Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (33): 5299-5304.doi: 10.3969/j.issn.2095-4344.2017.33.009
Previous Articles Next Articles
Behavior improvement and inflammatory regulation in Parkinson’s disease rats after neural stem cell transplantation
Zhou Jing1, Bao Li-wen1, Liang Jing2
- 1Medical School of Hubei Polytechnic Institute, Xiaogan 432100, Hubei Province, China; 2Sichuan Provincial People’s Hospital, Chengdu 610041, Sichuan Province, China
-
Revised:2017-08-18Online:2017-11-28Published:2017-12-01 -
About author:Zhou Jing, Master, Lecturer, Medical School of Hubei Polytechnic Institute, Xiaogan 432100, Hubei Province, China -
Supported by:the Science Research Project of Sichuan Provincial Health Department, No. 110238
CLC Number:
Cite this article
Zhou Jing, Bao Li-wen, Liang Jing. Behavior improvement and inflammatory regulation in Parkinson’s disease rats after neural stem cell transplantation[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(33): 5299-5304.
share this article
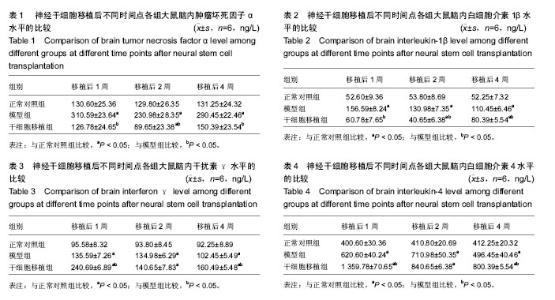
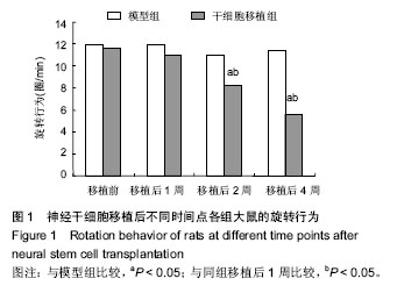
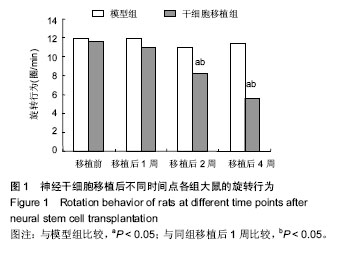
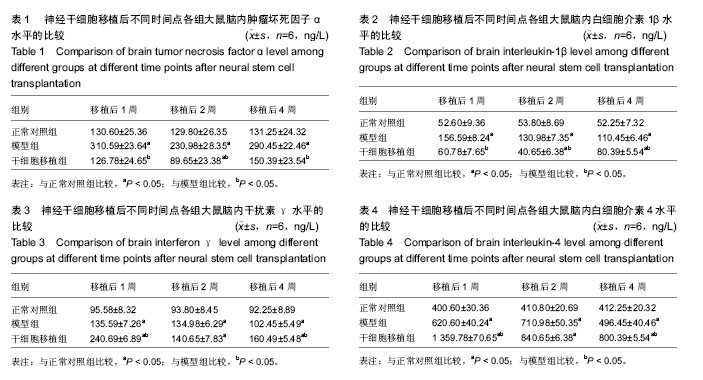
2.3 神经干细胞移植对帕金森病大鼠脑内肿瘤坏死因子α水平的影响 与正常对照组比较,模型组移植后不同时间点的肿瘤坏死因子α水平明显升高(P < 0.05);与模型组比较,干细胞移植组移植后不同时间点的肿瘤坏死因子α水平明显降低(P < 0.05);与正常对照组比较,干细胞移植组移植后2周的肿瘤坏死因子α水平明显降低(P < 0.05),其余时间点两组间比较差异无显著性意义,见表1。 2.4 神经干细胞移植对帕金森病大鼠脑内白细胞介素1β水平的影响 与正常对照组比较,模型组移植后不同时间点的白细胞介素1β水平均明显升高(P < 0.05);与模型组比较,干细胞移植组移植后不同时间点的白细胞介素1β水平均明显降低(P < 0.05);与正常对照组比较,干细胞移植组移植后2,4周的白细胞介素1β水平变化较大(P < 0.05),见表2。 2.5 神经干细胞移植对帕金森病大鼠脑内干扰素γ水平的影响 与正常对照组比较,模型组移植后不同时间点的干扰素γ水平均明显升高(P < 0.05);与模型组比较,干细胞移植组移植后1,4周的干扰素γ水平均明显升高(P < 0.05),移植2周时两组间比较差异无显著性意义;与正常对照组比较,干细胞移植组移植后不同时间点的干扰素γ水平均明显升高(P < 0.05),见表3。 2.6 神经干细胞移植对帕金森病大鼠脑内白细胞介素4水平的影响 与正常对照组比较,模型组移植后不同时间点的白细胞介素4水平均明显升高(P < 0.05);与模型组比较,干细胞移植组移植后1,4周的白细胞介素4水平均明显升高(P < 0.05),移植2周时两组间比较差异无显著性意义;与正常对照组比较,干细胞移植组移植后不同时间点的白细胞介素4水平均明显升高(P < 0.05),见表4。"
| [1] 谢姜,王斌,董方,等.人脐带间充质干细胞源的多巴胺分泌细胞治疗帕金森病模型大鼠的研究[J].天津医药,2017,45(1):21-24.[2] 马国祥,黄丽霞,刘刚,等.脐血干细胞诱导分化为多巴胺能神经元对帕金森病大鼠的治疗研究[J].中国药物经济学,2016,11(8): 77-80.[3] 肖振勇,卢国辉,覃军,等.人胎盘底蜕膜间充质干细胞纹状体移植治疗帕金森病模型大鼠的实验研究[J].右江医学,2016,44(5): 485-489.[4] 王晓晓,付文玉,庄文欣,等.慢病毒介导核受体相关因子1基因修饰骨髓间充质干细胞移植治疗帕金森病模型大鼠[J].解剖学报, 2015,46(6):742-749.[5] 张海龙,李智高,李玉,等.胚胎中脑神经干细胞移植治疗大鼠帕金森病模型[J].广东医学, 2016,37(1):49-51.[6] 吕颖,张念平,徐丽萍,等.神经干细胞移植治疗帕金森模型鼠的脑内炎症细胞因子变化[J].解剖学研究,2017,39(1):45-50.[7] 段奎甲.帕金森大鼠模型的神经干细胞移植治疗实验研究[D]. 昆明:昆明医科大学,2016.[8] Zuo FX, Bao XJ, Sun XC, et al. Transplantation of Human Neural Stem Cells in a Parkinsonian Model Exerts Neuroprotection via Regulation of the Host Microenvironment. Int J Mol Sci. 2015;16(11):26473-26492. [9] Cerri S, Greco R, Levandis G, et al. Intracarotid Infusion of Mesenchymal Stem Cells in an Animal Model of Parkinson's Disease, Focusing on Cell Distribution and Neuroprotective and Behavioral Effects. Stem Cells Transl Med. 2015;4(9):1073-1085.