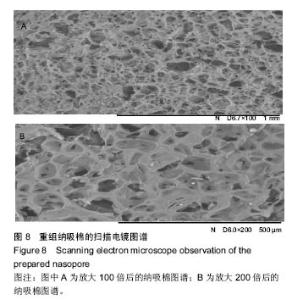
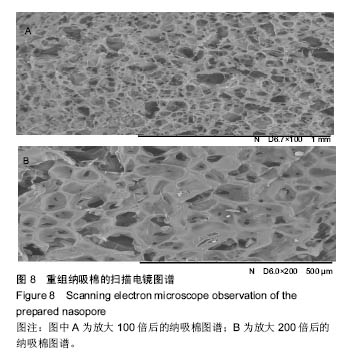
Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (10): 1532-1538.doi: 10.3969/j.issn.2095-4344.2017.10.010
Previous Articles Next Articles
Optimization of fiber reconstituted technology for preparation of nasopore using fish scale collagen
Li Jie1, Liu Dong-yan2, Qin Song2
- 1 Qingdao University of Science and Technology, Qingdao 266000, Shandong Province, China; 2 Yantai Institute of Coastal Zone Research, CAS, Yantai 2 64000, Shandong Province, China
-
Received:2016-12-13Online:2017-04-08Published:2017-05-08 -
Contact:Qin Song, Doctor, Investigator, Doctoral supervisor, Yantai Institute of Coastal Zone Research, CAS, Yantai 264000, Shandong Prov -
About author:Li Jie, Master, Qingdao University of Science and Technology, Qingdao 266000, Shandong Province, China -
Supported by:the Science and Technology Development Plan of Shandong Province, No. 2015GSF115032
CLC Number:
Cite this article
Li Jie, Liu Dong-yan, Qin Song. Optimization of fiber reconstituted technology for preparation of nasopore using fish scale collagen[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(10): 1532-1538.
share this article
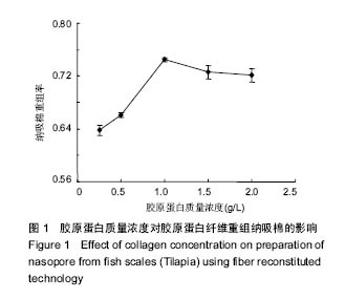
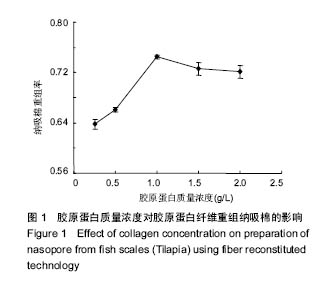
2.1 胶原蛋白质量浓度对纤维重组纳吸棉的影响 胶原蛋白质量浓度是影响纤维重组构建纳吸棉的因素之一。图1是不同质量浓度胶原蛋白对纤维重组纳吸棉的影响。从图中可以看出,随着胶原蛋白溶液质量浓度的升高,纤维重组率也随之增大,即纳吸棉生成量逐渐增加,在1 g/L胶原蛋白质量浓度下,纳吸棉生成量达到最高值。 该实验结果提示,随着胶原蛋白质量浓度的提高,胶原分子的重组程度也随之增加,有文献报道,胶原纤维重组存在临界质量浓度[16],临界质量浓度以上,胶原会重组构成纳吸棉,但在临界质量浓度以下,通过离心等测定方法不能检测到纳吸棉的形成。罗非鱼皮Ⅰ型胶原重组的临界质量浓度为0.2-0.3 g/L。但是,胶原质量浓度不能无限制增大,适量的胶原分子能促进重组纤维的生长,但过多却产生抑制作用。"
