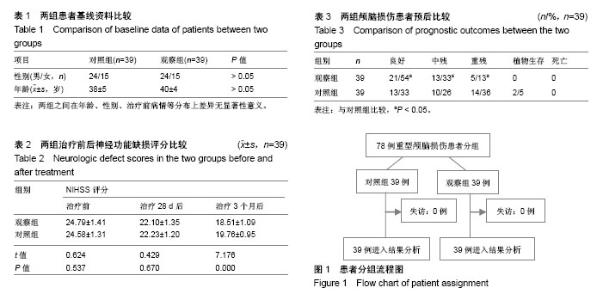
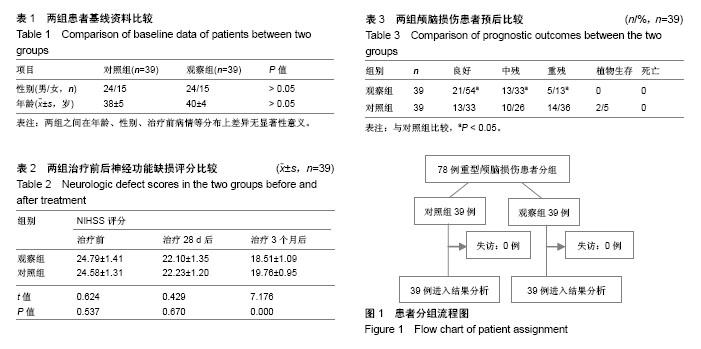
| [1] Titus DJ, Wilson NM, Freund JE, et al. Chronic Cognitive Dysfunction after Traumatic Brain Injury Is Improved with a Phosphodiesterase 4B Inhibitor. J Neurosci. 2016;36(27): 7095-7108.[2] Davis T, Ings A. National Institute of Health and Care Excellence. Head injury: triage, assessment, investigation and early management of head injury in children, young people and adults (NICE guideline CG 176). Arch Dis Child Educ Pract Ed.2015; 100(2):97-100. [3] Davis-López de Carrizosa MA, Morado-Díaz CJ, Morcuende S,etal.Nerve growth factor regulates the firing patternasandsyn- aptic composition of motoneurons.J Neurosci.2010;30(24): 8308-8319.[4] Khan A R, Liu M, Khan M W, et al. Progress in brain targeting drug delivery system by nasal route. J Control Release. 2017;268: 364-389. [5] 步星耀,闫兆月,郭晓鹤,等. 手术联合自体骨髓干细胞动员治疗重型颅脑损伤疗效观察[J]. 中华实用诊断与治疗杂志, 2013, 27(7): 657-659.[6] 焦继超,袁强,步星耀,等. NGF联合自体骨髓干细胞动员治疗重型颅脑损伤疗效观察[J].中国实用神经疾病杂志, 2015(2):4-6.[7] 姜金豆,步星耀,程培训,等.自体骨髓干细胞联合辛伐他汀治疗脊髓损伤[J]. 河南医学研究, 2010, 19(4):434-437.[8] 步星耀,赵红卫,钱宝延,等. 自体骨髓间质干细胞移植联合神经营养因子及综合康复治疗脊髓损伤[J]. 中华实用诊断与治疗杂志, 2009, 23(4):329-331.[9] Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6(4):268-286[10] Tian L, Guo R, Yue X, et al.Intranasal administration of nerve growth factor ameliorate β-amyloid deposition after traumatic brain injury in rats.Brain Res.2012;1440: 47-55.[11] Song JN, Liu ZW, Sui L,et al.Dynamic expression of nerve growth factor and its receptor TrkA after subarachnoid hemorrhage in rat brain.Neural Regen Res. 2016;11(8):1278-1284.[12] Hui L,Yuan J,Ren Z,et al.Nerve growth factor reduces apoptotic cell death in rat facial motor neurons after facial nerve injury. Neurosciences (Riyadh).2015;20(1):65-68.[13] 缪一艇,童凌云,王勇,等.神经生长因子对重型颅脑损伤患者的治疗效果及血清MMP-2、NSE影响研究[J]. 中华全科医学, 2016,14(12): 2040-2041.[14] 朱骏,赵建华.神经节苷脂联合神经生长因子对小儿脑损伤患者神经行为功能及临床疗效的影响[J].临床和实验医学杂志, 2017,16(1):82-85.[15] Md S, Mustafa G,Baboota S,et al. Nanoneurotherapeutics approach intended for direct nose tobrain delivery, Drug Dev Ind Pharm.2015;41(12):1922-34 Drug Dev. Ind. Pharm. (2015) 1-13.[16] 张慧,步星耀,杨冬谊,等. 神经生长因子经鼻脑靶向联合腰大池外引流治疗外伤性蛛网膜下腔出血临床观察[J].中华实用诊断与治疗杂志,2015,29(12):1234-1236.[17] Schaefer ML,Böttger B,Silver WL,et al.Trigeminal collaterals in the nasal epithelium and olfactorybulb: a potential route for direct modulation of olfactory information by trigeminal stimuli.J Comp Neurol.2002;444(3): 221-226.[18] Illum L.Nasal drug delivery—possibilities, problems and solutions. J Control Release. 2003;87(1-3):187-98.[19] Hanson LR, Frey WH 2nd.Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci.2008;9 Suppl 3:S5 [20] 林彦琛,云晨,钟士江.经鼻给予不同剂量NGF对G93A—SOD1小鼠的治疗作用[J].武警后勤学院学报:医学版,2016(9):701-704.[21] 张慧,步星耀,杨冬谊,等.神经生长因子经鼻脑靶向联合腰大池外引流治疗外伤性蛛网膜下腔出血临床观察[J].中华实用诊断与治疗杂志,2015,29(12):1234-1236.[22] Guo X, Bu X, Jiang J, et al. Enhanced neuroprotective effects of co-administration of G-CSF with simvastatin on intracerebral hemorrhage in rats. Turk Neurosurg. 2012;22(6):732-739. [23] Han X, Yang N, Cui Y, et al. Simvastatin mobilizes bone marrow stromal cells migrating to injured areas and promotes functional recovery after spinal cord injury in the rat. Neurosci Lett. 2012; 521(2):136-141.[24] 程培训,姜金豆,周长江,等.自体骨髓干细胞动员治疗持续植物状态临床研究[J].河南医学研究,2011,20(1):63-66[25] 李志营,夏云,郭晓鹤,等.自体骨髓干细胞动员联合醒脑静治疗脑损伤临床研究[J].中国实用神经疾病杂志,2016,19(7):71-73.[26] Wojakowski W,Landmesser U,Bachowski R,et al.Mobilization of stem and progenitor cells in cardiovascular diseases. Leukemia. 2012;26(1):23-33.[27] Spaeth EL,Kidd S,Marini FC.Tracking inflammation-induced mobilization of mesenchymal stem cells. Methods Mol Biol. 2012; 904:173-190.[28] 翟亚萍, 步星耀,王新军,等. 自体骨髓干细胞动员移植联合神经营养因子和综合康复治疗持续植物状态[J].中华实用诊断与治疗杂志, 2012,26(1):31-33.[29] 孙彦熙,步星耀,袁强,等. 颅脑损伤术后带肌蒂颅骨成形联合干细胞动员及综合康复治疗临床分析[J].中华实用诊断与治疗杂志, 2014, 28(10):989-991. |