Chinese Journal of Tissue Engineering Research ›› 2020, Vol. 24 ›› Issue (19): 3101-3107.doi: 10.3969/j.issn.2095-4344.2080
Previous Articles Next Articles
Immunoregulation of dental tissue-derived mesenchymal stem cells and its significance in oral diseases and tissue regeneration
Chen Qianqian, Shen Mengjie, Yang Kun, Liu Qi
- Department of Periodontology, Stomatological Hospital Affiliated to Zunyi Medical University, Zunyi 563003, Guizhou Province, China
-
Received:2019-12-02Revised:2019-12-05Accepted:2020-01-08Online:2020-07-08Published:2020-04-09 -
Contact:Liu Qi, MD, Professor, Department of Periodontology, Stomatological Hospital Affiliated to Zunyi Medical University, Zunyi 563003, Guizhou Province, China -
About author:Chen Qianqian, Master candidate, Physician, Department of Periodontology, Stomatological Hospital Affiliated to Zunyi Medical University, Zunyi 563003, Guizhou Province, China -
Supported by:the National Natural Science Foundation of China, No. 81860196 and 81760199
CLC Number:
Cite this article
Chen Qianqian, Shen Mengjie, Yang Kun, Liu Qi. Immunoregulation of dental tissue-derived mesenchymal stem cells and its significance in oral diseases and tissue regeneration[J]. Chinese Journal of Tissue Engineering Research, 2020, 24(19): 3101-3107.
share this article
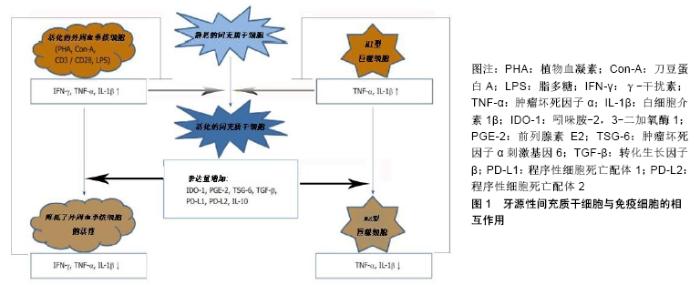
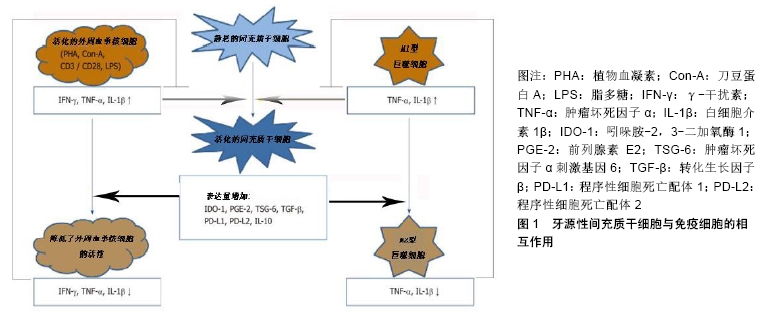
2.1 间充质干细胞介导免疫调节的基本机制 间充质干细胞是一种具有组织再生能力的细胞,并拥有强大的免疫调节能力[8]。在牙髓、人脱落乳牙、牙周韧带、根尖乳头、牙滤泡和牙龈等组织中发现了不同的间充质干细胞样细胞[8-9]。间充质干细胞免疫调节作用的潜在机制主要有酶的表达、可溶性因子的产生以及细胞间的接触[10-11]。首先间充质干细胞介导的人体免疫抑制主要由吲哚胺-2,3-二加氧酶1介导,该酶催化左旋色氨酸分解为犬尿氨酸,色氨酸的消耗导致不同免疫细胞受到抑制[12];其次参与间充质干细胞依赖性免疫调节的可溶性因子有前列腺素E2、肿瘤坏死因子α刺激基因6、肝细胞生长因子、转化生长因子β、白细胞介素10、半乳糖凝集素以及人白细胞抗原等[13]。研究发现前列腺素E2是由环氧合酶2产生的花生四烯酸级联的代谢产物,并影响先天和适应性免疫系统[14]。间充质干细胞可持续产生有效的免疫调节细胞因子转化生长因子β,其产生过程可以通过其他免疫调节细胞因子如白细胞介素4、白细胞介素13来增强[15]。抗炎细胞因子白细胞介素10可以由间充质干细胞本身或由间充质干细胞调节的免疫细胞产生[16];此外,细胞间接或直接接触也可以引起间充质干细胞的免疫抑制作用,这些作用主要通过程序性细胞死亡配体1、程序性细胞死亡配体2和膜结合人白细胞抗原所介导[17]。 2.2 牙源性间充质干细胞的免疫调节作用 牙组织来源的间充质干细胞与其他组织来源的间充质干细胞相似,可调节不同免疫细胞亚群的活性[18-19]。牙源性间充质干细胞的免疫调节活性在很大程度上取决于炎症细胞因子的激活,而这种炎症细胞因子通常是由免疫细胞产生的。牙源性间充质干细胞与活化的免疫细胞存在相互调节作用。牙源性间充质干细胞与外周血单核细胞共同培养,然后分析不同免疫细胞亚群的特异性标记和功能特征。在大多数共培养实验中,免疫细胞可以被刀豆蛋白A、植物血凝素、抗CD3/CD28抗体、脂多糖等不同的刺激物激活[20]。这些刺激物对于激活免疫细胞增殖和分化是至关重要的,它可以刺激牙源性间充质干细胞的免疫调节,见图1[21]。然而大多数这些刺激物对外周血单核细胞的激活是人为的,很难代表体内情况。 "
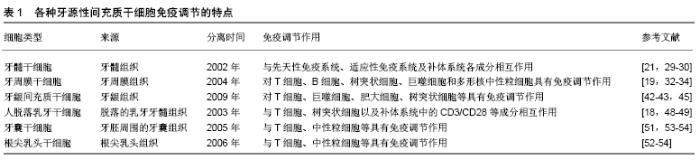
2.2.1 牙髓干细胞 GRONTHOS等[22]从牙髓中成功分离出了牙髓干细胞(dental pulp stem cells,DPSCs),并且发现牙髓干细胞与先天免疫系统、适应性免疫系统(T细胞、自然杀伤细胞、巨噬细胞)以及补体系统的各种成分之间相互作用。WADA等[19]研究发现牙髓干细胞以细胞间非接触的方式抑制同种异体植物血凝素活化或激活外周血单核细胞的增殖。另外经过γ-干扰素处理的牙髓干细胞能够抑制外周血单核细胞增殖。同时也发现经γ-干扰素处理后的牙髓干细胞可抑制T细胞增殖、减少白细胞介素17的产生并刺激调节性T细胞[23]。KWACK等[24]发现牙髓干细胞可抑制植物血凝素诱导的外周血单核细胞增殖,但对调节性T细胞的分化没有影响;与抗CD3/CD28抗体激活的外周血单核细胞共培养可抑制CD8+T细胞增殖和B细胞免疫球蛋白的产生,这种对T细胞和B细胞的调节作用通过增强γ-干扰素和转化生长因子β所介导。牙髓干细胞与植物血凝素活化的CD3+T细胞共培养可抑制T细胞增殖,诱导T细胞凋亡并刺激调节性T细胞分化,并且发现牙髓干细胞中肝细胞生长因子、人白细胞抗原、白细胞介素6和转化生长因子β的表达上调[25]。这种牙髓干细胞诱导T细胞凋亡的免疫调节作用,使其在体内具有抗炎作用[26]。成骨分化的牙髓干细胞也抑制植物血凝素激活的外周血单核细胞增殖[27]。