Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (10): 1539-1545.doi: 10.3969/j.issn.2095-4344.2017.10.011
Previous Articles Next Articles
Preparation of supported lipid bilayer membranes by thin film extrusion
Jia Jin, Ling Ying-chen, Fang Ying
- School of Bioscience and Bioengineering, South China University of Technology, Guangzhou 510006, Guangdong Province, China
-
Received:2017-01-27Online:2017-04-08Published:2017-05-08 -
Contact:Fang Ying, Associate professor, Master’s supervisor, School of Bioscience and Bioengineering, South China University of Technology, Guangzhou 510006, Guangdong Province, China -
About author:Jia Jin, Master, School of Bioscience and Bioengineering, South China University of Technology, Guangzhou 510006, Guangdong Province, China -
Supported by:the National Natural Science Foundation of China, No. 11672109, 11272125, 11432006
CLC Number:
Cite this article
Jia Jin, Ling Ying-chen, Fang Ying. Preparation of supported lipid bilayer membranes by thin film extrusion[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(10): 1539-1545.
share this article
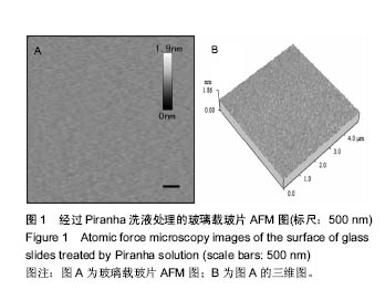
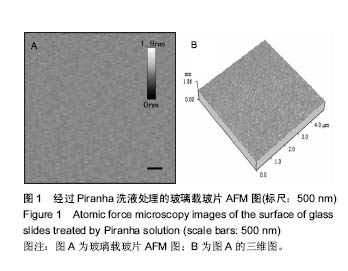
2.1 玻璃载玻片的表征 实验中所使用的玻璃载玻片均在100 ℃的Piranha洗液中浸泡60 min以上,通过此处理方法,不仅有效地去除了载玻片表面的有机物质,同时使载玻片表面发生羟基化,亲水性增强,有利于脂质体在其表面扩散形成均匀的脂双层膜。用AFM对玻璃载玻片的表面形貌进行观测,结果如图1所示,经Piranha洗液处理过的载玻片表面均方根粗糙度(root mean squared roughness, RMSR)为(0.026 8±0.000 1) nm。未经Piranha洗液处理玻璃载玻片的RMSR为(0.028 2±0.000 2) nm,以上结果说明Piranha洗液的处理并未明显增加玻璃载玻片的粗糙程度。"
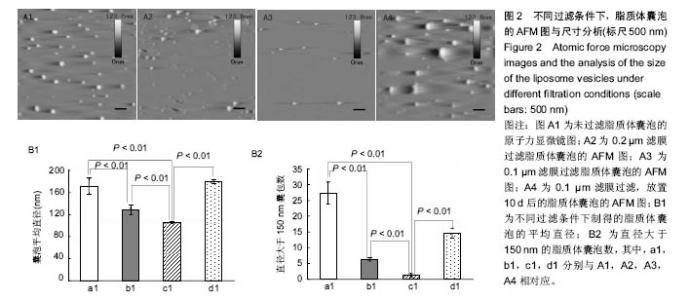
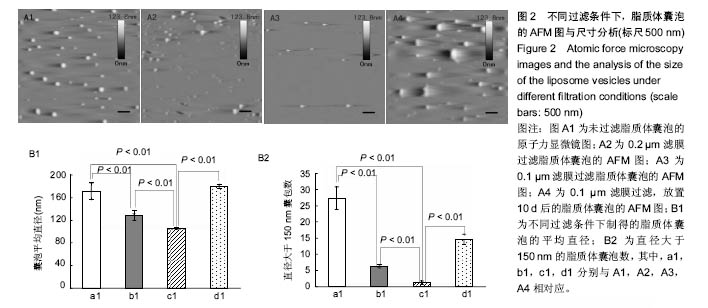
2.2 原子力显微镜检测脂质体囊泡的状态 实验使用不同孔径的滤膜来探究采用薄膜挤压法制备的脂质体囊泡的大小与分布,如图2A所示,对应囊泡的平均直径数值如图2B1所示。从图2A中可直观看出,采用薄膜挤压法过滤可有效减少大囊泡的出现,同时图2A结果表明,放置10 d后的脂质体半径明显增大。从图2B1中可看出过滤有效缩小了脂质体囊泡的尺寸,且采用0.1 μm滤膜过滤所形成的脂质体囊泡平均直径接近100 nm,极显著小于其他2组(P < 0.01)。由文献[21]报道,当脂质体囊泡直径小于150 nm时,所形成的脂双层膜更加均匀。图2B2为不同制备条件下,直径大于 150 nm的脂质体囊泡数,从图中可看出未过滤与采用孔径为0.2 μm滤膜过滤所得的脂质体囊泡尚有较多直径大于150 nm,极显著地高于采用孔径为0.1 μm滤膜过滤所得样品(P < 0.01)。上述结果表明,采用0.1 μm滤膜进行薄膜挤压法制备的脂质体囊泡更适合后续脂双层膜的制备。"
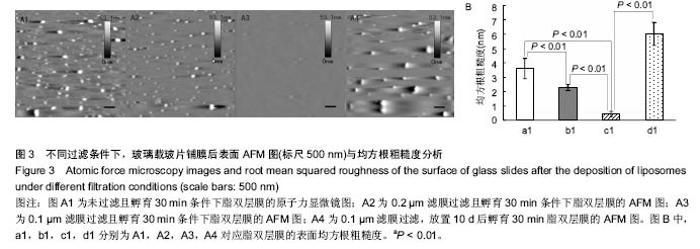
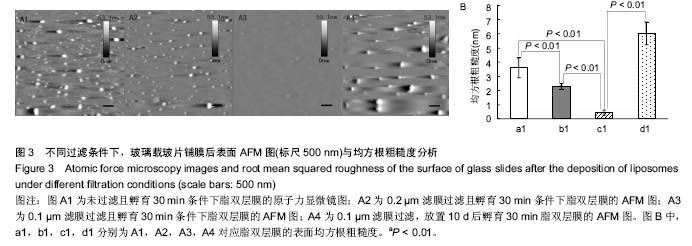
