Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (5): 1215-1224.doi: 10.12307/2026.005
Previous Articles Next Articles
Intra-articular injections for the treatment of osteoarthritis of the temporomandibular joint: different drugs with multiple combined treatment options
Bu Yangyang1, Ning Xinli2, Zhao Chen1
- 1Department of Prosthodontics, Hebei Key Laboratory of Stomatology, Hebei Technology Innovation Center of Oral Health, School and Hospital of Stomatology, Hebei Medical University, Shijiazhuang 050017, Hebei Province, China; 2Shexian County Hospital, Handan 056400, Hebei Province, China
-
Received:2024-11-07Accepted:2025-01-06Online:2026-02-18Published:2025-06-26 -
Contact:Zhao Chen, MD, Chief physician, Department of Prosthodontics, Hebei Key Laboratory of Stomatology, Hebei Technology Innovation Center of Oral Health, School and Hospital of Stomatology, Hebei Medical University, Shijiazhuang 050017, Hebei Province, China -
About author:Bu Yangyang, Master candidate, Physician, Department of Prosthodontics, Hebei Key Laboratory of Stomatology, Hebei Technology Innovation Center of Oral Health, School and Hospital of Stomatology, Hebei Medical University, Shijiazhuang 050017, Hebei Province, China -
Supported by:2022 Hebei Provincial Government Sponsored Research Project on Training of Excellent Talents in Clinical Medicine and Basic Subjects, No. 361029 (to ZC); Directive Subject Project of Medical Science Research Subjects Program of Hebei Provincial Health and Wellness Commission, No. 20230184 (to ZC); Subject Project of Handan Municipal Science and Technology Bureau, No. 23422083108ZC (to NXL)
CLC Number:
Cite this article