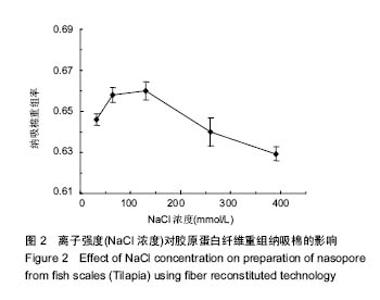
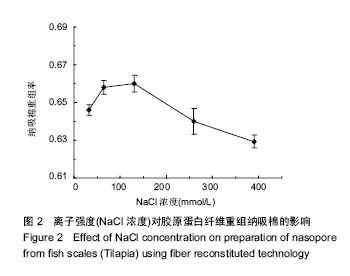
2.2 离子强度(NaCl浓度)对纤维重组纳吸棉效果的影响 离子强度(NaCl浓度)是决定纤维重组效果的重要参数之一。试验中通过控制PBS中NaCl浓度的方法调节离子强度的大小。离子强度对胶原蛋白纤维重组构建纳吸棉的影响如图2所示。从中可以看出,当溶液中有NaCl存在时,胶原表现明显的纤维重组现象。NaCl含量在35.5-130 mmol/L时,胶原蛋白纤维重组明显加快,盐粒子对聚集的阻滞作用力较弱,胶原分子表面的静电作用较大,分子间可通过静电作用聚集,说明NaCl在胶原重组过程中发挥重要作用,但浓度一旦超过130 mmol/L,胶原纤维重组率逐步下降,原因可能是NaCl通过屏蔽带电基团而增强了胶原分子间的斥力[17],胶原聚集受阻;实验表明适量的离子强度有利于胶原纤维重组过程,但是过量的离子强度阻碍胶原纤维重组效果。"
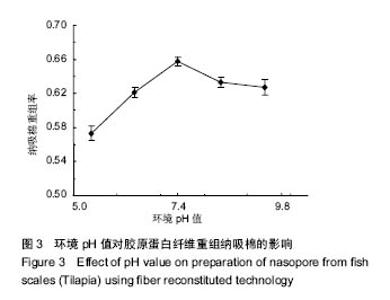
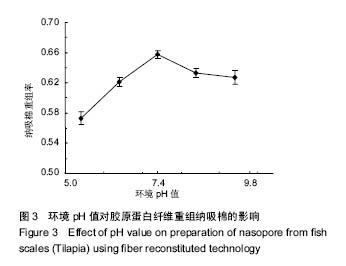
2.3 体系pH值对纤维重组纳吸棉效果的影响 溶液环境的pH值是决定纤维重组效果的最重要参数。pH值通过影响胶原分子间电荷排斥作用的强弱,从而影响分子间重组纤维的结合能力,进而影响纳吸棉纤维的重组效果[8]。 环境pH值对胶原蛋白纤维重组纳吸棉的影响如图3所示。从实验选择的5个pH值中,可以看出当pH值为5.4-7.4时,纤维重组率逐步升高;当pH值增大到7.4时,其重组效果达到最佳,随着pH值进一步增大,其重组率有所降低并渐渐维持恒定。 原因可能是:在H+浓度较高的条件下,胶原分子内肽键的酰胺基能和溶液中的H+形成-NH2+,由于电荷排斥作用,胶原分子多以线性单分子或小的聚集体形式存在,较难形成大的分子聚集体。随着pH值的增大,胶原蛋白分子间电荷排斥作用下降,分子间聚集程度增加,有助于大的纤维聚集体形成[18]。罗非鱼鱼皮酶促溶性胶原蛋白纤维重组快速上升阶段的起始pH值为5左右,接近胶原分子的等电点pH值[19]。"
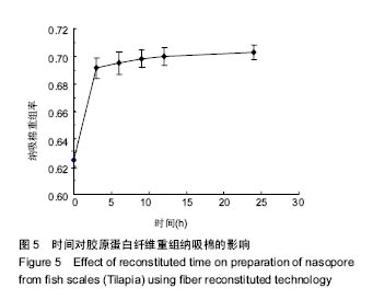
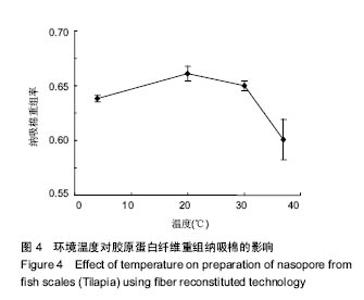
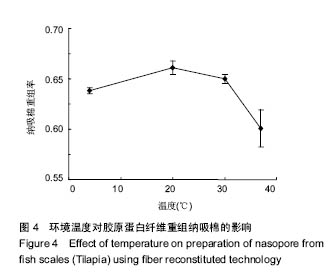
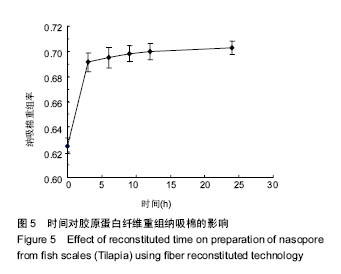
2.5 时间对纤维重组纳吸棉效果的影响 时间对胶原蛋白纤维重组构建纳吸棉的影响如图5所示,随着重组时间的延长,胶原蛋白重组效果明显增大,0-3 h增长速率最快,3 h后增长速度逐渐减慢,10 h达到峰值,以后胶原重组率基本维持不变,表明纤维重组在10 h已经达到平衡。由上述分析可知,胶原蛋白纤维重组构建纳吸棉时间为10 h较为合适。 单因素试验结果表明,在胶原质量浓度为1 g/L、离子强度(NaCl浓度)为130 mmol/L、调节pH值为7.4、环境温度为20 ℃、重组时间为10 h的条件下,罗非鱼鱼皮酶促溶性胶原蛋白纤维重组构建纳吸棉效果最好,从胶原质量浓度单因素来讲,适量的胶原分子能促进重组纤维的生长,但过多却产生抑制作用;从离子强度(NaCl浓度)单因素来讲,适量的离子强度(NaCl浓度)有利于胶原纤维重组过程,但是过量的离子强度(NaCl浓度)阻碍胶原纤维重组效果;从体系pH值单因素来考虑,当调节pH值达到蛋白等电点时,其蛋白纤维重组程度达到最大;调节环境温度达到鱼生长环境的最适温度,纤维重组率最好,一旦超过蛋白变性温度,三螺旋结构完全破坏,胶原蛋白不会重组形成纤维,可见胶原蛋白纤维重组程度与其结构的完整性关系很大,时间对胶原蛋白重组纤维的多少也起到作用。"
