Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (18): 4675-4684.doi: 10.12307/2026.746
Previous Articles Next Articles
Changes in the expression of six microRNAs in ovarian tissue from animal models of premature ovarian failure and in peripheral blood of patients with premature ovarian failure
Wang Xinyue1, Li Hongli1, 2, Guo Chunhui1, Chen Jibing1, 2, Yu Hua1, 2
- 1Guangxi University of Chinese Medicine, Nanning 530001, Guangxi Zhuang Autonomous Region, China; 2Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China
-
Received:2025-06-16Accepted:2025-09-11Online:2026-06-28Published:2025-12-06 -
Contact:Yu Hua, MS, Master’s supervisor, Guangxi University of Chinese Medicine, Nanning 530001, Guangxi Zhuang Autonomous Region, China; Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China -
About author:Wang Xinyue, MS, Guangxi University of Chinese Medicine, Nanning 530001, Guangxi Zhuang Autonomous Region, China -
Supported by:Guangxi Key Research & Development Program Project, No. 407312863109 (to YH [project participant]); 2025 University-Level Master’s Student Scientific Research Innovation Project of Guangxi University of Chinese Medicine, No. YCSY2025071 (to WXY)
CLC Number:
Cite this article
Wang Xinyue, Li Hongli, Guo Chunhui, Chen Jibing, Yu Hua. Changes in the expression of six microRNAs in ovarian tissue from animal models of premature ovarian failure and in peripheral blood of patients with premature ovarian failure[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4675-4684.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
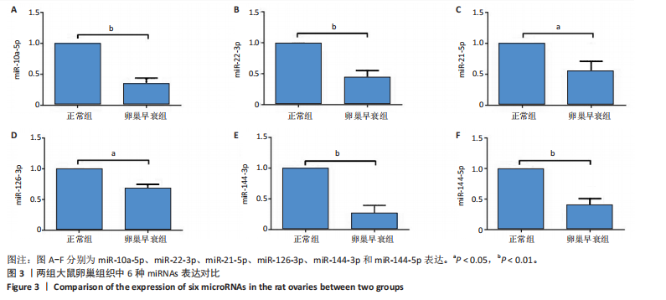
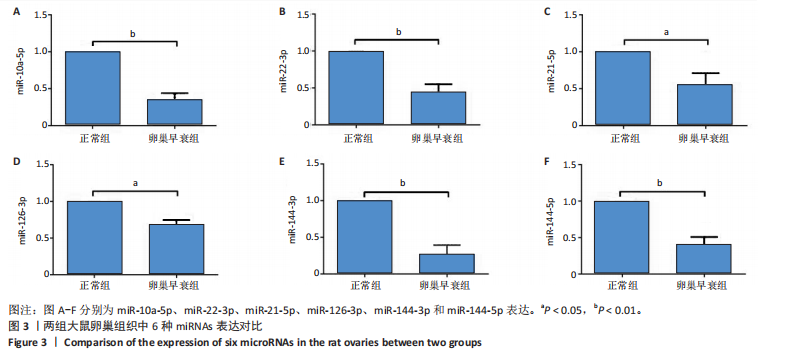
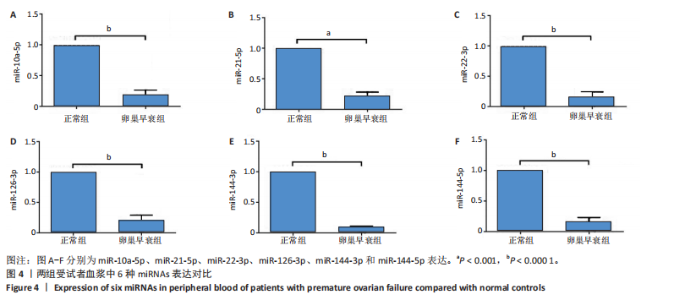
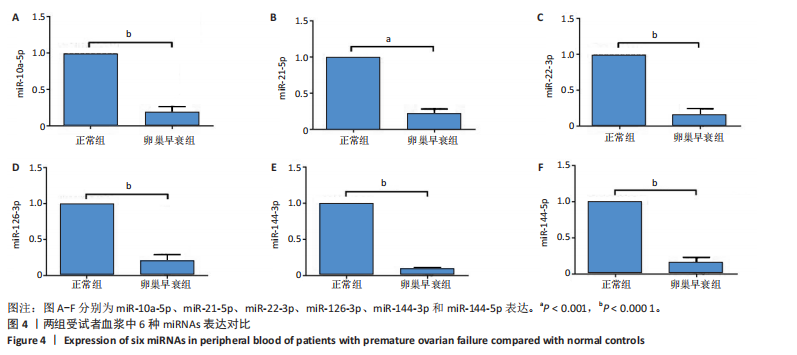
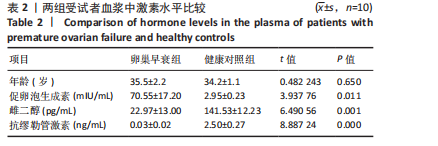
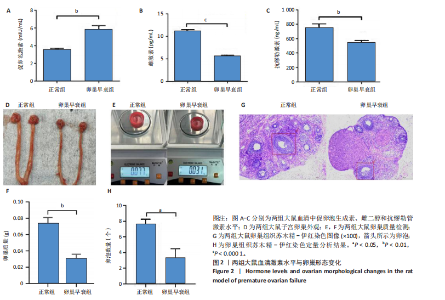
2.1 动物实验结果 2.1.1 实验动物数量分析 20只大鼠全部进入结果分析。 2.1.2 两组大鼠激素水平与卵巢形态比较 与正常组相比,卵巢早衰组大鼠血清雌二醇、抗缪勒管激素水平降低[雌二醇:(11.25±0.39),(5.70±0.19) pg/mL,P < 0.000 1;抗缪勒管激素:(758.53±82.31),(547.27± 53.13) ng/mL,P < 0.01),促卵泡生成素水平升高[(3.62±0.15),(5.90±0.72) mIU/mL,P < 0.01)],见图2A-C。 与正常组相比,卵巢早衰组大鼠卵巢体积减小,质量减少[(0.074±0.006),(0.031±0.005) g,P < 0.01],见图2D-F。苏木精-伊红染色结果显示卵巢早衰组大鼠卵泡数量少于正常组[(7.67±0.58),(3.33±1.16)个,P < 0.05)],见图2G,H。 2.1.3 两组大鼠卵巢组织中6种miRNAs表达比较 Q-PCR检测结果显示,卵巢早衰组大鼠卵巢组织中miR-10a-5p、miR-21-5p、miR-22-3p、miR-126-3p、miR-144-3p和miR-144-5p的表达均低于正常组(P < 0.05,P < 0.01),见图3。结果提示这些特定miRNAs的表达异常可能与卵巢早衰的病理过程密切相关。 2.2 临床样本检测结果 2.2.1 两组受试者血浆中激素水平比较 与健康对照组相比,卵巢早衰患者组血浆中促卵泡生成素水平升高(P < 0.05),雌二醇和抗缪勒管激素水平下降(P < 0.01),见表2。这一发现与卵巢早衰患者内分泌特征相符,进一步证实了促卵泡生成素、雌二醇和抗缪勒管激素在卵巢早衰诊断中的临床价值。 2.2.2 两组受试者血浆中6种miRNAs表达比较 Q-PCR检测结果显示,与健康对照组相比,卵巢早衰组受试者血浆中miR-10a-5p、miR-21-5p、miR-22-3p、miR-126-3p、miR-144-3p、miR-144-5p表"
