| [1] Leslie M. Beyond Clotting: The Powers of Platelets. Science. 2010; 328(5978):562-564.[2] Arnoczky SP, Delos D, Rodeo SA. What is platelet-rich plasma? Operat Tech Sports Med. 2011;19(3):142-148.[3] Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2014;21(12):739-748.[4] Semevolos SA, Youngblood CD, Grissom SK, et al. Evaluation of two platelet-rich plasma processing methods and two platelet-activation techniques for use in llamas and alpacas. Am J Vet Res. 2016;77(11):1288-1294.[5] Mazzucco L, Balbo V, Cattana E, et al. Not every PRP-gel is born equal: evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, regen PRP-Kit, Plateltex, and one Manual Procedure. Vox Sang. 2009;97(2): 110-118.[6] Marx RE. Platelet-rich Plasma(PRP): What is PRP and what is not PRP? Implant Dent. 2001;10(4):225-228.[7] Alsousou J, Thompson M, Hulley P, et al. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91(8): 987-996.[8] Cavallo C, Roffi A, Grigolo B, et al. Platelet-Rich Plasma: The Choice of Activation Method Affects the Release of Bioactive Molecules. Biomed Res Int. 2016;2016:6591717.[9] Fufa D, Shealy B, Jacobson M, Activation of platelet-rich plasma using soluble type I collagen. J Oral Maxillofac Surg. 2008;66(4):684-690.[10] Wasterlain AS, Braun HJ, Dragoo JL. Contents and Formulations of Platelet Rich Plasma. Operat Tech Orthop. 2012;22(1):33-42.[11] Lee KS. Platelet-rich plasma injectiton. Semin Musculoskelet Radiol. 2013;17(1):91-98.[12] Moore GW, Maloney JC, Archer RA, et al. Platelet-rich plasma for tissue regeneration can be stored at room temperature for at least five days. Br J Biomed Sci. 2016;1-7. [13] Nakatani Y, Agata H, Sumita Y, et al. Efficacy of freeze-dried platelet-rich plasma in bone engineering. Arch Oral Biol. 2016; 73:172-178.[14] Shiga Y, Orita S, Kubota G, et al. Freeze-Dried Platelet-Rich Plasma Accelerates Bone Union with Adequate Rigidity in Posterolateral Lumbar Fusion Surgery Model in Rats. Sci Rep. 2016;11(6):36715.[15] Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearance of disc degeneration. Spine(Phila Pa 1976).2005;30(1):5-14. [16] Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003; 5(3):120-130.[17] Wang SZ, Rui YF, Tan Q, et al. Enhancing intervertebral disc repair and regeneration through biology: platelet-rich plasma as an alternative strategy. Arthritis Res Ther. 2013;15(5):220.[18] Cho H, Holt DC 3rd, Smith R, et al. The Effects of Platelet-Rich Plasma on Halting the Progression in Porcine Intervertebral Disc Degeneration.Artif Organs. 2016;40(2): 190-195.[19] Khalaf K, Nikkhoo M, Ya-Wen Kuo, et al. Recovering the mechanical properties of denatured intervertebral discs through Platelet-Rich Plasma therapy. Conf Proc IEEE Eng Med Biol Soc. 2015; 933-936.[20] Obata S, Akeda K, Imanishi T, et al. Effect of autologous platelet-rich plasma-releasate on intervertebral disc degeneration in the rabbit anular puncture model: a preclinical study. Arthritis Res Ther. 2012;14(6):R241.[21] Gui K, Ren W, Yu Y, et al. Inhibitory effects of platelet-rich plasma on intervertebral disc degeneration: a preclinical study in a rabbit model. Med Sci Monit. 2015;21:1368-1375.[22] Gullung GB, Woodall JW, Tucci MA, et al. Platelet-rich plasma effects on degenerative disc disease: analysis of histology and imaging in an animal model.Evid Based Spine Care J. 2011;2(4):13-18.[23] Bendinelli P, Matteucci E, Dogliotti G, et al. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-κB inhibition via HGF. J Cell Phys. 2010;225(3):757-766.[24] Kim HJ, Yeom JS, Koh YG,et al. Anti-inflammatory effect of platelet-rich plasma on nucleus pulposus cells with response of TNF-α and IL-1. J Orthop Res. 2014;32(4):551-556.[25] Tuakli-Wosornu Y A, Terry A, Boachie-Adjei K, et al. Lumbar Intradiskal Platelet-Rich Plasma (PRP) Injections: A Prospective, Double-Blind, Randomized Controlled Study. PM R. 2015; 8(1):1-10.[26] Levi D, Horn S, Tyszko S, et al. Intradiscal Platelet-Rich Plasma Injection for Chronic Discogenic Low Back Pain: Preliminary Results from a Prospective Trial. Pain Med. 2016;17(6):1010-1022.[27] Bhatia R, Chopra G. Efficacy of Platelet Rich Plasma via Lumbar Epidural Route in Chronic Prolapsed Intervertebral Disc Patients-A Pilot Study. J Clin Diagn Res. 2016;10(9): UC05-UC07.[28] Nagae M, Ikeda T, Mikami Y, et al. Intervertebral disc regeneration using platelet-rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng Part A. 2007; 13(1):147-158.[29] Sawamura K, Ikeda T, Nagae M, et al. Characterization of in vivo effects of platelet-rich plasma and biodegradable gelatin hydrogel microspheres on degenerated intervertebral discs. Tissue Eng Part A. 2009;15(12):3719-3727.