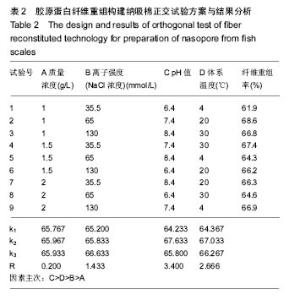
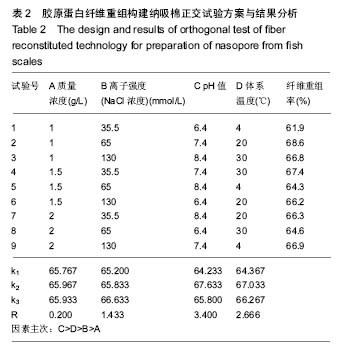
2.6 正交试验 在上述单因素试验的基础上,胶原质量浓度、离子强度(NaCl浓度)、体系pH值、体系温度为主要因素,进行四因素三水平正交试验,以胶原蛋白纤维重组率为考察指标,选用正交表L9(34)进行试验确定胶原蛋白纤维重组构建纳吸棉的最佳实验条件,结果分析见表2。 由表2可知,以胶原质量浓度、离子强度(NaCl浓度)、体系pH值、体系温度为主要因素,以纤维重组率为指标,对正交试验结果进行极差分析,发现各因素的影响程度依次为pH值、体系温度、离子强度(NaCl浓度)、胶原质量浓度;最佳试验组合为A1B2C2D2,即胶原质量浓度为1 g/L,离子强度(NaCl浓度)为65 mmol/L,pH 7.4体系温度为 20 ℃,重组时间为10 h。"
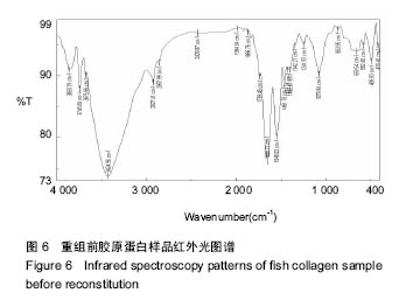
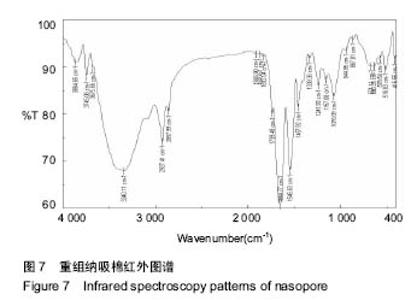
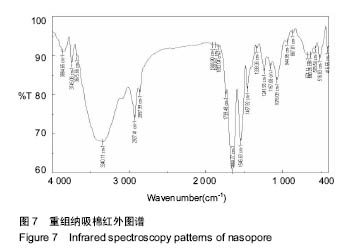
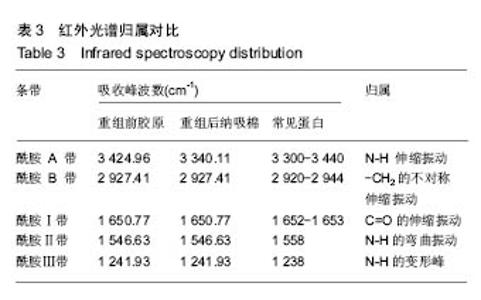
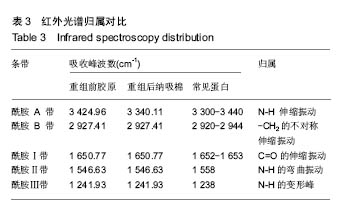
2.7 重组纳吸棉的红外光谱分析 蛋白质的二级结构与其分子内形成的不同氢键类型密切相关,傅里叶变换红外光谱(FTIR)技术是研究氢键的强有力手段,它可以在各种环境条件下得到几乎所有生物物质的红外光谱图。一般而言,N-H 伸缩振动产生的酰胺 A 带的吸收通常在3 300- 3 440 cm-1;-CH2的不对称伸缩振动产生的酰胺B带的吸收通常在2 920-2 944 cm-1;C=O伸缩振动产生的酰胺Ⅰ带的吸收通常在1 652-1 653 cm-1;酰胺Ⅱ带、Ⅲ带的吸收峰通常出现在1 558 cm-1、1 238 cm-1。 根据图6可知重组前胶原蛋白酰胺A带的特征吸收为 3 340.11 cm-1处的强吸收,酰胺B的特征吸收为 2 927.41 cm-1处的强吸收,酰胺Ⅰ带的特征吸收为 1 650.77 cm-1处的强吸收,酰胺Ⅱ带的特征吸收为 1 546.63 cm-1处的强吸收。1 241.93 cm-1处为胶原蛋白N-H弯曲引起的酰胺Ⅲ带的特征频率。"
| [1]Ghazanfari S, Khademhosseini A, Smit TH. Mechanisms of lamellar collagen formation in connective tissues. Biomaterials. 2016;97:74-84.[2]Ricard-Blum S, Ruggiero F.The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol (Paris). 2005;53(7):430-442.[3]曾名勇,张联英,刘尊英,等. 几种鱼皮胶原蛋白的理化特性及其影响因素[J]. 中国海洋大学学报:自然科学版, 2005,35(4): 608-612.[4]但卫华,王坤余,曾睿,等. 胶原的医学应用及其发展前景[J]. 生物医学工程与临床,2004,8(1):45-48,52.[5]赵颖,陆金婷,邓超,等. 纤维化胶原蛋白海绵的制备及其自组装工艺[J]. 