2.3 原子力显微镜检测脂双层膜的形态 为了观测不同制备条件下脂双层膜的表面形貌,实验采用原子力显微镜对脂双层膜进行扫描,结果如图3。 从图3A1中可以观察出未经过滤的脂质体在玻璃载玻片上所形成的脂双层膜表面尚有较多未铺展开的脂质体囊泡,图3A2未铺展开的脂质体囊泡较少,效果较佳,从图3A3中可看出经过孔径为0.1 μm滤膜过滤的脂质体在玻璃载玻片上形成的脂双层膜表面最为光滑,残存脂质体囊泡最少且尺寸最小,图3A4表明放置10 d的脂质体已不适于后续实验。图3B为不同条件下脂双层膜的均方根粗糙度,通过对比采用同样滤膜孔径过滤且未孵育样品的RMSR,发现未孵育样品的RMSR均大于孵育30 min的脂双层膜,其中采用0.1 μm滤膜过滤且未孵育样品的RMSR为(1.38±0.25)nm,这证明了孵育过程对脂质体在载玻片表面的铺展过程具有重要意义。 据以往研究显示,均匀脂双层膜的均方根粗糙度约为0.5 nm,实验中采用0.1 μm滤膜过滤且孵育30 min条件下制备所得的脂双层膜的RMSR为(0.423±0.181)nm[19],符合要求,且极显著小于其他3组(P < 0.01)。综上所述,采用0.1 μm滤膜过滤且孵育30 min条件下制备所得的脂双层膜在表面结构上更加接近生理细胞膜。"
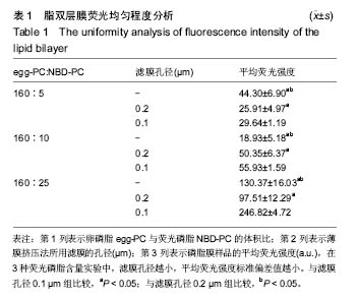
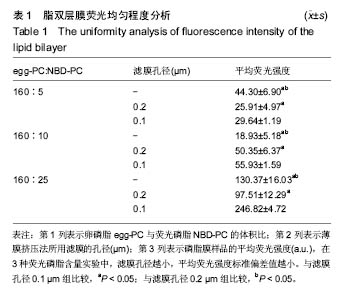
2.4 脂双层膜荧光均匀程度变化 为了检测不同制备条件下脂双层膜表面荧光分子的均匀程度,采用薄膜挤压法,分别以孔径为0.1 μm与0.2 μm的滤膜对脂质体分子过滤10次,保留未过滤样品作为对照,同时将160 μL egg-PC(10g/L)分别与5,10,25 μL NBD-PC(1 g/L)进行混合,共产生9组不同制备条件的脂质体。对9组样品制备的脂双层膜各进行9点取样,在每组样品表面相同位置选取9个直径为30 μm的圆作为观察区域,得9个取样区域的平均荧光强度,对其进行方差分析。结果表明,egg-PC与NBD-PC的配比和滤膜孔径均对脂双层膜的荧光均匀程度产生显著影响,前者F=1 842.38,P=1.48×10-62;后者F=264.21,P=6.91×10-34;且存在二因素的交互作用(P=1.24×10-39)。 从表1中可看出,NBD-PC的含量与挤压法中所采用滤膜孔径的大小均对9组样品荧光强度产生较大影响。滤膜孔径为0.1 μm的3组样品的标准偏差值均显著性小于同一配比下的其他2组(P < 0.05)。以上结果与之前采用原子力显微镜的检测结果一致,表明采用0.1 μm滤膜进行薄膜挤压法制备脂质体能够形成更加均匀的脂双层膜。"

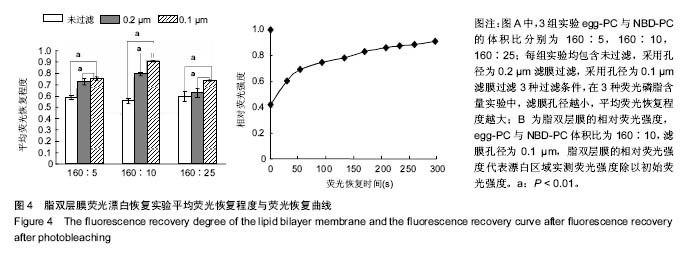
2.5 脂双层膜荧光漂白恢复情况 为了检测不同制备条件下脂双层膜的流动性,实验通过荧光漂白恢复技术对脂双层膜进行检测,漂白区域直径为30 μm,所得结果如图4A。从图4A中可见,滤膜孔径对荧光恢复程度具有较大影响,未过滤对照组与采用孔径为0.2 μm滤膜过滤实验组的荧光恢复程度均小于90%。从图4A中可见,采用孔径为 0.1 μm滤膜过滤的实验组,egg-PC与NBD-PC体积比为160∶10的样品,荧光恢复程度达到初始荧光强度的90%以上,荧光恢复曲线如图4B,经过计算得其扩散系数为(1.053±0.042) μm2/s,满足扩散系数大于1 μm2/s,符合优质脂双层膜的标准,表明此条件制备的脂双层膜具有良好的流动性。"
| [1]Bangham AD, Standish MM, Weissmann G. The action of steroids and streptolysin S on the permeability of phospholipid structures to cations. J Mol Biol. 1965;13(1): 253-259.[2]Dasa SSK, Suzuki R, Gutknecht M, et al. Development of target-specific liposomes for delivering small molecule drugs after reperfused myocardial infarction. J Control Release. 2015;220, Part A(5):56-67.[3]Dustin ML. Supported bilayers at the vanguard of immune cell activation studies. J Struct Biol. 2009;168(1):152-160.[4]Springer TA, Dustin ML. Integrin inside-out signaling and the immunological synapse. Curr Opin Cell Biol. 2012;24(1): 107-115.[5]Spedes PF, Bueno SM, Ram RBA, et al. Surface expression of the hRSV nucleoprotein impairs immunological synapse formation with T cells. Proc Natl Acad Sci U S A. 2014;111(31): E3214-E3223.