另外牙髓干细胞还影响体内巨噬细胞极化,将牙髓干细胞移植到单侧后肢骨骼肌中,会触发巨噬细胞M2极化并抑制坐骨神经炎症发生[28]。研究发现在牙髓干细胞分化、过表达缺氧诱导因子1或与外周血单核细胞共培养后,牙髓干细胞对NK细胞介导的溶解抗性明显增强[29]。此外,经过脂磷壁酸处理过的牙髓干细胞几乎表达了所有激活补体系统所需的因子[30]。牙髓干细胞表达的C3a和C5a可以通过激活补体系统,进而影响牙髓干细胞的增殖和活化[31-32]。 2.2.2 牙周膜干细胞 2004年SEO等首次从牙周膜中分离出了牙周膜干细胞(periodontal ligament stem cells,PDLSCs)。迄今为止,牙周膜干细胞已被证明在体内外对T细胞、B细胞、树突状细胞、巨噬细胞和多形核中性粒细胞具有免疫调节作用[33]。牙周膜干细胞可通过旁分泌机制抑制外周血单核细胞的增殖,而用γ-干扰素处理牙周膜干细胞后可增强这种能力[19]。LIU等[34]发现植物血凝素刺激的外周血单核细胞与γ-干扰素刺激的牙周膜干细胞共同培养,可抑制T细胞增殖、刺激调节性T细胞分化并且由T细胞产生的白细胞介素17也相对减少;同时也发现从发炎的牙周膜组织中分离出的牙周膜干细胞会抑制T细胞的Th1分化和γ-干扰素分泌。牙周膜干细胞通过间接可溶性介质和直接细胞间接触2种方式来抑制刀豆蛋白A刺激的外周血单核细胞增殖和γ-干扰素的产生[35]。另外牙周膜干细胞抑制植物血凝素刺激的外周血单核细胞增殖以及白细胞介素2和γ-干扰素的产生[36]。进一步的研究发现牙周膜干细胞的STRO1+、CD146+亚群通过抑制树突状细胞中非经典的主要组织相容性复合物样糖蛋白CD1b的表达来抑制T细胞增殖[37]。LIU等[38]发现人牙周膜干细胞移植在小型猪牙周炎模型中会抑制体液免疫应答。目前关于牙周膜干细胞对巨噬细胞的作用仍存在争议。NAGATA等[39] 报道牙周膜干细胞可抑制鼠单核细胞/巨噬细胞RAW 264.7细胞系中肿瘤坏死因子α的表达。ZHAO等[40]并未发现牙周膜干细胞对人单核细胞/巨噬细胞THP-1细胞系极化有任何影响,但是经脂多糖处理的牙周膜干细胞可促进巨噬细胞向炎性M1表型极化。牙周膜干细胞也可以通过白细胞介素6依赖性机制减少多形核中性粒细胞的凋亡[41]。在体外脂多糖刺激的牙周膜干细胞可降低外周血单核细胞中CD29的表达并抑制外周血单核细胞的跨内皮迁移[42],由此发现牙周膜干细胞不仅能对不同免疫细胞亚群产生影响,而且还影响组织的募集。 2.2.3 牙龈间充质干细胞 2009年ZHANG等[43]首次报道了关于牙龈间充质干细胞( gingival mesenchymal stem cells ,GMSCs)的分离、表征及其免疫调节特性,并且发现牙龈间充质干细胞可抑制植物血凝素诱导的外周血单核细胞增殖。另外人牙龈间充质干细胞可抑制小鼠CD4+ T细胞的增殖和Th1/Th2/Th17分化[44]。此外,牙龈间充质干细胞可抑制M1巨噬细胞的激活并促进其向M2表型极化[45]。有研究报道牙龈间充质干细胞也可以通过前列腺素E2依赖性机制抑制外周血单核细胞来源的树突状细胞成熟和分化;而且还可以通过该机制抑制人肥大细胞1(Human mast cell-1,HMC-1)释放炎性细胞因子,但对HMC-1细胞的增殖没有影响,另外活化的HMC-1细胞可通过肿瘤坏死因子α依赖性机制上调牙龈间充质干细胞环氧合酶2表达和前列腺素E2的产 生[46]。从牙龈组织中分离出来的牙龈成纤维细胞与牙龈间充质干细胞有许多类似的特性,同样具有免疫调节能力[47]。研究发现牙龈成纤维细胞抑制了刀豆蛋白A诱导的外周血单核细胞增殖。在牙龈成纤维细胞中,γ-干扰素诱导吲哚胺-2,3-二加氧酶基因表达水平的能力明显高于白细胞介素1β和肿瘤坏死因子α[48]。 2.2.4 人脱落乳牙干细胞 2003年MIURA等[49]首先从人脱落的乳牙中分离出间充质干细胞,并称其为人脱落乳牙干细胞(stem cells of human exfoliated deciduous teeth,SHEDs),由于这些细胞是从乳牙的牙髓中获得的,与恒牙来源的牙髓干细胞相比,它们具有更强的增殖率、更快的细胞增殖和更高的成骨分化能力。YAMAZA等[18]使用抗CD3/CD28抗体激活的外周血单核细胞和未成熟的CD4+T细胞与人脱落的乳牙干细胞进行共培养,结果发现人脱落乳牙干细胞抑制Th17分化,并且这种作用强于骨髓间充质干细胞。此外,单核细胞来源树突状细胞的分化、成熟以及T细胞活化能力受人脱落乳牙干细胞的影响,尤其是树突状细胞在暴露于人脱落乳牙间充质干细胞后会增强调节性T细胞分化能力,降低炎性细胞因子白细胞介素2、肿瘤坏死因子α、γ-干扰素的数量以及促进抗炎性白细胞介素10蛋白表达上升[50]。另外人脱落乳牙干细胞也促进了小鼠骨髓来源巨噬细胞向M2表型极化[51]。 2.2.5 牙囊干细胞和根尖乳头干细胞 2005年MORSCZECK等[52]首次从发育中的牙胚周围的外胚间充质组织中分离出牙囊干细胞(dental follicle stem cells,DFSCs)并进行了鉴定。SONOYAMAD等[53]成功将根尖乳头干细胞(stem cells from apical papilla,SCAPs)分离出来并进行了鉴定。目前只有少数研究涉及到这2种牙源性间充质干细胞的免疫调节作用。TOMIC等[54]发现用Toll样受体(TLR-3/TLR-4)激动剂处理人牙囊干细胞会抑制植物血凝素刺激的外周血单核细胞增殖,这种抑制作用由吲哚胺-2,3-二加氧酶和转化生长因子β介导。人牙囊干细胞感染了牙周病原体普氏杆菌或连翘坦氏菌后,中性粒细胞趋化性、吞噬活性会降低[55]。还有研究发现根尖乳头干细胞与植物血凝素刺激的猪外周血单核细胞共培养可抑制CD3+ T细胞的增殖[56]。 牙源性间充质干细胞的免疫调节特性取决于周围微环境。牙源性间充质干细胞在被γ-干扰素、肿瘤坏死因子α、白细胞介素1β等炎性细胞因子激活后,就会产生大量的免疫细胞,从而可以显著增强它们的免疫调节潜能[57]。活化的免疫细胞可以上调牙源性间充质干细胞中免疫调节蛋白的表达[58]。激活的免疫细胞在诱导牙源性间充质干细胞的免疫调节潜能中起着关键作用,并提示这些细胞类型之间存在紧密的相互调节。另外牙源性充质干细胞中免疫调节因子的表达,也受不同的炎性细胞因子调控[59-60]。 以上6种牙源性间充质干细胞免疫调节的特点总结见表1。 "