[10] Hoban DB, Howard L, Dowd E. GDNF-secreting mesenchymal stem cells provide localized neuroprotection in an inflammation-driven rat model of Parkinson's disease. Neuroscience. 2015;303:402-411. [11] Schwerk A, Altschüler J, Roch M, et al. Adipose-derived human mesenchymal stem cells induce long-term neurogenic and anti-inflammatory effects and improve cognitive but not motor performance in a rat model of Parkinson's disease. Regen Med. 2015;10(4):431-446. [12] Daviaud N, Garbayo E, Sindji L, et al. Survival, differentiation, and neuroprotective mechanisms of human stem cells complexed with neurotrophin-3-releasing pharmacologically active microcarriers in an ex vivo model of Parkinson's disease. Stem Cells Transl Med. 2015;4(6): 670-684. [13] Acquarone M, de Melo TM, Meireles F, et al. Mitomycin-treated undifferentiated embryonic stem cells as a safe and effective therapeutic strategy in a mouse model of Parkinson's disease. Front Cell Neurosci. 2015;9:97. [14] Gonzalez R, Garitaonandia I, Crain A, et al. Proof of concept studies exploring the safety and functional activity of human parthenogenetic-derived neural stem cells for the treatment of Parkinson's disease. Cell Transplant. 2015; 24(4):681-690.[15] Rockenstein E, Desplats P, Ubhi K, et al. Neuropeptide Treatment with Cerebrolysin Enhances the Survival of Grafted Neural Stem Cell in an α-Synuclein Transgenic Model of Parkinson's Disease. J Exp Neurosci. 2016;9(Suppl 2): 131-140.[16] Shin ES, Hwang O, Hwang YS, et al. Enhanced efficacy of human brain-derived neural stem cells by transplantation of cell aggregates in a rat model of Parkinson's disease. J Korean Neurosurg Soc. 2014;56(5):383-389.[17] Lopez WO, Nikkhah G, Maciaczyk J. Embryonic stem cells in neurology - current clinical transplantation trials in Parkinson's (PD) and Huntington's (HD) disease. Arq Neuropsiquiatr. 2014;72(12):978-979.[18] Teixeira FG, Carvalho MM, Panchalingam KM, et al. Impact of the Secretome of Human Mesenchymal Stem Cells on Brain Structure and Animal Behavior in a Rat Model of Parkinson's Disease. Stem Cells Transl Med. 2017;6(2): 634-646. [19] Safari M, Jafari B, Zarbakhsh S,et al. G-CSF for mobilizing transplanted bone marrow stem cells in rat model of Parkinson's disease. Iran J Basic Med Sci. 2016;19(12): 1318-1324.[20] Chen D, Fu W, Zhuang W,et al. Therapeutic effects of intranigral transplantation of mesenchymal stem cells in rat models of Parkinson's disease. J Neurosci Res. 2017;95(3): 907-917.[21] Aliaghaei A, Gardaneh M, Maghsoudi N, et al. Dopaminergic Induction of Umbilical Cord Mesenchymal Stem Cells by Conditioned Medium of Choroid Plexus Epithelial Cells Reduces Apomorphine-Induced Rotation in Parkinsonian Rats. Arch Iran Med. 2016;19(8):561-570.[22] Taouki I, Tasiudi E, Lalioti ME, et al. Geminin Participates in Differentiation Decisions of Adult Neural Stem Cells Transplanted in the Hemiparkinsonian Mouse Brain. Stem Cells Dev. 2017;26(16):1214-1222.