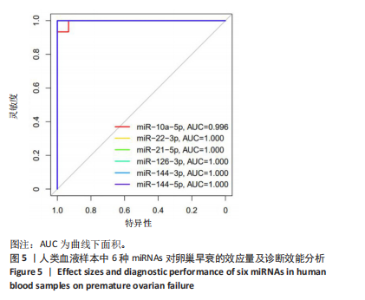
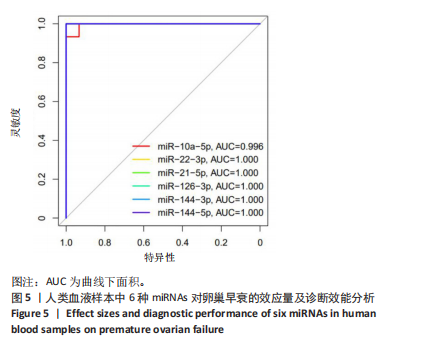
达均降低(P < 0.001,P < 0.000 1),见图4。提示这些miRNAs可能作为卵巢早衰的新型分子标志物,它们的表达异常与卵巢早衰病理机制存在潜在关联。 2.2.3 6种miRNAs的效应量及诊断效能分析结果 基于Q-PCR检测获得的2-ΔΔCt相对表达量数据,通过受试者工作特征曲线系统评估了各miRNA的诊断价值,见图5。受试者工作特征曲线分析显示,这6种miRNAs均展现出优异的诊断区分能力,其中miR-10a-5p(AUC=0.996,95%CI:0.989-1.000)具有近乎完美的诊断效能,而miR-22-3p、miR-21-5p、miR-126-3p、miR-144-3p和miR-144-5p则达到理论最佳诊断性能(AUC=1.000,95%CI:1.000-1.000)。这些结果从计量诊断学角度证实,此次研究所检测"

| [1] WELT CK. Primary Ovarian Insufficiency: A More Accurate Term for Premature Ovarian Failure. Clin Endocrinol (Oxf). 2008;68(4):499-509. [2] JANKOWSKA K. Premature Ovarian Failure. Prz Menopauzalny. 2017; 16(2):51-56. [3] 徐才秀,戴银英,郭苏苏.性激素六项检查在女性不孕症诊断中的临床应用价值分析[J].临床医学工程,2023,30(12):1701-1702. [4] HANSEN LM, BATZER FR, GUTMANN JN, et al. Evaluating Ovarian Reserve: Follicle Stimulating Hormone and Oestradiol Variability During Cycle Days 2-5. Hum Reprod. 1996;11(3):486-489. [5] SILLIMAN E, CHUNG EH, FITZPATRICK E, et al. Evaluation of at-Home Serum Anti-Mullerian Hormone Testing: A Head-to-Head Comparison Study. Reprod Biol Endocrinol. 2022;20(1):131. [6] TATANG C, ARREDONDO BT, BERGAMASCO A, et al. Human Papillomavirus Vaccination and Premature Ovarian Failure: A Disproportionality Analysis Using the Vaccine Adverse Event Reporting System. Drugs Real World Outcomes. 2022;9(1):79-90. [7] WESEVICH V, KELLEN AN, PAL L. Recent advances in understanding primary ovarian insufficiency. F1000Res. 2020;9:F1000 Faculty Rev-1101. [8] CHON SJ, UMAIR Z, YOON MS. Premature Ovarian Insufficiency: Past, Present, and Future. Front Cell Dev Biol. 2021;9:672890. [9] ARMENI E. Diagnostic Challenges in Suspected Premature Ovarian Insufficiency. Case Rep Womens Health. 2022;36:e453. [10] ASGARPOUR K, SHOJAEI Z, AMIRI F, et al. Exosomal Micrornas Derived From Mesenchymal Stem Cells: Cell-to-Cell Messages. Cell Commun Signal. 2020;18(1):149. [11] HUANG M, LIU Y, ZHANG L, et al. Advancements in Research On Mesenchymal Stem-Cell-Derived Exosomal Mirnas: A Pivotal Insight Into Aging and Age-Related Diseases. Biomolecules. 2024;14(11):1354. [12] TESFAYE D, GEBREMEDHN S, SALILEW-WONDIM D, et al. Micrornas: Tiny Molecules with a Significant Role in Mammalian Follicular and Oocyte Development. Reproduction. 