2.3 牙源性间充质干细胞在口腔疾病及组织再生中的免疫调节 口腔是各种微生物的栖息地,宿主-微生物稳态是维持口腔健康的重要因素[61]。口腔疾病通常与这种动态平衡的破坏和细菌侵入口腔组织有关。在炎症过程中,牙源性间充质干细胞暴露于不同的细菌和病毒产物 中[62],但是其在炎症反应过程中的确切作用仍在研究;另外有研究发现牙源性间充质干细胞的免疫调节作用对组织再生也有积极的影响。 2.3.1 牙源性间充质干细胞在口腔疾病中介导的免疫调节作用 目前关于牙源性间充质干细胞在口腔疾病发病机制中的免疫调节作用仍然不清楚。牙源性间充质干细胞有时会受到不同细菌刺激,这种炎症环境可能会对其免疫调节产生重大影响[63-64]。Toll样受体家族成员表达于不同牙源性间充质干细胞中,但它们对牙源性间充质干细胞免疫调节的作用尚不十分清楚[5-6]。研究发现Toll样受体3激动剂可增强牙髓干细胞和牙囊干细胞抑制外周血单核细胞增殖能力;而Toll样受体4激动剂可增强牙囊干细胞的免疫抑制特性。还有研究发现Toll样受体3、Toll样受体4的激活反而消除了牙源性间充质干细胞抑制T细胞激活的能力[65]。这种结果也提示了Toll样受体对不同牙源性间充质干细胞介导的免疫调节作用仍有待阐明。但是目前仅有少数研究探讨了Toll样受体激动剂对牙源性间充质干细胞与不同免疫细胞亚群之间的相互作用。牙源性间充质干细胞中其他免疫调节因子的表达也受细菌产物和Toll样受体激动剂的影响。在厌氧条件下,牙周病原体牙龈卟啉单胞菌和具核梭杆菌可诱导牙囊干细胞产生白细胞介素10[66]。研究发现细菌产物和Toll样受体激动剂还诱导各种颅颌面间充质干细胞中白细胞介素1β、白细胞介素6、白细胞介素8和单核细胞趋化蛋白1炎症介质的表达[67-68]。Toll样受体激动剂可能会同时激活牙源性间充质干细胞的促炎和抗炎特性,而这些细胞在炎症反应中的确切作用取决于炎症程度和周围微环境等多种因素。 与从健康组织分离的牙周膜干细胞相比,炎症组织提取的牙周膜干细胞能促进调节性T细胞分化和抑制Th17分化[34]。类似研究发现,炎症组织分离的牙周膜干细胞和外周血单核细胞共培养,白细胞介素2、肿瘤坏死因子α、γ-干扰素的表达水平更高,但白细胞介素10的水平相似[69]。而另一研究发现从健康组织和有慢性炎症的牙周组织中分离出的牙周膜干细胞在抑制外周血单核细胞增殖的能力或环氧合酶2和白细胞介素10的表达上没有任何差异;同时与刀豆蛋白A激活的外周血单核细胞共培养后,健康组织来源的牙周膜干细胞比炎症组织来源的牙周膜干细胞表现出更低的吲哚胺-2,3-二加氧酶和更高的肿瘤坏死因子α刺激基因6表达量[35]。来自牙髓炎牙齿的牙髓干细胞不能抑制外周血单核细胞增殖,但是通过γ-干扰素处理后能够抑制外周血单核细胞增殖[70]。有研究发现,从健康组织和炎症组织中分离出的牙髓干细胞在调节巨噬细胞功能的能力上并无差异,但是炎症组织提取的牙髓干细胞在吲哚胺-2,3-二加氧酶的表达水平上显著增加[71]。 目前只有少数体内研究探讨了炎症条件下牙齿组织中免疫调节因子的表达。在小鼠模型中,注射牙龈卟啉单胞菌引起的牙周疾病的严重程度与牙周组织中程序性细胞死亡配体1的表达呈负相关[72]。实验性牙周炎大鼠局部和全身应用经肿瘤坏死因子α刺激基因6转染的间充质干细胞可显著降低骨质流失、破骨细胞形成以及全身炎性细胞因子白细胞介素1β和肿瘤坏死因子α的表达水平[2]。这些结果表明,牙源性间充质干细胞介导的免疫调节在牙髓炎、牙龈炎、牙周炎等不同口腔疾病的进展中可能存在潜在作用,在未来仍需要深入研究。 2.3.2 牙源性间充质干细胞对组织再生的免疫调节作用 尽管体外实验已经验证了牙源性间充质干细胞具有多向分化能力,但是其在体内的分化机制还不是很清楚。研究发现移植的间充质干细胞寿命都很短[73],这使其在组织再生中的临床应用受到限制[74]。与其他组织的愈合和再生类似,口腔组织的愈合和再生也经历止血、炎症、增殖和重塑4个阶段[75]。炎症细胞因子在口腔组织愈合的不同阶段具有重要作用[76-77]。免疫细胞参与组织再生的每个阶段[78]。体外研究表明,人牙周膜干细胞的分化潜能取决于炎症微环境并与其免疫调节相关,人牙周膜干细胞中的肿瘤坏死因子α刺激基因6由骨形成蛋白2诱导产生,骨形成蛋白2目前被广泛用于临床中的骨再生。此外,骨形成蛋白2减轻了人巨噬细胞THP-1细胞系的炎症反应[79-80]。牙源性间充质干细胞的再生和免疫调节功能均受转化生长因子β的影响[81]。 人牙周膜干细胞移植促进了大鼠手术引起的牙周缺损的愈合过程,并且愈合过程中牙周组织肿瘤坏死因子α的mRNA水平降低[39]。将异基因骨髓间充质干细胞局部应用到牙周缺损中,不仅有利于组织再生,而且还降低局部白细胞介素1β、肿瘤坏死因子α、γ-干扰素等表达水平[82]。将人脱落乳牙干细胞移植到牙周缺损中会刺激牙周组织再生,并伴随着M2巨噬细胞比例增加[51]。尽管这些研究没有提供任何证据表明牙源性间充质干细胞的免疫调节作用对组织再生是必不可少的,但可以表明牙源性间充质干细胞的组织再生伴随着炎症反应的免疫调节。CAO等[7]将一种携带肝细胞生长因子的腺病毒转染人牙髓干细胞,随后将其注射到小型猪牙周骨缺损模型中,12周后发现牙周骨再生显著提高。牙龈内注射肿瘤坏死因子α刺激基因6可促进大鼠牙龈切除后伤口的早期愈合,并降低白细胞介素1β和过氧化物酶的表达水平[83]。将经过γ-干扰素处理的骨髓间充质干细胞膜片移植到小鼠颅骨缺损中会诱导骨再生,这在未经处理的细胞膜片中未观察到,此外未经处理的骨髓间充质干细胞膜片移植可诱导T细胞浸润至移植区域[84]。有学者研究发现牙髓干细胞激活补体系统可以促进牙髓再生[85]。牙源性间充质干细胞的成骨、成脂、成神经等多向分化在γ-干扰素激活吲哚胺-2,3-二加氧酶后会发生改变[86]。另外牙源性间充质干细胞的分化能力也受肿瘤坏死因子α刺激基因6的影响[87-88]。牙源间充质干细胞的再生和免疫调节能力是紧密相连的,但这2种功能之间的确切关系还有待进一步阐述。 "

| [1] WADA N, GRONTHOS S, BARTOLD PM. Immunomodulatory effects of stem cells. Periodontol 2000. 2013;63(1):198-216. [2] YANG H, APRECIO RM, ZHOU X, et al. Therapeutic effect of TSG-6 engineered iPSC-derived MSCs on experimental periodontitis in rats: a pilot study. PLoS One. 2014;9(6):e100285. [3] 周典,燕飞,周泽堃,等.炎症微环境下间充质干细胞的免疫学研究及调节作用[J].中国组织工程研究,2018,22(17):2747-2754. [4] MESTAS J, HUGHES CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731-2738. [5] MEKHEMAR MK, ADAM-KLAGES S, KABELITZ D, et al. TLR-induced immunomodulatory cytokine expression by human gingival stem/ progenitor cells. Cell Immunol. 