中国组织工程研究,2015,19(30):4820-4826.[6]汪海波,梁艳萍,李云雁,等. 交联方法对草鱼皮胶原蛋白海绵性能的影响[J]. 水产学报,2013,37(1):132-140.[7]de Wild M, Pomp W, Koenderink GH. Thermal memory in self-assembled collagen fibril networks. Biophys J. 2013; 105(1):200-210.[8]Li Y,Asadi A,Monroe MR,et al. pH effects on collagen fibrillogenesis in vitro : Electrostatic interactions and phosphate binding. Materials Science & Engineering C. 2009;29(5):1643-1649.[9]Burduk PK, Wierzchowska M, Grze?kowiak B, et al. Clinical outcome and patient satisfaction using biodegradable (NasoPore) and non-biodegradable packing, a double-blind, prospective, randomized study. Braz J Otorhinolaryngol. 2016. [Epub ahead of print][10]赵国锋,王东海.纳吸棉在鼻内镜术后鼻腔填塞的应用[J]. 中国误诊学杂志, 2010,10(3):524-525.[11]Zeng SK, Zhang CH, Lin H, et al. Isolation and characterisation of acid-solubilised collagen from the skin of Nile tilapia (Oreochromis niloticus). Food Chemistry. 2009; 116(4):879-883.[12]Yan M, Li B, Zhao X, et al. Effect of concentration, pH and ionic strength on the kinetic self-assembly of acid-soluble collagen from walleye pollock (Theragra chalcogramma) skin. Food Hydrocolloids.2012;29:199-204.[13]中国农业大学,农业部奶及奶制品质量监督检验测试中心,农业部食品质量监督检验测试中心,农业部乳品质量监督检验测试中心. 中华人民共和国农业行业标准NY/T1678-2008乳与乳制品中蛋白质的测定双缩脲比色法[J]. 农产品质量与安全, 2008(6):32-33.[14]方林,黄红海.ATR-FTIR光谱技术在高聚物研究中的应用[J]. 化学工程师,2008,22(3):33-35,50.[15]Zheng X, Hu J, Chen Y, et al. AFM study of the effects of collagenase and its inhibitors on dentine collagen fibrils. J Dent. 2012;40(2):163-171.