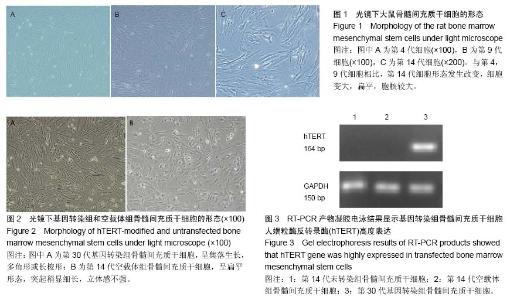
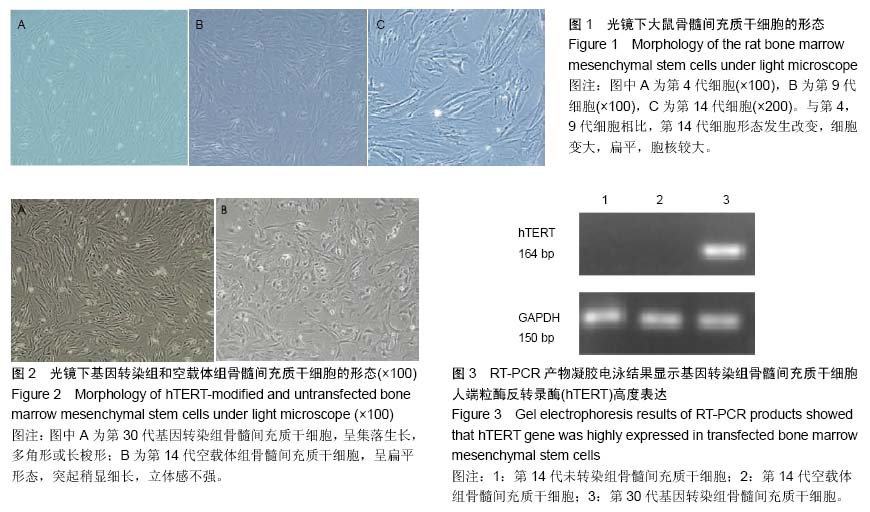
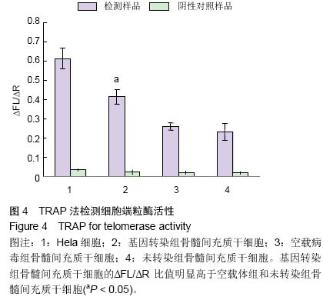
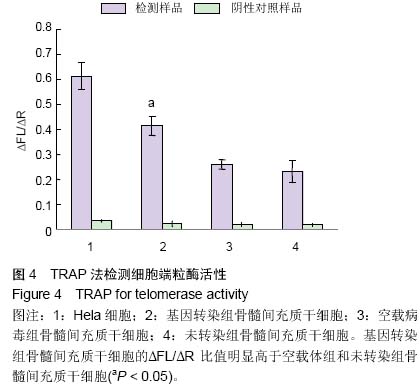
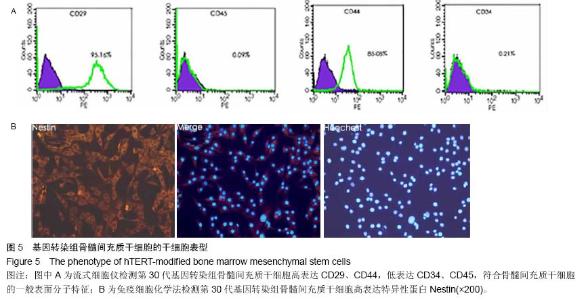
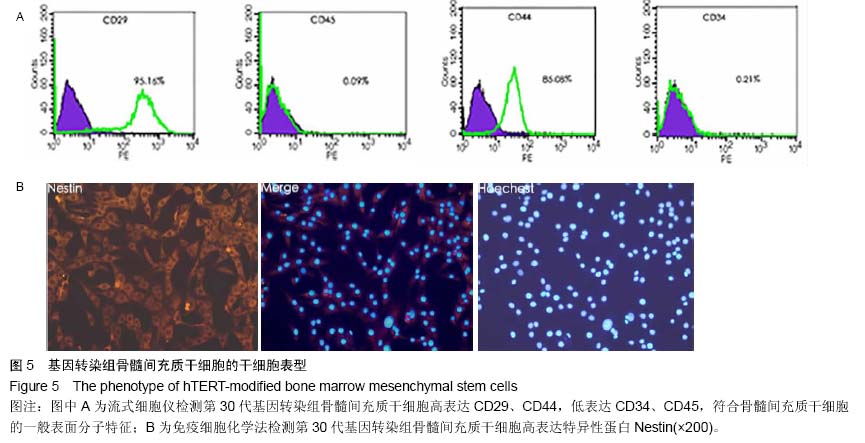
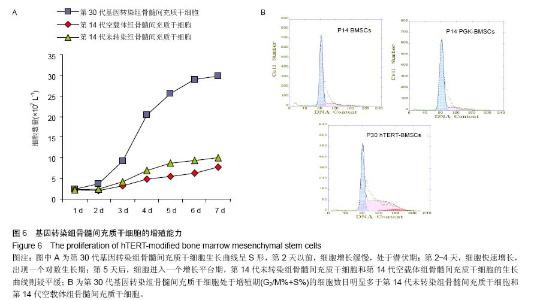
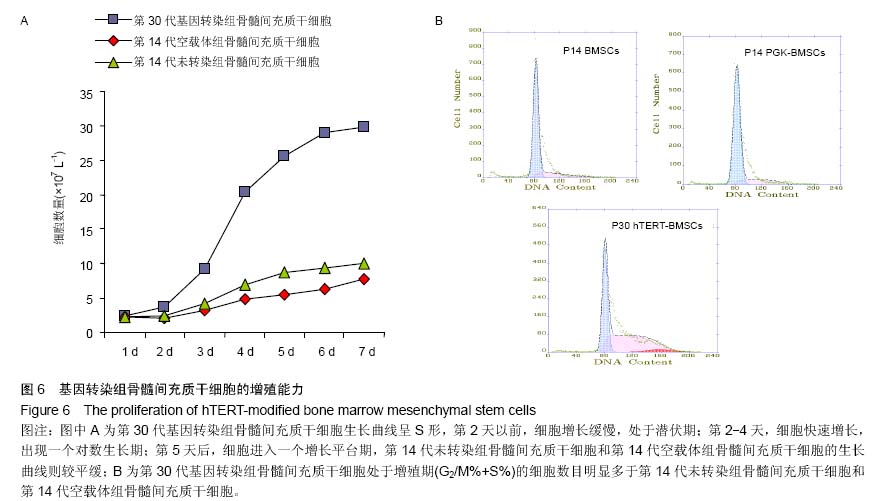
| [1] Jeon Y. Cell based therapy for the management of chronic pain. Korean J Anesthesiol. 2011;60(1):3-7.
[2] Franchi S, Castelli M, Amodeo G, et al. Adult stem cell as new advanced therapy for experimental neuropathic pain treatment. Biomed Res Int. 2014;2014:470983.
[3] Purcell M, Kruger A, Tainsky MA. Gene expression profiling of replicative and induced senescence. Cell Cycle. 2014;13(24): 3927-3937.
[4] Tuan R. Boning up on telomerase. Nat Biotechnol. 2002;20(6): 560-561.
[5] Toblin RL, Mack KA, Perveen G, et al. A population-based survey of chronic pain and its treatment with prescription drugs. Pain. 2011;152(6):1249-1255.
[6] 安珂,田玉科,杨辉,等.构建大鼠前甘丙肽原基因修饰永生化星形胶质细胞株对镇痛研究的意义[J].中国临床康复, 2005,9(14): 109-111.
[7] An K, Xu Y, Yang H, et al. Subarachnoid transplantation of immortalized galanin-overexpressing astrocytes attenuates chronic neuropathic pain. Eur J Pain. 2010; 14(6):595-601.