Bu Yangyang, Ning Xinli, Zhao Chen. Intra-articular injections for the treatment of osteoarthritis of the temporomandibular joint: different drugs with multiple combined treatment options[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1215-1224.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
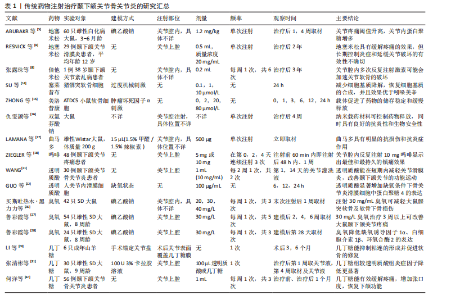
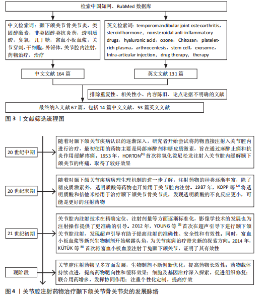
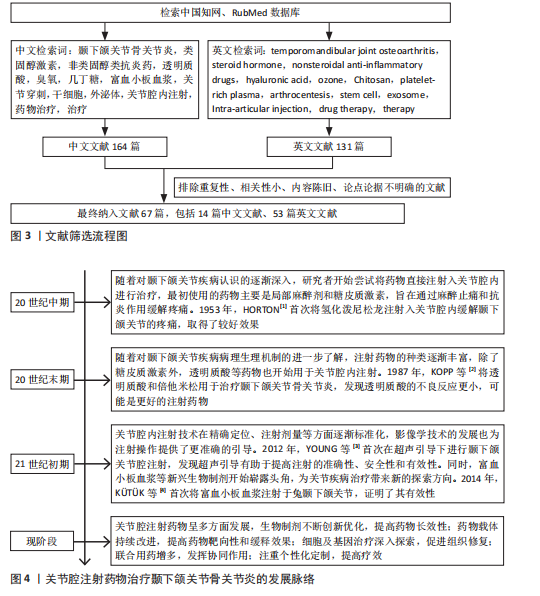
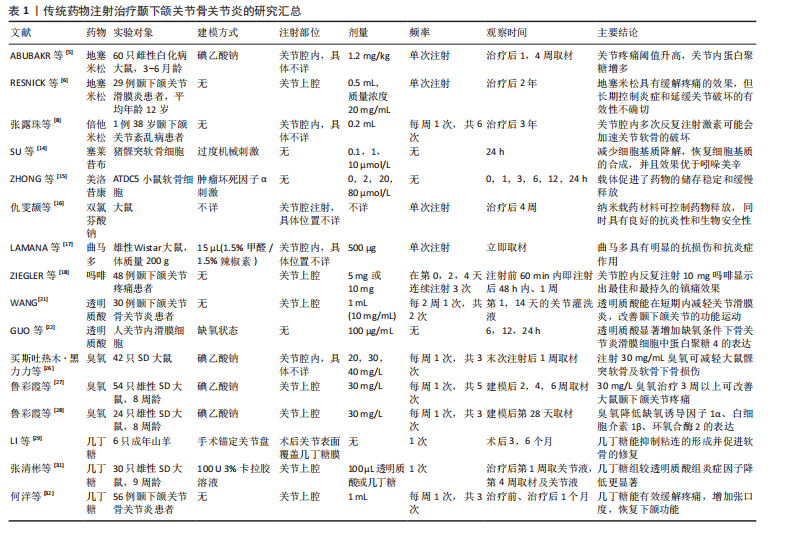
节炎治疗中常用的类固醇药物包括氢化可的松、甲泼尼龙、地塞米松和曲安奈德等,这些药物能够在短时间内减轻疼痛并提高关节的活动能力。ABUBAKR等[5]研究发现,在大鼠急性颞下颌关节骨关节炎早期关节内注射地塞米松明显提高了大鼠的疼痛阈值,并且关节内蛋白聚糖明显增加。RESNICK等[6]将29例幼年特发性颞下颌关节骨关节炎患者纳入研究,在关节腔内注射类固醇药物后89%的患者疼痛消失,并且最大张口度明显增加、滑膜炎症明显降低。但也有研究报道,长期使用类固醇药物可能会带来一些不良反应,如反复注射可能会增加感染的风险,引起关节软骨及周围组织的损伤,全身应用也会出现免疫系统抑制、骨质疏松等不良反应[7]。张露珠等[8]的研究发现,对颞下颌关节骨关节炎患者进行糖皮质激素关节腔内注射治疗可以有效减轻关节疼痛,然而,多次反复注射可能会加速关节软骨的破坏,其机制可能是影响了线粒体功能进而诱导了软骨细胞凋亡。有报道指出,单次关节腔内注射氢化可的松1年后患者出现了髁突溶解这一并发症[9]。因此,目前类固醇类药物在颞下颌关节骨关节炎治疗中的应用已经较为少见。 2.2.2 非类固醇类抗炎药 非类固醇类抗炎药是一类不含类固醇结构的抗炎药物,主要是通过抑制环氧合酶的活性减少前列腺素的合成,从而发挥解热、镇痛和抗炎效果。非类固醇类抗炎药能有效缓解关节疼痛、减轻关节症状、改善关节功能[10],其机制可能与阻断三叉神经节中Nav1.7 mRNA和甘油三酯酶蛋白表达上调有关[11]。环氧合酶分为3种亚型:环氧合酶1、环氧合酶2、环氧合酶3,环氧合酶2主要是环氧合酶的诱导型亚型,促炎细胞因子白细胞介素1β可诱导环氧合酶2高表达,从而增加骨关节炎组织中前列腺素E2的产生[12]。大多数非类固醇类抗炎药,如乙酰水杨酸(阿司匹林)、布洛芬、萘普生和酮洛芬等,是环氧合酶的非选择性抑制剂,存在较大的胃肠道不良反应及心血管风险[13]。近年来,随着对颞下颌关节骨关节炎病理机制的深入研究,非类固醇类抗炎药的应用也在不断发展,新型非类固醇类抗炎药的研发提高了药物的疗效和安全性,例如选择性环氧合酶2抑制剂对环氧合酶2的选择性更高,减少了对环氧合酶1的抑制,从而降低了胃肠道出血的风险。SU等[14]的研究发现,塞来昔布通过下调环氧合酶2、前列腺素E2、基质金属蛋白酶的表达减少软骨细胞基质降解,上调Ⅱ型胶原蛋白和蛋白聚糖的表达增加软骨细胞基质的合成,从而发挥对髁突软骨细胞的保护作用。另外,一些药物递送系统可以提高非类固醇类抗炎药的生物利用度,降低注射频率、注射浓度,从而降低并发症的发生,例如ZHONG等[15]将美洛昔康主动负载于脂质体内,促进了药物的储存稳定和缓慢释放;仇雯颉等[16]构建了一种光响应二硫化钼纳米片装载双氯芬酸钠的药物递送系统,在体外研究中发现其具有卓越的抗炎性能,能有效减少给药频率、提高药物利用率。新型非类固醇抗炎药具有广阔的应用前景,但其长期疗效和安全性尚需进一步观察。 