[23] Napoli E, Borlongan CV. Cell Therapy in Parkinson's Disease: Host Brain Repair Machinery Gets a Boost From Stem Cell Grafts. Stem Cells. 2017;35(6):1443-1445.[24] Chumarina M, Azevedo C, Bigarreau J, et al. Derivation of mouse embryonic stem cell lines from tyrosine hydroxylase reporter mice crossed with a human SNCA transgenic mouse model of Parkinson's disease. Stem Cell Res. 2017;19:17-20.[25] Takahashi H, Ishikawa H, Tanaka A. Regenerative medicine for Parkinson's disease using differentiated nerve cells derived from human buccal fat pad stem cells. Hum Cell. 2017;30(2):60-71.[26] Kawano E, Toriumi T, Iguchi S, et al. Induction of neural crest cells from human dental pulp-derived induced pluripotent stem cells. Biomed Res. 2017;38(2):135-147.[27] Lax E, Sapozhnikov DM. Adult Neural Stem Cell Multipotency and Differentiation Are Directed by the Methyl-CpG-Binding Protein MBD1. J Neurosci. 2017;37(16):4228-4230.[28] 刘定华,顾鲁军,韩伯军,等.自体骨髓间充质神经干细胞移植治疗帕金森病的疗效观察[J].中华物理医学与康复杂志,2016,38(3): 194-198.[29] 王明国,李智高,杨智勇,等. 多巴胺神经元联合中脑神经干细胞移植治疗帕金森病[J].中国微侵袭神经外科杂志, 2016,21(3): 127-130.[30] Zuo FX, Bao XJ, Sun XC, et al. Transplantation of Human Neural Stem Cells in a Parkinsonian Model Exerts Neuroprotection via Regulation of the Host Microenvironment. Int J Mol Sci. 2015;16(11):26473-26492. [31] Chen NN, Wei F, Wang L, et al. Tumor Necrosis Factor Alpha Induces Neural Stem Cell Apoptosis Through Activating p38 MAPK Pathway. Neurochem Res. 2016;41(11):3052-3062.[32] Eckert A, Huang L, Gonzalez R, et al. Bystander Effect Fuels Human Induced Pluripotent Stem Cell-Derived Neural Stem Cells to Quickly Attenuate Early Stage Neurological Deficits After Stroke. Stem Cells Transl Med. 2015;4(7):841-851. [33] Wang L, Wei FX, Cen JS, et al. Early administration of tumor necrosis factor-alpha antagonist promotes survival of transplanted neural stem cells and axon myelination after spinal cord injury in rats. Brain Res. 2014;1575:87-100. [34] Ben-Menachem-Zidon O, Ben-Menahem Y, Ben-Hur T, et al. Intra-hippocampal transplantation of neural precursor cells with transgenic over-expression of IL-1 receptor antagonist rescues memory and neurogenesis impairments in an Alzheimer's disease model. Neuropsychopharmacology. 2013;38(13):2736.[35] Green HF, Treacy E, Keohane AK, et al. A role for interleukin-1β in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol Cell Neurosci. 2012;49(3):311-321. [36] Wang X, Fu S, Wang Y, et al. Interleukin-1beta mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway. Mol Cell Neurosci. 2007;36(3):343-354.[37] Gao M, Dong Q, Zhang H, et al. Syringe needle skull penetration reduces brain injuries and secondary inflammation following intracerebral neural stem cell transplantation. Exp Ther Med. 2017;13(3):885-890.[38] Wang Y, Zhou K, Li T, et al. Inhibition of autophagy prevents irradiation-induced neural stem and progenitor cell death in the juvenile mouse brain. Cell Death Dis. 2017;8(3):e2694.