Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (25): 4044-4049.doi: 10.3969/j.issn.2095-4344.2017.25.018
Previous Articles Next Articles
Different types of allogeneic hematopoietic stem cell transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia: therapeutic efficacy and safety
Hu Xiao-hui1, Zhou Jie2, Zhou Hai-xia1, Zhang Ri1, Wu De-pei1
- 1Jiangsu Provincial Hematology Institute, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China; 2ICU, Suzhou Municipal Hospital, Suzhou 215008, Jiangsu Province, China
-
Revised:2017-07-19Online:2017-09-08Published:2017-10-09 -
Contact:Zhang Ri, Doctoral supervisor, Chief physician, Jiangsu Provincial Hematology Institute, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China -
About author:Hu Xiao-hui, M.D., Associate chief physician, Master’s supervisor, Jiangsu Provincial Hematology Institute, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China -
Supported by:Jiangsu Province Science and Education Project for Medicine Revitalization - Clinical Medicine Center, No. ZX201102; National Clinical Specialist Construction Projects; Health and Public Welfare Special Funds, No. 201202017
CLC Number:
Cite this article
Hu Xiao-hui, Zhou Jie, Zhou Hai-xia, Zhang Ri, Wu De-pei. Different types of allogeneic hematopoietic stem cell transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia: therapeutic efficacy and safety[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(25): 4044-4049.
share this article
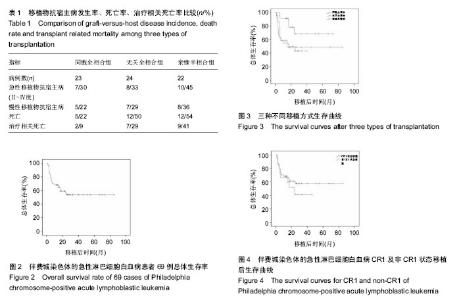
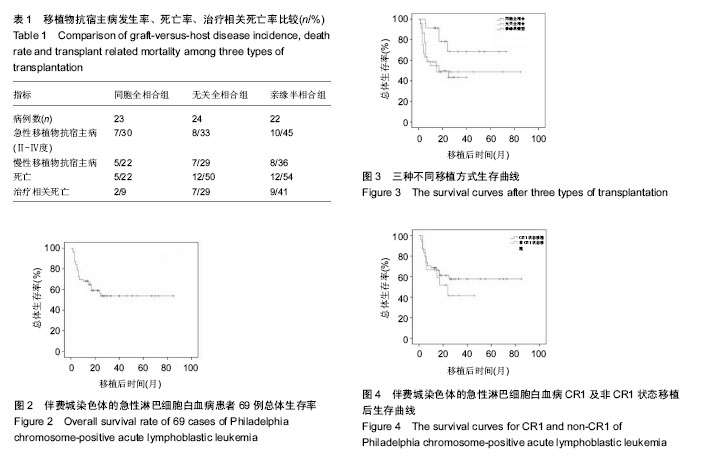
2.2 干细胞回输数量 同胞全相合组回输单个核细胞中位数7.06(1.7-13.11)×108/kg,CD34+细胞中位数4.18 (1.02-6.55)×106/kg;无关全相合组单个核细胞中位数7.76 (4.75-15.00)×108/kg,CD34+细胞中位数4.38(2.84-10.00)× 106/kg;单倍型组单个核细胞中位数9.65(1.27-20.39)× 108/kg,CD34+细胞中位数3.22(1.73-8.56)×106/kg。 2.3 造血重建 同胞全相合及无关全相合组全部造血重建,中性粒细胞≥0.5×109 L-1的中位时间分别为12(10- 15) d,12(11-15) d;血小板≥20×109 L-1的中位时间分别为13(10-30) d,12.5(10-15) d。亲缘单倍型移植组有2例巨核系未达到植活指标,其余重建患者中性粒细胞≥0.5× 109 L-1的中位时间为13(10-21) d,血小板≥20×109 L-1的中位时间为21(11-66) d。移植后定期监测供受者嵌合度水平,68例患者移植2周后供受者嵌合度持续大于95%。1例单倍型移植患者移植后1个月时供受者嵌合度下降至25.4%,考虑植入失败。 2.4 移植物抗宿主病 ①同胞全相合移植患者中,发生急性移植物抗宿主病7例,发生率30%(7/23),其中Ⅱ度移植物抗宿主病4例,Ⅲ-Ⅳ度3例;发生慢性移植物抗宿主病5例,发生率22%(5/23),其中3例为局限性,2例广泛性;②无关全相合移植患者中,发生急性移植物抗宿主病8例,发生率33%(8/24),其中Ⅱ度4例,Ⅲ-Ⅳ度4例;发生慢性移植物抗宿主病7例,发生率29%(7/24),4例为局限性,3例广泛性;③亲缘单倍型移植患者中,发生急性移植物抗宿主病10例,发生率45%(10/22),其中Ⅱ度5例,Ⅲ度3例,Ⅳ度2例;发生慢性移植物抗宿主病8例,发生率36%(8/22),4例为局限性,4例广泛性。统计学分析显示,各移植组间急慢性移植物抗宿主病发生率差异无显著性意义(P > 0.05),见表1。 2.5 白血病复发 随访至2014年11月底,同胞全相合移植组患者共有4例(17%)复发,其中1例发生中枢神经系统白血病,经三联鞘注化疗后缓解并存活;无关全相合组5例(21%)复发;单倍型移植组3例(14%)复发,其中1例发生中枢神经系统白血病,经三联鞘注治疗后脑脊液幼稚细胞转阴,但最终死于重度慢性移植物抗宿主病。各移植组间复发率比较,差异无显著性意义(P > 0.05),见表1。 2.6 其他并发症 移植后动态监测CMV-DNA,EBV-DNA拷贝数,发现CMV血症35例,EBV血症10例,接受系统性抗病毒、美罗华等治疗。移植后出现发热、咳嗽、低氧血症等患者,及时行肺部CT、肺功能等检查,并进行相应的抗感染及抗移植物抗宿主病治疗。 2.7 疗效分析 69例患者自移植日起,随访至2014年11月底,患者3年总体生存率54%(图2)。 同胞全相合组中位随访时间23.8(5.5-60)个月,死亡5例,其中移植相关死亡2例、复发死亡3例,1例患者移植后出现中枢神经系统白血病经三联鞘注化疗后缓解并生存,总体生存率68%。 无关全相合组中位随访时间17.4(1.5-60)个月,死亡12例,其中移植相关死亡7例,复发死亡5例,3年总体生存率为49%。 单倍型组中位随访时间17.7(1.5-40)个月,死亡12例,其中移植相关死亡9例,复发死亡3例,3年总体生存率为43%(图3)。 同胞全相合组与无关供体全相合组、亲缘单倍型组分别比较,差异均有显著性意义(P均< 0.05),而无关供体全相合组与亲缘单倍型组差异无显著性意义。 54例CR1状态下行移植患者,3年总体生存率58%;15例于非CR1状态移植患者,3年总体生存率为41%,但两组间差异无显著性意义,见图4。"