2018;326:60-67. [6] FAWZY EL-SAYED KM, KLINGEBIEL P, DÖRFER CE. Toll-like Receptor Expression Profile of Human Dental Pulp Stem/Progenitor Cells. J Endod. 2016;42(3):413-417. [7] CAO Y, LIU Z, XIE Y, et al. Adenovirus-mediated transfer of hepatocyte growth factor gene to human dental pulp stem cells under good manufacturing practice improves their potential for periodontal regeneration in swine. Stem Cell Res Ther. 2015;6:249. [8] 刘鑫,李洋.间充质干细胞对免疫细胞的影响及其在自身免疫性疾病中的应用[J].东南大学学报(医学版),2017,36(5):881-885. [9] 刘雪梅,刘尧.牙源性干细胞与炎症相互作用研究进展[J].中国实用口腔科杂志,2015,8(1):52-55. [10] MA OK, CHAN KH. Immunomodulation by mesenchymal stem cells: Interplay between mesenchymal stem cells and regulatory lymphocytes. World J Stem Cells. 2016;8(9):268-278. [11] WANG L, ZHAO Y, SHI S. Interplay between mesenchymal stem cells and lymphocytes: implications for immunotherapy and tissue regeneration. J Dent Res. 2012;91(11):1003-1010. [12] PRENDERGAST GC, SMITH C, THOMAS S, et al. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014;63(7):721-735. [13] FONTAINE MJ, SHIH H, SCHÄFER R, et al. Unraveling the Mesenchymal Stromal Cells' Paracrine Immunomodulatory Effects. Transfus Med Rev. 2016;30(1):37-43. [14] KALINSKI P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188(1):21-28. [15] NEMETH K, KEANE-MYERS A, BROWN JM, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A. 2010; 107(12):5652-5657. [16] NAJAR M, RAICEVIC G, FAYYAD-KAZAN H, et al. Bone Marrow Mesenchymal Stromal Cells Induce Proliferative, Cytokinic and Molecular Changes During the T Cell Response: The Importance of the IL-10/CD210 Axis. Stem Cell Rev Rep. 2015;11(3):442-452. [17] TIPNIS S, VISWANATHAN C, MAJUMDAR AS. Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDO. Immunol Cell Biol. 2010;88(8):795-806. [18] YAMAZA T, KENTARO A, CHEN C, et al. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther. 2010;1(1):5. [19] WADA N, MENICANIN D, SHI S, et al. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol. 2009;219(3): 667-676. [20] ARALA-CHAVES MP, HOPE L, KORN JH, et al. Role of adherent cells in immune responses to phytohemagglutinin and concanavalin A. Eur J Immunol. 1978;8(2):77-81. [21] ANDRUKHOV O, BEHM C, BLUFSTEIN A, et al. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: Implication in disease and tissue regeneration. World J Stem Cells. 2019;11(9): 604-617. [22] GRONTHOS S, MANKANI M, BRAHIM J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625-13630. [23] ÖZDEMIR AT, ÖZGÜL ÖZDEMIR RB, KIRMAZ C, et al. The paracrine immunomodulatory interactions between the human dental pulp derived mesenchymal stem cells and CD4 T cell subsets. Cell Immunol. 2016; 310:108-115. [24] KWACK KH, LEE JM, PARK SH, et al. Human Dental Pulp Stem Cells Suppress Alloantigen-induced Immunity by Stimulating T Cells to Release Transforming Growth Factor Beta. J Endod. 2017;43(1): 100-108. [25] DEMIRCAN PC, SARIBOYACI AE, UNAL ZS, et al. Immunoregulatory effects of human dental pulp-derived stem cells on T cells: comparison of transwell co-culture and mixed lymphocyte reaction systems. Cytotherapy. 