2018;155(3):R121-R135. [13] JU C, LIU D. Exosomal Micrornas From Mesenchymal Stem Cells: Novel Therapeutic Effect in Wound Healing. Tissue Eng Regen Med. 2023;20(5):647-660. [14] YANG M, LIN L, SHA C, et al. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal Mir-144-5P Improves Rat Ovarian Function After Chemotherapy-Induced Ovarian Failure by Targeting Pten. Lab Invest. 2020;100(3):342-352. [15] LIU M, XIAO B, ZHU Y, et al. Microrna-144-3P Protects Against Chemotherapy-Induced Apoptosis of Ovarian Granulosa Cells and Activation of Primordial Follicles by Targeting Map3K9. Eur J Med Res. 2023;28(1):264. [16] XIAO GY, CHENG CC, CHIANG YS, et al. Exosomal Mir-10a Derived From Amniotic Fluid Stem Cells Preserves Ovarian Follicles After Chemotherapy. Sci Rep.2016;6:23120. [17] SANDHU R, REIN J, D’ARCY M, et al. Overexpression of Mir-146a in Basal-Like Breast Cancer Cells Confers Enhanced Tumorigenic Potential in Association with Altered P53 Status. Carcinogenesis. 2014;35(11):2567-2575. [18] SUN B, MA Y, WANG F, et al. Mir-644-5P Carried by Bone Mesenchymal Stem Cell-Derived Exosomes Targets Regulation of P53 to Inhibit Ovarian Granulosa Cell Apoptosis. Stem Cell Res Ther. 2019;10(1):360. [19] GAO T, CHEN Y, HU M, et al. Microrna-22-3P in Human Umbilical Cord Mesenchymal Stem Cell-Secreted Exosomes Inhibits Granulosa Cell Apoptosis by Targeting Klf6 and Atf4-Atf3-Chop Pathway in Pof Mice. Apoptosis. 2023;28(7-8):997-1011. [20] FU X, HE Y, WANG X, et al. Overexpression of Mir-21 in Stem Cells Improves Ovarian Structure and Function in Rats with Chemotherapy-Induced Ovarian Damage by Targeting Pdcd4 and Pten to Inhibit Granulosa Cell Apoptosis. Stem Cell Res Ther. 2017;8(1):187. [21] ZHANG Q, SUN J, HUANG Y, et al. Human Amniotic Epithelial Cell-Derived Exosomes Restore Ovarian Function by Transferring Micrornas Against Apoptosis. Mol Ther Nucleic Acids. 2019;16:407-418. [22] ZHANG Q, BU S, SUN J, et al. Paracrine Effects of Human Amniotic Epithelial Cells Protect Against Chemotherapy-Induced Ovarian Damage. Stem Cell Res Ther. 2017;8(1):270. [23] LIU T, LIN J, CHEN C, et al. Microrna-146B-5P Overexpression Attenuates Premature Ovarian Failure in Mice by Inhibiting the Dab2Ip/Ask1/P38-Mapk Pathway and Gammah2a.X Phosphorylation. Cell Prolif. 2021;54(1):e12954. [24] QU Q, LIU L, CUI Y, et al. Mir-126-3P Containing Exosomes Derived From Human Umbilical Cord Mesenchymal Stem Cells Promote Angiogenesis and Attenuate Ovarian Granulosa Cell Apoptosis in a Preclinical Rat Model of Premature Ovarian Failure. Stem Cell Res Ther. 2022;13(1):352. [25] HONG DS, KANG YK, BORAD M, et al. Phase 1 Study of Mrx34, a Liposomal Mir-34a Mimic, in Patients with Advanced Solid Tumours. Br J Cancer. 