Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (4): 621-626.doi: 10.3969/j.issn.2095-4344.2017.04.022
Previous Articles Next Articles
Research progress of extracellular vesicles
Wang Jin1, Chen Jian-ying2
- 1Graduate School of Guangdong Medical University, Zhanjiang 524001, Guangdong Province, China; 2Third Department of Cardiology, Affiliated Hospital of Guangdong Medical University, Zhanjiang 524001, Guangdong Province, China
-
Received:2016-11-26Online:2017-02-08Published:2017-03-13 -
Contact:Corresponding author: Chen Jian-ying, Chief physician, Master’s supervisor, Third Department of Cardiology, Affiliated Hospital of Guangdong Medical University, Zhanjiang 524001, Guangdong Province, China -
About author:Wang Jin, Master, Graduate School of Guangdong Medical University, Zhanjiang 524001, Guangdong Province, China -
Supported by:the National Natural Science Foundation of China, No. 81370242
CLC Number:
Cite this article
Wang Jin1, Chen Jian-ying2 . Research progress of extracellular vesicles[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(4): 621-626.
share this article
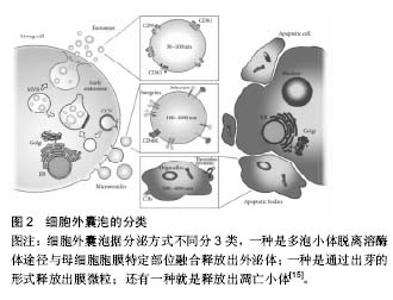
2.1 细胞外囊泡定义分类 细胞外囊泡是由脂质双分子层包绕形成的球状膜性囊泡,由细胞分泌产生,分子直径在4 000 nm间,其中包括外泌体、膜微粒、微囊泡及凋亡小体等,外泌体定义较为明确[12-13]。关于细胞外囊泡的分类,依据细胞来源命名分类是其常见分类方法之一。以膜微粒为例,内皮细胞来源的膜微粒命名为内皮细胞膜微粒、血小板来源的膜微粒、间充质干细胞来源的膜微粒等。此外,根据分子大小、释放方式的不同可将细胞外囊泡分为3种类型,即外泌体、膜微粒及凋亡小体。见图2。 2.2 细胞外囊泡形成、释放机制及过程 细胞外囊泡主要存在于细胞生存的微环境中,如细胞培养上清以及各种体液(血液、淋巴液、唾液、尿液、精液及乳汁)等[14]。几乎所有的细胞都可自发或在一定刺激条件下产生和释放细胞外囊泡,如上皮细胞、未成熟树突状细胞等会自发的产生和释放细胞外囊泡[15]。刺激条件主要包括细"
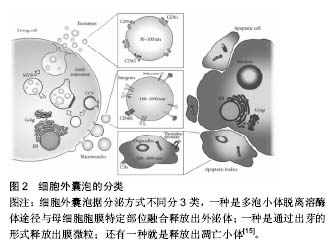
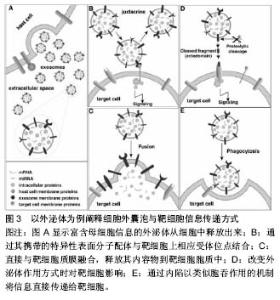
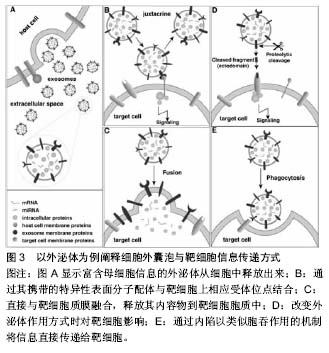
胞活化、氧化应激、细胞癌性转化、放射损伤、细胞死亡和(或)凋亡等[16],如骨髓间充质干细胞经低氧或低营养诱导后会分泌释放细胞外囊泡[17]。 细胞外囊泡形成、释放的分子机制不完全清楚,可能与细胞膜重构和细胞骨架改变有关。既往蛋白质组学、基因芯片分析及RT-PCR等显示细胞外囊泡选择性地将母细胞来源的多种生物活性物质包装进其双分子层膜结构中或携带于膜表面,如脂质(如富含胆固醇和鞘磷)、细胞因子、趋化因子、生长因子、特异性及非特异性蛋白、DNA、mRNA、microRNA、lncRNA、circRNA等[14,18-21]。 外泌体:分子直径30-100 nm,也有很多学者认为在150 nm范围内的,来源于细胞质膜内陷的初级核内体的微粒都属于外泌体,这类囊泡在细胞内部形成,与一般的出芽方式不同。其形成释放过程是,母细胞释放颗粒物质到质膜内陷形成的核内体腔内囊泡中,含有腔内囊泡的核内体成为次级核内体,也称作多囊泡胞内体或多泡小体。随后,多泡小体离开溶酶体途径而与母细胞胞膜特定部位融合,之后胞内体中的微小囊泡朝向内体囊腔的内部形成芽泡,继而母细胞将这种芽泡以外泌的形式释放到细胞外。由于外泌体特殊的分泌方式,其所含蛋白组分有别于其他细胞外囊泡,不含有内质网内的蛋白质,而高表达与主要组织相容性复合体以及整合素等多种蛋白质相互作用的四分子交联体超蛋白家族,如CD9,CD81,CD63以及热休克蛋白,如热休克蛋白60,70,90和部分细胞内源性蛋白如Alix,Tsg101等,低表达磷脂酰丝氨酸。 微囊泡:分子直径100-1 000 nm,也有文献称之为脱落囊泡或Ectosomes。