[30] Hou Y, Shi G, Shi J, et al. Study design: in vitro and in vivo assessment of bone morphogenic protein 2 combined with platelet-rich plasma on treatment of disc degeneration. Int Orthop. 2016; 40(6):1143-1155.[31] Mietsch A, Neidlinger-Wilke C, Schrezenmeier H, et al. Evaluation of platelet-rich plasma and hydrostatic pressure regarding cell differentiation in nucleus pulposus tissue engineering. J Tissue Eng Regen Med. 2013;7(3):244-252.[32] Tarantino R, Donnarumma P, Mancarella C, et al. Posterolateral arthrodesis in lumbar spine surgery using autologous platelet-rich plasma and cancellous bone substitute: an osteoinductive and osteoconductive effect. Global Spine J. 2014;4(3):137-142. [33] Sys J, Weyler J, Van Der Zijden T, et al. Platelet-rich plasma in mono-segmental posterior lumbar interbody fusion. Eur Spine J. 2011;20(10):1650-1657.[34] Hee HT, Majd ME, Holt RT, et al. Do autologous growth factors enhance transforaminal lumbar interbody fusion? Eur Spine J. 2003;12(4):400-407.[35] Li H, Zou X, Xue Q, et al. Anterior lumbar interbody fusion with carbon fiber cage loaded with bioceramics and platelet-rich plasma. An experimental study on pigs. Eur Spine J.2004;13(4):354-358.[36] Kamoda H, Ohtori S, Ishikawa T, et al. The effect of platelet-rich plasma on posterolateral lumbar fusion in a rat model. J Bone Joint Surg Am. 2013;95(12):1109-1116.[37] Kamoda H, Yamashita M, Ishikawa T, et al. Platelet-rich plasma combined with hydroxyapatite for lumbar interbody fusion promoted bone formation and decreased an inflammatory pain neuropeptide in rats. Spine. 2012;37(20): 1727-1733.[38] Cinotti G, Corsi A, Sacchetti B, et al. Bone ingrowth and vascular supply in experimental spinal fusion with platelet-rich plasma. Spine. 2013;38(5):385-391.[39] Okamoto S, Ikeda T, Sawamura K, et al. Positive effect on bone fusion by the combination of platelet-rich plasma and a gelatin β-tricalcium phosphate sponge: a study using a posterolateral fusion model of lumbar vertebrae in rats. Tissue Eng Part A. 2012;18(18):157-166.[40] Boddu K, Saha S, Brish EL, et al. Epidural injection of platelet-rich plasma (PRP) for treatment of dural tears :a novel intervention. Reg Anesth Pain Med. 2010; 35(5):1098.[41] Anitua E, Pino A, Orive G. Plasma rich in growth factors promotes dermal fibroblast proliferation, migration and biosynthetic activity. J Wound Care. 2016; 25(11):680-687.[42] Singla V, Batra YK, Bharti N, et al. Steroid versus Platelet-Rich Plasma in Ultrasound-Guided Sacroiliac Joint Injection for Chronic Low Back Pain. Pain Practice. 2016. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Yu Chengxiang, Liu Lehong, Li Wenbo, Chen Jinshi, Ran Chunlei, Wang Zhongping. Correlation between spine-pelvic sagittal parameters and prognosis of vertebroplasty in the treatment of thoracolumbar osteoporotic vertebral compression fractures [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1412-1417. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [5] | Liu Yuhang, Zhou Jianqiang, Xu Xuebin, Qu Xingyue, Li Ziyu, Li Kun, Wang Xing, Li Zhijun, Li Xiaohe, Zhang Shaojie. Establishment and validation of finite element model of lower cervical spine in 6-year-old children [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 870-874. |
| [6] | Li Yuqiao, Sun Tianwei, Ma Bin, Zhou Zhaohong, Dong Runbei, Wu Haiyang. A comparative study of imaging parameters and quality of life scores between subtypes of lumbar spondylolisthesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 943-948. |
| [7] | Li Jian, Bao Zhengqi, Zhou Pinghui, Zhu Ruizhi, Li Zhixiang, Wang Jinzi. Effects of posterior single open-door laminoplasty and anterior cervical corpectomy fusion on cervical sagittal balance parameters in the treatment of multilevel cervical spondylotic myelopathy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 949-953. |
| [8] | Yi Xinrong, Jia Fuquan, He Xin, Zhang Shaojie, Ren Xiaoyan, Li Zhijun. Establishment of cervical bone age equation for male adolescents aged 8-16 years old in Hohhot based on thin-slice CT [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 954-958. |
| [9] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [10] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [11] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [12] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [13] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [14] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [15] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||