[6]Wu J, Fang Y, Zarnitsyna VI, et al. A coupled diffusion-kinetics model for analysis of contact-area FRAP experiment. Biophys J. 2008;95(2):910-919.[7]Tolentino TP, Wu J, Zarnitsyna VI, et al. Measuring diffusion and binding kinetics by contact area FRAP. Biophys J. 2008; 95(2):920-930. [8]Crites TJ, Maddox M, Padhan K, et al. Supported lipid bilayer technology for the study of cellular interfaces. Current Protocols in Cell Biology. John Wiley, Sons, Inc. 2001.[9]Santo IE, Campardelli, Albuquerque EC, et al. Liposomes size engineering by combination of ethanol injection and supercritical processing. J Pharm Sci. 2015;104(11): 3842-3850.[10]Sakai H, Gotoh T, Imura T, et al. Preparation and properties of liposomes composed of various phospholipids with different hydrophobic chains using a supercritical reverse phase evaporation method. J Oleo Sci. 2008;57(11):613-621.[11]Grimaldi N, Andrade F, Segovia N, et al. Lipid-Based nanovesicles for nanomedicine. Chem Soc Rev. 2016;45: 6520-6545.[12]Woodall AA, Britton G, Jackson MJ. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: relationship between carotenoid structure and protective ability. Biochim Biophys Acta. 1997; 1336(3):575-586.[13]Zucker D, Marcus D, Barenholz Y, et al. Liposome drugs' loading efficiency: a working model based on loading conditions and drug's physicochemical properties. J Control Release. 2009;139(1):73-80.[14]Takechi-Haraya Y, Sakai-Kato K, Abe Y, et al. Observation of liposomes of differing lipid composition in aqueous medium by means of atomic force microscopy. Microscopy. 2016.[15]Chada N, Sigdel KP, Gari RRS, et al. Glass is a viable substrate for precision force microscopy of membrane proteins. Sci Rep. 2015;(5):12550.[16]Suarez-Germa C, Morros A, Montero MT, et al. Combined force spectroscopy, AFM and calorimetric studies to reveal the nanostructural organization of biomimetic membranes. Chem Phys Lipids. 2014;(183):208-217.[17]Seu KJ, Cambrea LR, Everly RM, et al. Influence of lipid chemistry on membrane fluidity: tail and headgroup interactions. Biophys J. 2006;91(10):3727-3735.[18]Hu XY, Lei H, Zhang XQ, et al. Strong hydrophobic interaction between graphene oxide and supported lipid bilayers revealed by AFM. Microsc Res Tech. 2016;79(8):721-726.