形成过程相对简单,是直接通过出芽方式从母细胞膜表面脱落产生,大小不均一,高表达磷脂酰丝氨酸,没有特定的表面分子标记物,但和外泌体一样表达母细胞来源的表面标记物[22]。如EMV表达CD31,PMV表达CD42b,白细胞来源的表达CD45,网织红细胞来源的表达转铁蛋白受体分子,抗原呈递细胞来源的富含MHC-Ⅰ,Ⅱ。微囊泡中也含有如金属蛋白酶等外泌体中不包含的一些物质。 凋亡小体:是细胞程序性死亡或凋亡晚期释放的微粒,文章不赘述,这类囊泡颗粒较大,分子直径为500- 4 000 nm,具有Annexin V高亲和力,富含死细胞(凋亡细胞)碎片的浓缩DNA,能将这些DNA运载入巨噬细胞细胞核中。 2.3 细胞外囊泡分离提取和鉴定方法 目前,学界普遍认同差速离心法是提取细胞外囊泡的标准方法[23]。先低速离心去除死亡的细胞和大的细胞碎片,再高速离心去除可溶性蛋白质、蛋白质聚集体及其他与细胞外囊泡共沉淀的杂质,即可分离得到微囊泡和外泌体。对细胞外囊泡纯度要求较高时可采用密度梯度离心或免疫磁珠分选的方法来纯化细胞外囊泡。前者是在超速离心力作用下,使蔗糖溶液形成从低到高连续分布的密度阶层,是一种区带分离法。免疫磁珠分选技术则是利用抗原抗体特异性结合的原理,用包被抗标记物抗体的磁珠与细胞外囊泡孵育后结合,这样就能将特定的细胞外囊泡分选出来。此外,分离提取细胞外囊泡的方法还有诸如微孔过滤技术、微流控技术、高效液相色谱法以及细胞外囊泡提取试剂盒。随着研究的深入,目前对细胞外囊泡也进行蛋白、DNA/RNA提取,有学者使用传统方法提取,也有研究者用BioVision等公司的提取试剂盒,总的来说各种技术各有利弊。关于细胞外囊泡的鉴定,已有许多技术能表征以及分析纳米颗粒和纳米囊泡。其中包括:蛋白免疫印迹、流式细胞学、酶联免疫吸附分析、动态光散射、透射电镜检查法、纳米颗粒跟踪分析技术。蛋白免疫印迹和酶联免疫吸附分析技术是对细胞外囊泡中蛋白进行分析,流式细胞学技术能分析细胞外囊泡携带的细胞表面抗原标记物;透射电镜检查法技术可以分析细胞外囊泡分子直径和形态结构;纳米颗粒跟踪分析技术可实时地对低浓度的细胞外囊泡悬浮液中50-1 000 nm直径范围内特定的细胞外囊泡进行分子波峰及浓度测定。 2.4 细胞外囊泡生物学功能 2.4.1 细胞外囊泡所携带信息物质 目前Vesiclepedia中记录了来自33种不同种类的538份研究统计分析出细胞外囊泡中含有92 897种蛋白,27 642 种mRNAs, 4 934种 miRNAs和584种脂质等(数据是在2015年9月前收录)[24]。蛋白质谱分析发现这些蛋白包括血管内皮生长因子、碱性成纤维细胞生长因子、血小板衍生生长因子、转化生长因子β、MAPK通路信号分子、RHO通路信号分子、Tie-2/TEK及细胞黏附分子等。这些蛋白质分子部分是酶类,酶催化具有高效性,能够放大其效应,相比RNA,进入内皮细胞的蛋白质能够即时迅速发挥作用,在维持血管新生以及维持血管功能及修复损伤组织等方面起主要作用。Ratajczak等[25]证实小鼠胚胎干细胞来源的MVs能把蛋白质和mRNA转运到造血祖细胞中,并使其重新编程,这一作用经RNase处理微粒后不复存在,表明有微粒转运的mRNA不仅能稳定存在于靶细胞,还能在靶细胞内被翻译成相应蛋白。近年来有研究者报道在这类囊泡中发现lncRNA、circRNA等noncodingRNA及gDNA[20-21,26]。microRNA (miRNA)是一类长约22 nt且高度保守的单链小分子RNA,本身不编码蛋白质但能够在转录后水平调控基因的表达。且一个miRNA能够调控多个mRNA,而一个mRNA能同时被多个miRNA调控,目前已知miRNA的功能有调控细胞的增殖、分化和代谢,并与心血管疾病、肿瘤、白血病等疾病的发生、发展有着密切联系。lncRNA即长链非编码RNA,是一类本身不编码蛋白、转录本长度超过200 nt的长链非编码RNA,能干扰mRNA的剪切,他在表观遗传调控、转录调控以及转录后调控等多层面调控基因的表达。circRNA即环状RNA,是一类特殊的非编码RNA分子,富含miRNA结合位点,在细胞中起到miRNA海绵的作用,进而解除miRNA对靶基因的抑制作用,升高靶基因的表达水平。Collino等[27]通过miRNA芯片对比骨髓MSC和肝脏MSC与两者分泌的微粒发现一些miRNA同时存在于微粒和来源母细胞中,一些miRNA只存在于母细胞中,一些miRNA则只存在微粒中。Chen等[19]的一项人脐带间充质干细胞来源的微囊泡成血管相关蛋白分析中关于细胞外囊泡中蛋白质成分分析也有类似发现,目前这种现象发生的机制仍不明确有待进一步研究。 2.4.2 细胞外囊泡参与细胞间物质、信息传递 在生理、病理条件下,细胞外囊泡将其携带生物信息运输到周边靶细胞或经血液循环及体液运输而被远处组织细胞摄取,进而对靶细胞遗传组进行重新编排,使靶细胞获得新的功能或失去某功能甚至死亡[28]。一项研究表明成纤维细胞来源的、携带有miR-195的细胞外囊泡能够在胆管癌大鼠体内富集,降低肿瘤大小,改善治疗鼠的存活[29]。细胞外囊泡作用于靶细胞参与细胞间信息传递主要有3种方式:第1种,通过其携带的特异性表面分子配体与靶细胞上相应受体位点结合;第2种,直接与靶细胞质膜融合,释放其内容物到靶细胞胞质中。第3种,通过内陷以类似胞吞作用的机制将信息直接传递给靶细胞(图3)。 2.5 细胞外囊泡参与疾病的发生发展 中枢神经细胞来源的细胞外囊泡可以进入血液循环并在外周循环中发挥其促增殖、分化及抗凋亡的作用,在阿尔茨海默病和帕金森病等退行性疾病及其他中枢神经系统疾病中发挥着重要作用。内皮细胞来源细胞外囊泡本身与机体高凝倾向和小血管炎症等微循环障碍疾病有密切关系[30]。T细胞来源的外泌体可通过磷脂酰丝氨酸识别单核细胞膜表面的受体,诱导胆固醇堆积,进而促进动脉粥样硬化形成[31]。类风湿性关节炎患者关节滑液来源的纤维母细胞分泌的细胞外囊泡携带的TNF-α结合于T淋巴细胞后抵抗机体活化诱导的细胞死亡,从而加重关节炎症状[32]。细胞外囊泡也参与体内自噬过程,有研究指出,经烟雾提取物诱导人气道上皮细胞后收集到的HBEC-细胞外囊泡中miR-210表达量上调,后者作"