2.2.3 阿片类镇痛药 目前,阿片类镇痛药在颞下颌关节骨关节炎治疗中的应用处于谨慎探索阶段。阿片类镇痛药是一类作用于中枢神经系统的强效止痛药物,主要是通过与中枢神经系统的阿片受体结合抑制神经递质释放,阻止痛觉信号传递,同时也作用于外周神经末梢的阿片受体,发挥镇痛及减轻关节周围炎症反应的作用。对于颞下颌关节骨关节炎患者,当非类固醇类抗炎药等常规止痛药物效果不佳时,常考虑使用阿片类镇痛药(如可待因、曲马多等口服制剂),可有效缓解疼痛、提高患者生活质量。近年来,关节腔注射阿片类镇痛药也逐渐受到关注。研究发现,将少量阿片类药物直接注射到颞下颌关节腔内可在局部发挥镇痛作用,减少全身用药的不良反应,并且能更精准地作用于病变部位[17-18]。然而,长期使用阿片类镇痛药存在成瘾风险,还可能出现恶心、呕吐等常见不良反应。因此,有专家提出将阿片类镇痛药与非类固醇抗炎药联合使用,可发挥协同镇痛作用,同时减少阿片类药物的用量,降低成瘾风险和不良反应的发生率[19]。近年来,低毒高效的新型阿片类镇痛药陆续进入临床试验阶段,以期为颞下颌关节骨关节炎的治疗提供更安全有效的药物选择[20]。 2.2.4 透明质酸 透明质酸是临床上用于治疗膝骨关节炎时最常用的注射药物,目前也逐渐应用于颞下颌关节骨关节炎的治疗。透明质酸是一种天然多糖,是关节滑液的关键成分之一,能够润滑关节、改善关节活动,同时还能抑制炎症因子(如白细胞介素1、肿瘤坏死因子α)的产生和释放,从而减轻炎症对关节软骨的损害,进而促进软骨的修复。WANG等[21]研究表明,透明质酸关节腔注射后可在短时间内降低关节滑液内的血管内皮生长因子水平。GUO等[22]研究表明,透明质酸显著增加了缺氧条件下骨关节炎滑膜细胞中蛋白聚糖4表达,而蛋白聚糖4是一种关节软骨表面润滑剂。然而,有研究指出透明质酸在关节腔内注射后停留时间短且代谢速度快,导致长期疗效并不理想,因此,建议将透明质酸与其他药物联合注射或与非药物疗法结合使用,以期提高治疗效果[23]。FERREIRA等[24]对透明质酸治疗颞下颌关节骨关节炎的效果进行了系统评价,结果显示目前高质量的临床证据尚不足以证明透明质酸能有效治疗颞下颌关节骨关节炎,为了得出可靠的结论,还需要进行更多的临床试验来验证。 2.2.5 医用臭氧 关节腔内注射臭氧(O3)是目前治疗颞下颌关节骨关节炎的一种新兴疗法。医用臭氧是一种由3个氧原子组成的强氧化剂,具有抗氧化特性,能够保护软骨免受氧化损伤,还具有抗炎和镇痛效果,能够有效抑制炎症递质的释放,减轻关节局部的炎症反应,并通过抑制疼痛感受器、改善局部血液循环减轻患者的疼痛症状;此外,还能刺激软骨细胞增殖和分化,促进组织修复[25]。臭氧的疗效与其注射浓度和疗程密切相关。买斯吐热木·黑力力等[26]研究发现,在颞下颌关节腔内注射30 μg/mL医用臭氧可以减轻颞下颌关节骨关节炎大鼠髁突软骨及软骨下骨的损伤,然而,当臭氧质量浓度上升到40 μg/mL时,反而会加重颞下颌关节骨关节炎的病变程度。鲁彩霞等[27]使用30 μg/mL医用臭氧治疗颞下颌关节骨关节炎,结果表明医用臭氧治疗3周以上可有效改善大鼠的颞下颌关节疼痛,通过减少关节内缺氧诱导因子1α、白细胞介素1β、环氧合酶2的表达来缓解SD大鼠颞下颌关节骨关节炎引起的疼痛[28]。然而,关于医用臭氧的最佳治疗浓度、剂量和治疗频率等尚未形成统一标准,仍需进一步深入研究以完善其临床应用。 2.2.6 几丁糖 几丁糖是由甲壳素脱乙酰化得到的一种天然高分子多糖,具有良好的生物相容性、可降解性、抗菌性和生物活性,安全性较高,在预防术后关节粘连方面效果显著。动物实验证明,几丁糖能够抑制成纤维细胞的增殖、减少胶原纤维的形成,从而防止术后关节内粘连[29]。另外,几丁糖有一定的黏稠度和润滑性,能阻断周围组织在愈合过程中的粘连;同时,几丁糖还能刺激关节内的滑膜细胞及软骨细胞增殖,加速组织修复过程[30]。张清彬等[31]在大鼠颞下颌关节骨关节炎模型中应用几丁糖进行治疗,与透明质酸治疗组相比,几丁糖治疗组的白细胞介素1β、白细胞介素6、 白细胞介素8及肿瘤坏死因子α等炎症因子表达水平降低更为显著。