2020;122(11):1630-1637. [26] BEG MS, BRENNER AJ, SACHDEV J, et al. Phase I Study of Mrx34, a Liposomal Mir-34a Mimic, Administered Twice Weekly in Patients with Advanced Solid Tumors. Invest New Drugs. 2017;35(2):180-188. [27] REID G, KAO SC, PAVLAKIS N, et al. Clinical Development of Targomirs, a Mirna Mimic-Based Treatment for Patients with Recurrent Thoracic Cancer. Epigenomics. 2016;8(8):1079-1085. [28] VAN ZANDWIJK N, PAVLAKIS N, KAO SC, et al. Safety and Activity of Microrna-Loaded Minicells in Patients with Recurrent Malignant Pleural Mesothelioma: A First-in-Man, Phase 1, Open-Label, Dose-Escalation Study. Lancet Oncol. 2017;18(10):1386-1396. [29] BALDARI S, DI ROCCO G, MAGENTA A, et al. Extracellular Vesicles-Encapsulated Microrna-125B Produced in Genetically Modified Mesenchymal Stromal Cells Inhibits Hepatocellular Carcinoma Cell Proliferation. Cells. 2019;8(12):1560. [30] GALLANT-BEHM CL, PIPER J, LYNCH JM, et al. A Microrna-29 Mimic (Remlarsen) Represses Extracellular Matrix Expression and Fibroplasia in the Skin. J Invest Dermatol. 2019;139(5):1073-1081. [31] DONOSO-QUEZADA J, AYALA-MAR S, GONZALEZ-VALDEZ J. The Role of Lipids in Exosome Biology and Intercellular Communication: Function, Analytics and Applications. Traffic. 2021;22(7):204-220. [32] KOGA Y, YASUNAGA M, MORIYA Y, et al. Exosome Can Prevent Rnase From Degrading Microrna in Feces. J Gastrointest Oncol. 2011;2(4): 215-222. [33] KRYLOVA SV, FENG D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int J Mol Sci. 2023;24(2):1337. [34] QU Q, LIU L, CUI Y, et al. Mir-126-3P Containing Exosomes Derived From Human Umbilical Cord Mesenchymal Stem Cells Promote Angiogenesis and Attenuate Ovarian Granulosa Cell Apoptosis in a Preclinical Rat Model of Premature Ovarian Failure. Stem Cell Res Ther. 2022;13(1):352. [35] WEN SW, ZHANG YF, LI Y, et al. Characterization and Effects of Mir-21 Expression in Esophageal Cancer. Genet Mol Res. 2015;14(3):8810-8818. [36] WANG S, AURORA AB, JOHNSON BA, et al. The Endothelial-Specific Microrna Mir-126 Governs Vascular Integrity and Angiogenesis. Dev Cell. 2008;15(2):261-271. [37] FISH JE, SANTORO MM, MORTON SU, et al. Mir-126 Regulates Angiogenic Signaling and Vascular Integrity. Dev Cell. 2008;15(2):272-284. [38] YANG X, ZHOU Y, PENG S, et al. Differentially Expressed Plasma Micrornas in Premature Ovarian Failure Patients and the Potential Regulatory Function of Mir-23a in Granulosa Cell Apoptosis. Reproduction. 