| [1] Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166:189-197.[2] Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269-288.[3] Chen SC, Brown PR, Rosie DM. Extraction procedures for use prior to HPLC nucleotide analysis using microparticle chemically bonded packings. J Chromatogr Sci. 1977;15(6):218-221.[4] Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967-978.[5] Johnstone RM, Adam M, Hammond JR, et al. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412-9420.[6] Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19(2):43-51.[7] Sahoo S, Losordo DW. Exosomes and Cardiac Repair After Myocardial Infarction. Circ Res. 2014;114:333-344.[8] Yu F, Jun A,Yusuke Y, et al. Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis. J Extracell Vesicles. 2015;4:28388.[9] Yang Y, Li YY. Exosomal transfer of miR-30a between cardiomyocytes regulates autophagy after hypoxia. J Mol Med .2016;94(6):711-724.[10] Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195-208.[11] Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials an ISEV position paper. J Extracell Vesicles. 2015;4:30087.[12] Wu YT, Deng WT. Exosomes: improved methods to characterize their morphology,RNA content, and surface protein biomarkers. Analyst. 2015;140(19):6631-6642.[13] Yin M, Loyer X, Boulanger CM. Extracellular vesicles as new pharmacological targets to treat atherosclerosis. Eur J Pharmacol. 2015;763(Pt A):90-103.[14] Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-383.[15] Pugholm LH, Revenfeld AL, Søndergaard EK, et al. Antibody-Based Assays for Phenotyping of Extracellular Vesicles. Biomed Res Int. 2015;2015:524817.[16] Kalra H, Simpson RJ, Ji H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10(12):e1001450.[17] Chen JY, An R, Liu ZJ, et al. Therapeutic effects of mesenchymal stem cell-derived microvesicles on pulmonary arterial hypertension in rats. Acta Pharmacologica Sinica. 2014;35(9):1121-1128.[18] Witwer KW, Buzás EI, Bemis LT, et al.Standardization of sample collection,isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:20360. [19] Chen JY, Liu ZJ, Hong MM, et al. Proangiogenic Compositions of Microvesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells. PLoS One. 2014;9(12):e115316.