[19]Morenno-Cencerrado A, Tharad S, Iturri J, et al. Time influence on the interaction between Cyt2Aa2 and lipid/cholesterol bilayers. Microsc Res Tech. 2016;79(11): 1017-1023.[20]Silva-L Pez EI, Edens LE, Barden AO, et al. Conditions for liposome adsorption and bilayer formation on BSA passivated solid supports. Chem Phys Lipids. 2014;(183):91-99.[21]Dayani Y, Malmstadt N. Lipid bilayers covalently anchored to carbon nanotubes. Langmuir. 2012;28(21):8174-8182. [22]Jass J, TJ Rnhage T, Puu G. From liposomes to supported, planar bilayer structures on hydrophilic and hydrophobic surfaces: an atomic force microscopy study. Biophys J. 2000;79(6):3153-3163.[23]Borrell JH, Montero MT, Morros A, et al. Unspecific membrane protein-lipid recognition: combination of AFM imaging, force spectroscopy, DSC and FRET measurements. J Mol Recognit. 2015;(28):679-686.[24]Kam LC. Capturing the nanoscale complexity of cellular membranes in supported lipid bilayers. J Struct Biol. 2009; 168(1):3-10.[25]刘国立,于昆仑,白江博,等.脱细胞羊膜与医用膜修复腱鞘缺损防治肌腱粘连的比较[J].中国组织工程研究, 2016,20(21): 3117-3123.[26]Kalb E, Frey S, Tamm LK. Formation of supported planar bilayers by fusion of vesicles to supported phospholipid monolayers. Biochim Biophys Acta. 1992;1103(2):307-316. [27]Yoshina-Ishii C, Chan YH, Johnson JM, et al. Diffusive dynamics of vesicles tethered to a fluid supported bilayer by single-particle tracking. Langmuir. 2006;22(13):5682-5689.[28]Zhang HY, Hill RJ. Concentration dependence of lipopolymer self-diffusion in supported bilayer membranes. J R Soc Interface. 2011;8(54):127-143.[29]Watanabe R, Soga N, Yamanaka T, et al. High-throughput formation of lipid bilayer membrane arrays with an asymmetric lipid composition. Sci Rep. 2014;(4):70-76.[30]Goksu EI, Vanegas JM, Blanchette CD, et al. AFM for structure and dynamics of biomembranes. Biochim Biophys Acta. 2009;1788(1):254-266.[31]方颖,向雪,凌颖琛,等.细胞接触面内的扩散阻塞效应的估计[J].医用生物力学,2007,22(1):30-34. [32]Xiang X, Sun J, Wu JH, et al. A FRET-Based biosensor for imaging SYK activities in living cells. Cell Mol Bioeng. 2011; 4(4):670-677. [33]Khalili S, Jahangiri A, Hashemi ZS, et al. Structural pierce into molecular mechanism underlying Clostridium perfringens Epsilon toxin function. Toxicon. 