2011;13(10):1205-1220. [26] ZHAO Y, WANG L, JIN Y, et al. Fas ligand regulates the immunomodulatory properties of dental pulp stem cells. J Dent Res. 2012;91(10):948-954. [27] STRUYS T, MOREELS M, MARTENS W, et al. Ultrastructural and immunocytochemical analysis of multilineage differentiated human dental pulp- and umbilical cord-derived mesenchymal stem cells. Cells Tissues Organs. 2011;193(6):366-378. [28] OMI M, HATA M, NAKAMURA N, et al. Transplantation of dental pulp stem cells suppressed inflammation in sciatic nerves by promoting macrophage polarization towards anti-inflammation phenotypes and ameliorated diabetic polyneuropathy. J Diabetes Investig. 2016;7(4):485-496. [29] TSENG HC, CACALANO N, JEWETT A. Split anergized Natural Killer cells halt inflammation by inducing stem cell differentiation, resistance to NK cell cytotoxicity and prevention of cytokine and chemokine secretion. Oncotarget. 2015;6(11):8947-8959. [30] CHMILEWSKY F, JEANNEAU C, LAURENT P, et al. Pulp fibroblasts synthesize functional complement proteins involved in initiating dentin-pulp regeneration. Am J Pathol. 2014;184(7):1991-2000. [31] CARDOSO CR, GARLET GP, MOREIRA AP, et al. Characterization of CD4+CD25+ natural regulatory T cells in the inflammatory infiltrate of human chronic periodontitis. J Leukoc Biol. 2008;84(1):311-318. [32] RUFAS P, JEANNEAU C, ROMBOUTS C, et al. Complement C3a Mobilizes Dental Pulp Stem Cells and Specifically Guides Pulp Fibroblast Recruitment. J Endod. 2016;42(9):1377-1384. [33] GONG Y, WEI B, YU L, et al. Type 2 diabetes mellitus and risk of oral cancer and precancerous lesions: a meta-analysis of observational studies. Oral Oncol. 2015;51(4):332-340. [34] LIU D, XU J, LIU O, et al. Mesenchymal stem cells derived from inflamed periodontal ligaments exhibit impaired immunomodulation. J Clin Periodontol. 2012;39(12):1174-1182. [35] LI C, WANG X, TAN J, et al. The immunomodulatory properties of periodontal ligament stem cells isolated from inflamed periodontal granulation. Cells Tissues Organs. 2014;199(4):256-265. [36] XIA Y, TANG HN, WU RX, et al. Cell Responses to Conditioned Media Produced by Patient-Matched Stem Cells Derived From Healthy and Inflamed Periodontal Ligament Tissues. J Periodontol. 2016;87(5):e53-63. [37] SHIN C, KIM M, HAN JA, et al. Human periodontal ligament stem cells suppress T-cell proliferation via down-regulation of non-classical major histocompatibility complex-like glycoprotein CD1b on dendritic cells. J Periodontal Res. 2017;52(1):135-146. [38] LIU O, XU J, DING G, et al. Periodontal ligament stem cells regulate B lymphocyte function via programmed cell death protein 1. Stem Cells. 2013;31(7):1371-1382. [39] NAGATA M, IWASAKI K, AKAZAWA K, et al. Conditioned Medium from Periodontal Ligament Stem Cells Enhances Periodontal Regeneration. Tissue Eng Part A. 2017;23(9-10):367-377. [40] ZHAO L, KANG I, FANG X, et al. Gamma-tocotrienol attenuates high-fat diet-induced obesity and insulin resistance by inhibiting adipose inflammation and M1 macrophage recruitment. Int J Obes (Lond). 