2012;144(2):235-244. [39] NIE M, YU S, PENG S, et al. Mir-23a and Mir-27a Promote Human Granulosa Cell Apoptosis by Targeting Smad5. Biol Reprod. 2015; 93(4):98. [40] 娄季武,何凤屏,刘彦慧,等.原发性卵巢功能不全患者血清外泌体miRNA-146、HIF-1α和ROS的表达及临床意义[C]//四川省国际医学交流促进会.医学护理创新学术交流会论文集(智慧医学篇).东莞市妇幼保健院;粤北人民医院,2024:244-248.DOI:10.26914/c.cnkihy.2024.049396. [41] 仝慧杰,刘丽丽,范志刚,等.卵巢早衰患者血浆中Mirna-503的作用及对内皮祖细胞的影响[J].实用医学杂志,2019,35(17):2765-2769. [42] ALDAKHEEL FM, ABUDERMAN AA, ALDURAYWISH SA, et al. Microrna-21 Inhibits Ovarian Granulosa Cell Proliferation by Targeting Snhg7 in Premature Ovarian Failure with Polycystic Ovary Syndrome. J Reprod Immunol. 2021;146:103328. [43] DU R, CHENG X, JI J, et al. Mechanism of Ferroptosis in a Rat Model of Premature Ovarian Insufficiency Induced by Cisplatin. Sci Rep. 2023;13(1):4463. [44] LEE EH, HAN SE, PARK MJ, et al. Establishment of Effective Mouse Model of Premature Ovarian Failure Considering Treatment Duration of Anticancer Drugs and Natural Recovery Time. J Menopausal Med. 2018;24(3):196-203. [45] SZELIGA A, CALIK-KSEPKA A, MACIEJEWSKA-JESKE M, et al. Autoimmune Diseases in Patients with Premature Ovarian Insufficiency-Our Current State of Knowledge. Int J Mol Sci. 2021;22(5):2594. [46] CAO LB, LEUNG CK, LAW PW, et al. Systemic Changes in a Mouse Model of Vcd-Induced Premature Ovarian Failure. Life Sci. 2020;262:118543. [47] NOURI N, SHAREGHI-OSKOUE O, AGHEBATI-MALEKI L, et al. Role of Mirnas Interference On Ovarian Functions and Premature Ovarian Failure. Cell Commun Signal. 2022;20(1):198. |
| [1] | Li Zhifei, Han Bin, Liu Qiuli, Zhang Zhanming, Wei Haokai, Zuo Kuangshi, Zhang Yisheng. Cervical motion characteristics in patients with cervical spondylotic radiculopathy based on motion capture technology [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2286-2293. |
| [2] | Zhou Sirui, Xu Yukun, Zhao Kewei. Ideas and methods of anti-melanogenesis of Angelica dahurica extracellular vesicles [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1747-1754. |
| [3] | Wang Baiyan, Yang Shu, Wang Yiming, Wu Mengqing, Xiao Yu, Guo Zixuan, Zhang Boyi, Feng Shuying. Exosome-delivered CRISPR/Cas system enables gene editing in target cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1839-1849. |
| [4] | Pan Hongfei, Zhuang Zhenbing, Xu Baiyun, Yang Zhangyang, Lin Kairui, Zhan Bingqing, Lan Jinghan, Gao Heng, Zhang Nanbo, Lin Jiayu. Inhibitory effects of different concentrations of auranofin on M1 macrophage function and its therapeutic potential in diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1390-1397. |