| [1] Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166-178.[2] Terwey TH, Kim TD, Arnold R. Allogeneic hematopoietic stem cell transplantation for adult acute lymphocytic leukemia. Curr Hematol Malig Rep. 2009;4(3):139-147.[3] Fielding AK. How I treat Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2010;116(18): 3409-3417.[4] Faderl S, Kantarjian HM, Thomas DA, et al. Outcome of Philadelphia chromosome-positive adult acute lymphoblastic leukemia. Leuk Lymphoma. 2000;36(3-4):263-273.[5] Thomas X, Thiebaut A, Olteanu N, et al. Philadelphia chromosome positive adult acute lymphoblastic leukemia: characteristics, prognostic factors and treatment outcome. Hematol Cell Ther. 1998;40(3):119-128.[6] Dombret H, Gabert J, Boiron JM, et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia--results of the prospective multicenter LALA-94 trial. Blood. 2002;100(7):2357-2366.[7] Bassan R, Rohatiner AZ, Lerede T, et al. Role of early anthracycline dose-intensity according to expression of Philadelphia chromosome/BCR-ABL rearrangements in B-precursor adult acute lymphoblastic leukemia. Hematol J. 2000;1(4):226-234.[8] Gleissner B, Gökbuget N, Bartram CR, et al. Leading prognostic relevance of the BCR-ABL translocation in adult acute B-lineage lymphoblastic leukemia: a prospective study of the German Multicenter Trial Group and confirmed polymerase chain reaction analysis. Blood. 2002;99(5): 1536-1543.[9] Fielding AK, Rowe JM, Richards SM, et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood. 2009;113(19):4489-4496.[10] Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103(12):4396-4407.[11] Tanguy-Schmidt A, Rousselot P, Chalandon Y, et al. Long-term follow-up of the imatinib GRAAPH-2003 study in newly diagnosed patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: a GRAALL study. Biol Blood Marrow Transplant. 2013;19(1): 150-155.[12] Yanada M, Ohno R, Naoe T. Recent advances in the treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia. Int J Hematol. 2009;89(1):3-13.[13] Mizuta S, Matsuo K, Yagasaki F, et al. Pre-transplant imatinib-based therapy improves the outcome of allogeneic hematopoietic stem cell transplantation for BCR-ABL-positive acute lymphoblastic leukemia. Leukemia. 2011;25(1):41-47.[14] Lee HJ, Thompson JE, Wang ES, et al. Philadelphia chromosome-positive acute lymphoblastic leukemia: current treatment and future perspectives. Cancer. 2011;117(8): 1583-1594.[15] Chalandon Y, Thomas X, Hayette S, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711-3719.[16] Hatta Y, Mizuta S, Ohtake S, et al. Promising outcome of imatinib-combined chemotherapy followed by allogeneic hematopoietic stem cell transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia: results of the Japan Adult Leukemia Study Group (JALSG) Ph+ALL202 Regimen. Blood. 2009;114:3090.[17] Tanguy-Schmidt A, Rousselot P, Chalandon Y, et al. Long-term follow-up of the imatinib GRAAPH-2003 study in newly diagnosed patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: a GRAALL study. Biol Blood Marrow Transplant. 2013;19(1): 150-155.[18] Huang XJ, Liu DH, Liu KY, et al. Treatment of acute leukemia with unmanipulated HLA-mismatched/haploidentical blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2009;15(2):257-265.[19] Chen XH, Gao L, Zhang X, et al. HLA-haploidentical blood and bone marrow transplantation with anti-thymocyte globulin: long-term comparison with HLA-identical sibling transplantation. Blood Cells Mol Dis. 2009;43(1):98-104.