2015; 39(3):438-446. [41] WANG Q, DING G, XU X. Periodontal Ligament Stem Cells Regulate Apoptosis of Neutrophils. Open Med (Wars). 2017;12:19-23. [42] KUKOLJ T, TRIVANOVIĆ D, DJORDJEVIĆ IO, et al. Lipopolysaccharide can modify differentiation and immunomodulatory potential of periodontal ligament stem cells via ERK1,2 signaling. J Cell Physiol. 2018;233(1): 447-462. [43] ZHANG Q, SHI S, LIU Y, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183(12):7787-7798. [44] CHEN M, SU W, LIN X, et al. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013;65(5):1181-1193. [45] ZHANG QZ, SU WR, SHI SH, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28(10):1856-1868. [46] SU WR, ZHANG QZ, SHI SH, et al. Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells. 2011;29(11): 1849-1860. [47] DU L, YANG P, GE S. Isolation and characterization of human gingiva-derived mesenchymal stem cells using limiting dilution method. J Dent Sci. 2016;11(3):304-314. [48] NISAPAKULTORN K, MAKRUDTHONG J, SA-ARD-IAM N, et al. Indoleamine 2,3-dioxygenase expression and regulation in chronic periodontitis. J Periodontol. 2009;80(1):114-121. [49] MIURA M, GRONTHOS S, ZHAO M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003; 100(10):5807-5812. [50] SILVA FDE S, RAMOS RN, DE ALMEIDA DC, et al. Mesenchymal stem cells derived from human exfoliated deciduous teeth (SHEDs) induce immune modulatory profile in monocyte-derived dendritic cells. PLoS One. 2014;9(5):e98050. [51] GAO X, SHEN Z, GUAN M, et al. Immunomodulatory Role of Stem Cells from Human Exfoliated Deciduous Teeth on Periodontal Regeneration. Tissue Eng Part A. 2018;24(17-18):1341-1353. [52] MORSCZECK C, GÖTZ W, SCHIERHOLZ J, et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005; 24(2):155-165. [53] SONOYAMA W, LIU Y, YAMAZA T, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34(2):166-171. [54] TOMIC S, DJOKIC J, VASILIJIC S, et al. Immunomodulatory properties of mesenchymal stem cells derived from dental pulp and dental follicle are susceptible to activation by toll-like receptor agonists. Stem Cells Dev. 2011;20(4):695-708. [55] HIEKE C, KRIEBEL K, ENGELMANN R, et al. Human dental stem cells suppress PMN activity after infection with the periodontopathogens Prevotella intermedia and Tannerella forsythia. Sci Rep. 2016;6:39096. [56] DING G, LIU Y, AN Y, et al. Suppression of T cell proliferation by root apical papilla stem cells in vitro. Cells Tissues Organs. 2010;191(5): 357-364. [57] KRAMPERA M. Mesenchymal stromal cell 'licensing': a multistep process. Leukemia. 2011;25(9):1408-1414. [58] ANDRUKHOV O, HONG JS, ANDRUKHOVA O, et al. Response of human periodontal ligament stem cells to IFN-γ and TLR-agonists. Sci Rep. 2017;7(1):12856. [59] MAHANONDA R, SA-ARD-IAM N, MONTREEKACHON P, et al. IL-8 and IDO expression by human gingival fibroblasts via TLRs. J Immunol. 