[16]Prockop DJ,Hulmes DJS. Assembly of Collagen Fibrils de Novo from Soluble Precursors: Polymerization and Copolymerization of Procollagen, pN-Collagen, and Mutated Collagens. In: Birk DE, Mecham RP, editors. Extracellular Matrix Assembly and Structure. San Diego: Academic Press, 1994:47-90.[17]Li X, Li Y, Hua Y, et al. Effect of concentration, ionic strength and freeze-drying on the heat-induced aggregation of soy proteins. Food Chemistry. 2007; 104(4):1410-1417.[18]Marelli B, Ghezzi CE, Zhang YL, et al. Fibril formation pH controls intrafibrillar collagen biomineralization in vitro and in vivo. Biomaterials. 2015;37:252-259.[19]温祖谍. 胶原的两性及其等电点[J]. 皮革科技, 1980(4): 32-37.[20]赵燕,鲁亮,杨玲,等.草鱼皮胶原的体外自组装动力学研究[J]. 食品科学, 2014, 35(11): 21-26. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [3] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [4] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [5] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [6] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [9] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [10] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [11] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [12] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [13] | Wang Kun, He Benxiang. Asperosaponin VI therapy for Achilles tendinopathy in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 211-217. |
| [14] | Yang Xue, Wang Baoqun, Jiang Xiaowen, Zou Shengcan, Ming Jinfa, Lin Shasha. Preparation and properties of biodegradable plant polysaccharide hemostatic microspheres [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2607-2611. |
| [15] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||