[39] Choi JY, Kim JY, Kim JY, et al. M2 Phenotype Microglia-derived Cytokine Stimulates Proliferation and Neuronal Differentiation of Endogenous Stem Cells in Ischemic Brain. Exp Neurobiol. 2017;26(1):33-41.[40] Uchida K, Takano S, Matsumoto T, et al. Transforming growth factor activating kinase 1 regulates extracellular matrix degrading enzymes and pain-related molecule expression following tumor necrosis factor-α stimulation of synovial cells: an in vitro study. BMC Musculoskelet Disord. 2017;18(1):283.[41] Simpson NJ, Ferguson AV. The pro-inflammatory cytokine tumor necrosis factor alpha excites subfornical organ neurons. J Neurophysiol. 2017 Jun 21. [Epub ahead of print][42] Li HB, Wu F, Miao HC, et al. Effects of Polysaccharide of Gastrodia Elata Blume and Electro-Acupuncture on Expressions of Brain-Derived Neurotrophic Factor and Stem Cell Factor Protein in Caudate Putamen of Focal Cerebral Ischemia Rats. Med Sci Monit Basic Res. 2016;22:175-180.[43] Holló K, Ducza L, Hegyi Z, et al. Interleukin-1 receptor type 1 is overexpressed in neurons but not in glial cells within the rat superficial spinal dorsal horn in complete Freund adjuvant-induced inflammatory pain. J Neuroinflammation. 2017;14(1):125.[44] Lei XD, Sun Y, Cai SJ, et al. Role of tumor necrosis factor-alpha in zebrafish retinal neurogenesis and myelination. Int J Ophthalmol. 2016;9(6):831-837.[45] Guadagno J, Swan P, Shaikh R, et al. Microglia-derived IL-1β triggers p53-mediated cell cycle arrest and apoptosis in neural precursor cells. Cell Death Dis. 2015;6:e1779.[46] Mammari N, Vignoles P, Halabi MA, et al. Interferon gamma effect on immune mediator production in human nerve cells infected by two strains of Toxoplasma gondii. Parasite. 2015;22:39.[47] Ahn J, Lee J, Kim S. Interferon-gamma inhibits the neuronal differentiation of neural progenitor cells by inhibiting the expression of Neurogenin2 via the JAK/STAT1 pathway. Biochem Biophys Res Commun. 2015;466(1):52-59. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Fan Yiming, Liu Fangyu, Zhang Hongyu, Li Shuai, Wang Yansong. Serial questions about endogenous neural stem cell response in the ependymal zone after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1137-1142. |
| [4] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [5] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [6] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [7] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [8] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [9] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [10] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [11] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [12] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [13] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [14] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [15] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||