2007;178(2):1151-1157. [60] TAKEGAWA D, NAKANISHI T, HIRAO K, et al. Modulatory roles of interferon-γ through indoleamine 2, 3-dioxygenase induction in innate immune response of dental pulp cells. J Endod. 2014;40(9):1382-1387. [61] OLSEN I, LAMBRIS JD, HAJISHENGALLIS G. et al. Porphyromonas gingivalis disturbs host-commensal homeostasis by changing complement function. J Oral Microbiol. 2017;9(1):1340085. [62] 王瑜,徐慕晗,郑凡君,等.间充质干细胞在炎症性疾病治疗中的基础研究和临床应用[J].中国细胞生物学学报,2019,41(4):561-572. [63] 易桥,卢燕勤,黄宏宇,等.间充质干细胞在免疫调节过程中的作用与应用进展[J].中国组织工程研究,2016,20(41):6216-6224. [64] STAGG J. Immune regulation by mesenchymal stem cells: two sides to the coin. Tissue Antigens. 2007;69(1):1-9. [65] LIOTTA F, ANGELI R, COSMI L, et al. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 2008;26(1):279-289. [66] BIEDERMANN A, KRIEBEL K, KREIKEMEYER B, et al. Interactions of anaerobic bacteria with dental stem cells: an in vitro study. PLoS One. 2014;9(11):e110616. [67] BINDAL P, RAMASAMY TS, KASIM NHA, et al. Immune responses of human dental pulp stem cells in lipopolysaccharide-induced microenvironment. Cell Biol Int. 2018;42(7):832-840. [68] ANDRUKHOV O, ANDRUKHOVA O, ÖZDEMIR B, et al. Soluble CD14 Enhances the Response of Periodontal Ligament Stem Cells to P. gingivalis Lipopolysaccharide. PLoS One. 2016;11(8):e0160848. [69] TANG HN, XIA Y, YU Y, et al. Stem cells derived from "inflamed" and healthy periodontal ligament tissues and their sheet functionalities: a patient-matched comparison. J Clin Periodontol. 2016;43(1):72-84. [70] SONODA S, YAMAZA H, MA L, et al. Interferon-gamma improves impaired dentinogenic and immunosuppressive functions of irreversible pulpitis-derived human dental pulp stem cells. Sci Rep. 2016;6:19286. [71] LEE S, ZHANG QZ, KARABUCAK B, et al. DPSCs from Inflamed Pulp Modulate Macrophage Function via the TNF-α/IDO Axis. J Dent Res. 2016;95(11):1274-1281. [72] ZHANG J, WANG CM, ZHANG P, et al. Expression of programmed death 1 ligand 1 on periodontal tissue cells as a possible protective feedback mechanism against periodontal tissue destruction. Mol Med Rep. 2016;13(3):2423-2430. [73] 陈晓,苏佳灿.骨质疏松研究热点:骨髓间充质干细胞分化命运[J].第二军医大学学报,2017,38(4):397-404. [74] 刘开蕾,刘学恒.牙源性间充质干细胞及其在牙周组织再生治疗中的应用[J].中华口腔医学研究(电子版),2015,9(1):81-84. [75] HÄMMERLE CH, GIANNOBILE WV. Biology of soft tissue wound healing and regeneration--consensus report of Group 1 of the 10th European Workshop on Periodontology. J Clin Periodontol. 2014;41 Suppl 15:S1-5. [76] MORAND DN, DAVIDEAU JL, CLAUSS F, et al. Cytokines during periodontal wound healing: potential application for new therapeutic approach. Oral Dis. 2017;23(3):300-311. [77] MIYAUCHI M, SATO S, KITAGAWA S, et al. Cytokine expression in rat molar gingival periodontal tissues after topical application of lipopolysaccharide. Histochem Cell Biol. 2001;116(1):57-62. [78] JULIER Z, PARK AJ, BRIQUEZ PS, et al. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017;53:13-28. [79] ZHANG J, LI ZG, SI YM, et al. The difference on the osteogenic differentiation between periodontal ligament stem cells and bone marrow mesenchymal stem cells under inflammatory microenviroments. Differentiation. 2014;88(4-5):97-105. [80] JAMES AW, LACHAUD G, SHEN J, et al. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng Part B Rev. 2016; 22(4):284-297. [81] NG F, BOUCHER S, KOH S, et al. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112(2):295-307. [82] DU J, SHAN Z, MA P, et al. Allogeneic bone marrow mesenchymal stem cell transplantation for periodontal regeneration. J Dent Res. 2014;93(2): 183-188. [83] BELTRAN SR, SVOBODA KK, KERNS DG, et al. Anti-inflammatory protein tumor necrosis factor-α-stimulated protein 6 (TSG-6) promotes early gingival wound healing: an in vivo study. J Periodontol. 2015; 86(1):62-71. [84] TAKESHITA K, MOTOIKE S, KAJIYA M, et al. Xenotransplantation of interferon-gamma-pretreated clumps of a human mesenchymal stem cell/extracellular matrix complex induces mouse calvarial bone regeneration. Stem Cell Res Ther. 2017;8(1):101. [85] WANG Y, CHEN X, CAO W, et al. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009-1016. [86] CROITORU-LAMOURY J, LAMOURY FM, CARISTO M, et al. Interferon-γ regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO). PLoS One. 2011;6(2):e14698. [87] LEE RH, YU JM, FOSKETT AM, et al. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc Natl Acad Sci U S A. 2014; 111(47):16766-16771. [88] QI Y, JIANG D, SINDRILARU A, et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J Invest Dermatol. 2014;134(2):526-537. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [4] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [5] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [6] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [7] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [8] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [9] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [10] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [11] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [12] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [13] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [14] | Kong Desheng, He Jingjing, Feng Baofeng, Guo Ruiyun, Asiamah Ernest Amponsah, Lü Fei, Zhang Shuhan, Zhang Xiaolin, Ma Jun, Cui Huixian. Efficacy of mesenchymal stem cells in the spinal cord injury of large animal models: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1142-1148. |
| [15] | Chen Junyi, Wang Ning, Peng Chengfei, Zhu Lunjing, Duan Jiangtao, Wang Ye, Bei Chaoyong. Decalcified bone matrix and lentivirus-mediated silencing of P75 neurotrophin receptor transfected bone marrow mesenchymal stem cells to construct tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 510-515. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||