[20] Cai J, Han Y, Ren HM, et al. Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J Mol Cell Biol. 2013;5(4):227-238.[21] Lasda E, Parker R. Circular RNAs Co-Precipitate with Extracellular Vesicles: a Possible Mechanism for circRNA Clearance. PLoS One. 2016;11(2):e0148407. [22] Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013; 27:31-39.[23] Sunkara V, Woo HK, Cho YK. Emerging techniques in the isolation and characterization of extracellular vesicles and their roles in cancer diagnostics and prognostics. Analyst. 2015.[24] Iraci N, Leonardi T, Gessler F, et al. Focus on extracellular vesicles: physiological role and signalling properties of extracellular membrane vesicles. Int J Mol Sci. 2016;17(2):171. [25] Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cellderived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847-856.[26] Takahashi K, Yan IK. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol Cancer Res. 2014;12(10):1377-1387. [27] Collino F, Deregibus MC, Bruno S, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5(7):e11803.[28] Mause SF, Weber C. Microparticles Protagonists of a Novel Communication Network for Intercellular Information Exchange. Circ Res. 2010;107(9):1047-1057.[29] Li L, Piontek K, Ishida M, et al. Extracellular vesicles carry miR-195 to intrahepatic cholangiocarcinoma andimprove survival in a rat model.Hepatology. 2016.[30] Helbing T, Olivier C, Bode C, et al. Role of microparticles in endothelial dysfunction and arterial hypertension. World J Cardiol. 2014;6(11):1135-1139.[31] Zakharova L, Svetlova M, Fomina AF. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J Cell Physiol. 2007;212:174-181.[32] Chaput N, Théry C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011; 33:419-440.[33] Montoro-García S, Shantsila E, Tapp LD, et al. Small-size circulating microparticles in acute coronary syndromes: relevance to fibrinolytic status, reparative markers and outcomes. Atherosclerosis. 2013;227(2):313-322.[34] Lorenzen JM, Thum T. Circulating and urinary microRNAs in kidney disease. Clin J Am Soc Nephrol. 2012;7(9):1528-1533.[35] Fleitas T, Martínez-Sales V, Vila V, et al. Circulating endothelial cells and microparticles as prognostic markers in advanced non-small cell lung cancer. PLoS One. 2012;7(10):e47365.[36] Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177-182.