| [5] | Peng Zhiwei, Chen Lei, Tong Lei. Luteolin promotes wound healing in diabetic mice: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1398-1406. |
| [6] | Cao Xinyan, Yu Zifu, Leng Xiaoxuan, Gao Shiai, Chen Jinhui, Liu Xihua. Effect of repetitive transcranial magnetic stimulation and transcranial direct current stimulation on motor function and gait in children with cerebral palsy: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1539-1548. |
| [7] | Guo Ying, Tian Feng, Wang Chunfang. Potential drug targets for the treatment of rheumatoid arthritis: large sample analysis from European databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1549-1557. |
| [8] | Sun Yajie, Zhao Xinchen, Bo Shuangling. Spatiotemporal expression of bone morphologic protein 7 in mouse kidney development [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1156-1161. |
| [9] | Li Haojing, Wang Xin, Song Chenglin, Zhang Shengnan, Chen Yunxin. Therapeutic efficacy of extracorporeal shock wave therapy in the upper trapezius muscle area combined with exercise control training in patients with chronic non-specific neck pain [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1162-1170. |
| [10] | Liu Yu, Lei Senlin, Zhou Jintao, Liu Hui, Li Xianhui. Mechanisms by which aerobic and resistance exercises improve obesity-related cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1171-1183. |
| [11] | Yu Huifen, Mo Licun, Cheng Leping. The position and role of 5-hydroxytryptamine in the repair of tissue injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1196-1206. |
| [12] | Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Advance in the mechanisms underlying miRNAs in steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1207-1214. |
| [13] | Bu Yangyang, Ning Xinli, Zhao Chen. Intra-articular injections for the treatment of osteoarthritis of the temporomandibular joint: different drugs with multiple combined treatment options [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1215-1224. |
| [14] | Wen Fan, Xiang Yang, Zhu Huan, Tuo Yanfang, Li Feng. Exercise improves microvascular function in patients with type 2 diabetes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1225-1235. |
| [15] | Liu Xinyue, Li Chunnian, Li Yizhuo, Xu Shifang. Regeneration and repair of oral alveolar bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1247-1259. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||