[20] Kanda Y, Chiba S, Hirai H, et al. Allogeneic hematopoietic stem cell transplantation from family members other than HLA-identical siblings over the last decade (1991-2000). Blood. 2003;102(4):1541-1547.[21] Szydlo R, Goldman JM, Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15(5): 1767-1777.[22] Shaw BE, Gooley TA, Malkki M, et al. The importance of HLA-DPB1 in unrelated donor hematopoietic cell transplantation. Blood. 2007;110(13):4560-4566.[23] Hambach L, Spierings E, Goulmy E. Risk assessment in haematopoietic stem cell transplantation: minor histocompatibility antigens. Best Pract Res Clin Haematol. 2007;20(2):171-187.[24] Ruggeri L, Mancusi A, Burchielli E, et al. NK cell alloreactivity and allogeneic hematopoietic stem cell transplantation. Blood Cells Mol Dis. 2008;40(1):84-90.[25] Ichinohe T, Maruya E, Saji H. Long-term feto-maternal microchimerism: nature's hidden clue for alternative donor hematopoietic cell transplantation. Int J Hematol. 2002;76(3): 229-237.[26] Giebel S, Labopin M, Gorin NC, et al. Improving results of autologous stem cell transplantation for Philadelphia-positive acute lymphoblastic leukaemia in the era of tyrosine kinase inhibitors: a report from the Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation. Eur J Cancer. 2014;50(2):411-417.[27] Wetzler M, Watson D, Stock W, et al. Autologous transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia achieves outcomes similar to allogeneic transplantation: results of CALGB Study 10001 (Alliance). Haematologica. 2014;99(1):111-115.[28] Shin HJ, Chung JS, Cho GJ. Imatinib interim therapy between chemotherapeutic cycles and in vivo purging prior to autologous stem cell transplantation, followed by maintenance therapy is a feasible treatment strategy in Philadelphia chromosome-positive acute lymphoblastic leukemia. Bone Marrow Transplant. 2005;36(10):917-918.[29] Ribera JM, Oriol A, González M, et al. Concurrent intensive chemotherapy and imatinib before and after stem cell transplantation in newly diagnosed Philadelphia chromosome- positive acute lymphoblastic leukemia. Final results of the CSTIBES02 trial. Haematologica. 2010;95(1):87-95.[30] Linker C, Damon L, Martin T, et al. Autologous hematopoietic cell transplantation for high-risk ALL. Bone Marrow Transplant. 2011;46(3):460-461.[31] Bassan R, Rossi G, Pogliani EM, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. 2010;28(22):3644-3652. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [3] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [4] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [5] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [6] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [9] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [10] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [11] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [12] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [13] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [14] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| [15] | Fang Xiaoyang, Tang Tian, Wang Nan, Qian Yuzhang, Xie Lin. Repair and regenerative therapies of the annulus fibrosus [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1582-1587. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||