[37] Bank IE, Timmers L, Gijsberts CM, et al. The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease. Exp Rev Mol Diagn. 2015;15(12): 1577-1588. [38] Wisgrill l, Lamm C. Peripheral blood microvesicles secretion is influenced by storage time, temperature, and anticoagulants. Cytometry A. 2016;89(7):663-672.[39] Ong SG, Wu JC. Exosomes as potential alternatives to stem cell therapy in mediating cardiac regeneration. Circ Res. 2015; 117:7-9. [40] Ong SG, Lee WH. Cross talk of combined gene and cell therapy in ischemic heart disease role of exosomal microrna transfer. circulation. 2014;130(suppl 1):S60-S69.[41] Lai RC, Yeo RW, Tan KH, et al. Exosomes for drug delivery a novel application for the mesenchymal stem cell. Biotechnol Adv. 2013;31:543-551.[42] Yim N, Ryu SW. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun. 2016;7:12277.[43] Ohno S, Takanashi M, Sudo K, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185-191.[44] Ibrahim AG, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2(5):606-619. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Wu Weiyue, Guo Xiaodong, Bao Chongyun. Application of engineered exosomes in bone repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1102-1106. |
| [3] | Zhou Hongqin, Wu Dandan, Yang Kun, Liu Qi. Exosomes that deliver specific miRNAs can regulate osteogenesis and promote angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1107-1112. |
| [4] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [5] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [6] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [7] | Huang Chuanjun, Zou Yu, Zhou Xiaoting, Zhu Yangqing, Qian Wei, Zhang Wei, Liu Xing. Transplantation of umbilical cord mesenchymal stem cells encapsulated in RADA16-BDNF hydrogel promotes neurological recovery in an intracerebral hemorrhage rat model [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 510-515. |
| [8] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [9] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [10] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [11] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [12] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [13] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [14] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [15] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||