2017;127:90-99. [34]Broecker J, Eger BT, Ernst OP. Crystallogenesis of Membrane Proteins Mediated by Polymer-Bounded Lipid Nanodiscs. Structure. 2017;25(2):384-392.[35]Tian Y, Schwieters CD, Opella SJ, et al. High quality NMR structures: a new force field with implicit water and membrane solvation for Xplor-NIH. J Biomol NMR. 2017;67(1):35-49. [36]Yousefi N, Tufenkji N. Probing the Interaction between Nanoparticles and Lipid Membranes by Quartz Crystal Microbalance with Dissipation Monitoring. Front Chem. 2016;4:46. [37]Piai A, Fu Q, Dev J, et al. Optimal Bicelle Size q for Solution NMR Studies of the Protein Transmembrane Partition. Chemistry. 2017;23(6):1361-1367. [38]Dai X, Yin Q, Wan G, et al. Effects of concentrations on the transdermal permeation enhancing mechanisms of borneol: a coarse-grained molecular dynamics simulation on mixed-bilayer membranes. Int J Mol Sci. 2016;17(8)E1349.[39]Postic G, Ghouzam Y, Gelly JC. OREMPRO web server: orientation and assessment of atomistic and coarse-grained structures of membrane proteins. Bioinformatics. 2016; 32(16):2548-2550.[40]Kim MC, Gunnarsson A, Tabaei SR, et al. Supported lipid bilayer repair mediated by AH peptide. Phys Chem Chem Phys. 2016;18(4):3040-3047. [41]Tabaei SR, Jackman JA, Kim M, et al. Biomembrane Fabrication by the Solvent-assisted Lipid Bilayer (SALB) Method. J Vis Exp. 2015. doi: 10.3791/53073.[42]Umegawa Y, Tanaka Y, Nobuaki M, et al. (13) C-TmDOTA as versatile thermometer compound for solid-state NMR of hydrated lipid bilayer membranes. Magn Reson Chem. 2016; 54(3):227-233. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [5] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [6] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [9] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [10] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [11] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [12] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [13] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [14] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| [15] | Fang Xiaoyang, Tang Tian, Wang Nan, Qian Yuzhang, Xie Lin. Repair and regenerative therapies of the annulus fibrosus [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1582-1587. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||