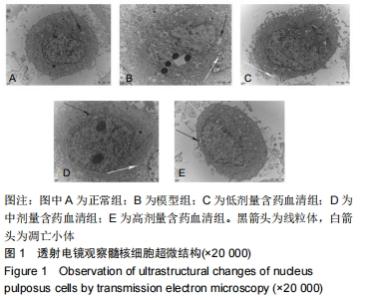
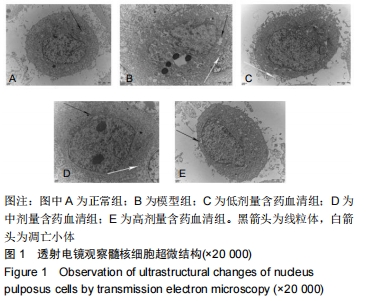
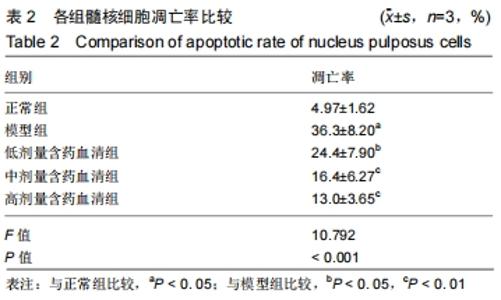
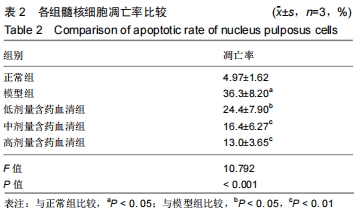
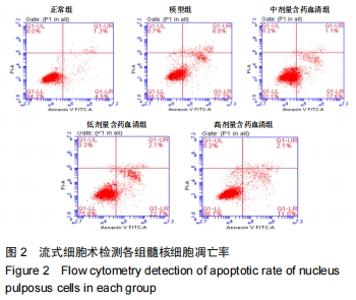
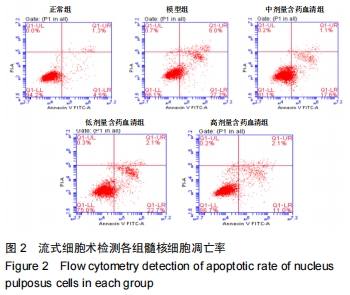
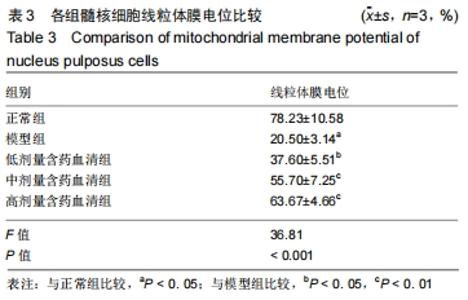
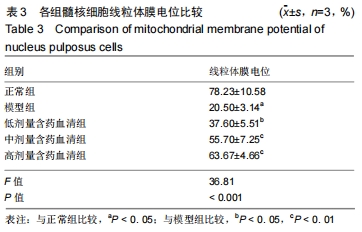
[1]ZHENG CJ, CHEN J. Disc degeneration implies low back pain. Theor Biol Med Model. 2015;12:24.
[2]王宪峰,张爱平,孙中仪,等.人退变椎间盘组织中核因子κB信号通路的变化[J].中华医学杂志,2017,97(17):1324-1329.
[3]ERWIN WM, ISLAM D, INMAN RD, et al. Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: implications for the mechanisms of intervertebral disc degeneration. Arthritis Res Ther. 2011;13(6):R215.
[4]KADOW T, SOWA G, VO N, et al. Molecular basis of intervertebral disc degeneration and herniations: what are the important translational questions? Clin Orthop Relat Res. 2015;473(6):1903-1912.
[5]PENNINGER JM, KROEMER G. Mitochondria, AIF and caspases-- rivaling for cell death execution. Nat Cell Biol. 2003;5(2):97-99.
[6]孙浩林,李淳德.原代培养差速贴壁法分离纯化大鼠腰椎间盘髓核细胞[J].中国组织工程研究与临床康复,2011,15(46):8569-8573.
[7]肖韩艳,刘颖,张岩,等.姜黄素对Aβ_(25-35)诱导的PC12细胞线粒体内细胞色素C表达的影响[J].中国实验方剂学杂志,2017,23(23):116-121.
[8]孙鹏,王拥军,施杞,等.益气化瘀方对椎间盘软骨细胞凋亡相关因子的作用[J].中国骨伤,2006,19(8):449-451.
[9]梁祖建,直彦亮.林一峰教授从督脉论治腰椎间盘突出症经验介绍[J].新中医,2010,42(1):33-34.
[10]林一峰,牛维.脊柱退行性疾病从督脉论治探讨[J].安徽中医学院学报, 2002,21(5):4-6.
[11]汤家铭,陈民利.医学实验动物学[M].北京:中国中医药出版社,2012: 209-211.
[12]林一峰,张震,刘海全,等.补肾壮督方预防腰椎间盘退变的实验研究[J].中医临床研究,2016,8(13):16-18.
[13]叶乔峰,朱金墙,王丹丹,等.中药干预细胞凋亡线粒体通路研究进展[J].中国中医药信息杂志,2013,20(8):100-103.
[14]ZHAO CQ, JIANG LS, DAI LY. Programmed cell death in intervertebral disc degeneration. Apoptosis. 2006;11(12):2079-2088.
[15]朱玉山,陈佺.线粒体与细胞凋亡调控[J].生命科学,2008,20(4):506-513.
[16]刘丽君,彭建新,洪华珠,等.线粒体在细胞凋亡中的变化与作用[J].细胞生物学杂志,2005,27(2):117-120.
[17]DING F, SHAO ZW, YANG SH, et al. Role of mitochondrial pathway in compression-induced apoptosis of nucleus pulposus cells. Apoptosis. 2012;17(6):579-590.
[18]XU F, REN L, SONG M, et al. Fas- and Mitochondria-Mediated Signaling Pathway Involved in Osteoblast Apoptosis Induced by AlCl3. Biol Trace Elem Res. 2018;184(1):173-185.
[19]曹军军,杨茂伟,郭宝磊,等.高糖通过线粒体途径诱导成骨细胞凋亡[J].中国生物化学与分子生物学报, 2012,28(12):1109-1114.
[20]BOSOI CR, YANG X, HUYNH J, et al. Systemic oxidative stress is implicated in the pathogenesis of brain edema in rats with chronic liver failure. Free Radic Biol Med. 2012;52(7):1228-1235.
[21]朱赛君,沈明勤,许尤琪.益肺散结方对小鼠Lewis肺癌组织Bcl-2、Bax、MMP-9表达的影响[J].现代中西医结合杂志, 2019,28(9):920-923,971.
[22]杨禹晗,陈超英,袁占鹏.玉竹提取物对心肌缺血再灌注损伤大鼠心肌细胞线粒体损伤及凋亡的保护作用[J].中国实验方剂学杂志, 2018,24(16): 136-140.
|