[8] Xu Y, Tian XB, An K, et al. Lumbar transplantation of immortalized enkephalin-expressing astrocytes attenuates chronic neuropathic pain. Eur J Pain. 2008;12(4):525-533.
[9] Bonab MM, Alimoghaddam K, Talebian F, et al. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14.
[10] Blackburn EH, Collins K. Telomerase: an RNP enzyme synthesizes DNA. Cold Spring Harb Perspect Biol. 2011;3(5). pii: a003558.
[11] Sagong M, Park CK, Kim SH, et al. Human telomerase reverse transcriptase-immortalized porcine monomyeloid cell lines for the production of porcine reproductive and respiratory syndrome virus. J Virol Methods. 2012;179(1): 26-32.
[12] Zou L, Zhang P, Luo C, et al. shRNA-targeted hTERT suppress cell proliferation of bladder cancer by inhibiting telomerase activity. Cancer Chemother Pharmacol. 2006; 57(3):328-334.
[13] Bourgine P, Le Magnen C, Pigeot S, et al. Combination of immortalization and inducible death strategies to generate a human mesenchymal stromal cell line with controlled survival. Stem Cell Res. 2014;12(2):584-598.
[14] Liang XJ, Chen XJ, Yang DH, et al. Differentiation of human umbilical cord mesenchymal stem cells into hepatocyte-like cells by hTERT gene transfection in vitro. Cell Biol Int. 2012; 36(2):215-221.
[15] Piper SL, Wang M, Yamamoto A, et al. Inducible immortality in hTERT-human mesenchymal stem cells. J Orthop Res. 2012; 30(12):1879-1885. |