何洋等[32]对56例颞下颌关节骨关节炎患者进行了临床研究,对照组仅接受关节腔冲洗治疗,实验组在接受关节腔冲洗后注射几丁糖,实验组患者术后1个月的疼痛程度和张口度均较对照组明显改善,并且关节液中肿瘤坏死因子α含量较对照组显著降低。几丁糖还能为干细胞提供物理支撑和附着位点,提高干细胞移植的治疗效果,并且还具有延缓药物释放的作 用[33]。一般情况下,关节腔内注射几丁糖适用于早中期骨关节炎患者,不适用于软骨破坏严重的晚期患者。 传统药物注射治疗颞下颌关节骨关节炎的研究汇总,见表1。 2.3 新型生物制剂注射治疗 2.3.1 血小板浓缩物 血小板浓缩物是一种从全血中分离制备出的富含血小板的生物制品,其中含有多种生物活性因子,包括转化生长因子β、血小板源性生长因子等,能刺激软骨细胞、滑膜细胞等增殖和分化,促进胶原蛋白和蛋白聚糖的合成,有助于关节软骨的修复,同时还可以抑制炎症细胞的活化,发挥抗炎作用。目前研究较多的血小板浓缩物是富血小板血浆。LIU等[34]通过体外研究发现,富血小板血浆对白细胞介素1β诱导的软骨细胞损伤具有保护作用,其机制可能是激活了丝裂原活化蛋白激酶和磷脂酰肌醇-3-激酶/蛋白激酶B信号通路。在动物实验中,COSKUN等[35]发现富血小板血浆促进了软骨细胞增殖和软骨基质形成,抑制了破骨细胞的形成和炎症因子的表达。体内外研究表明,富血小板血浆具有促进软骨修复的作用。在临床研究中,富血小板血浆注射治疗能显著减轻颞下颌关节骨关节炎引起的相关疼痛及增大开口度,并且在短期内缓解疼痛、长期改善开口度方面优于透明质酸,未发现有严重并发症发生[36-37]。 浓缩生长因子是在富血小板血浆的基础上,通过特殊的激活剂或技术使血小板中的生长因子进一步释放并浓缩,获得更纯净、浓度更高的生长因子,能够更迅速、强烈地促进细胞地增殖和分化。WANG等[38]通过手术方法制造山羊双侧髁突软骨缺损,建立颞下颌关节骨关节炎,浓缩生长因子治疗组显示出髁突软骨的修复和再生,而生理盐水组髁突表面凹凸不平,修复效果不明显。HADDAD等[39]提出血小板浓缩物治疗颞下颌关节骨关节炎的各项试验之间存在较大异质性,包括血小板浓缩物的制备方法和质量、试验对象的种类、患病程度、治疗次数及观察时间的不同,可能会产生不同的结果,因此,仍需完善血小板浓缩物的制备流程以及更多的同质性样本、多中心、长期随机对照试验来验证结果的稳定性。 2.3.2 间充质干细胞 间充质干细胞具有自我更新和多向分化潜力,能促进软骨和骨组织的修复,同时还可以分泌多种细胞因子减轻细胞炎症反应。间充质干细胞主要来源于骨髓、脂肪组织、牙髓、脐带和胚胎干细胞等,其中骨髓间充质干细胞是治疗颞下颌关节骨关节炎最常用的细胞来源[40]。近年来,间充质干细胞在颞下颌关节骨关节炎的治疗中显示出良好的效果[41-42]。KIM等[43]将人脐带间"

充质干细胞注射入颞下颌关节骨关节炎兔的关节腔内,发现人脐带间充质干细胞可以上调生长因子、细胞外基质和抗炎因子,下调促炎因子,表现出突出的促软骨再生潜力。KHAZENI等[44]分别将骨髓间充质干细胞和脂肪间充质干细胞注射入颞下颌关节骨关节炎大鼠的关节腔内,发现二者均有效缓解关节内滑膜炎症,并且脂肪间充质干细胞表现出更优的治疗效果。尽管间充质干细胞疗法在治疗颞下颌关节骨关节炎方面取得了显著成效,但仍然存在许多挑战:炎症环境和氧化应激可能会影响移植间充质干细胞的分化能力;此外,细胞供者的年龄、扩增后细胞质量、扩增后细胞的衰老和去分化都影响干细胞的活性。MA等[45]利用可注射的多功能水凝胶递送牙髓干细胞,提高了牙髓干细胞在氧化应激下的抗凋亡能力,并显著改善了关节的炎症微环境,增加了牙髓干细胞在大鼠颞下颌关节中的植入率,促进了巨噬细胞的M2极化,并进一步增强了牙髓干细胞的软骨修复潜力。间充质干细胞在组织修复和再生中的治疗效果可能归因于旁分泌信号,特别是外泌体。邢超等[46]研究发现,骨髓间充质干细胞来源外泌体具有促进髁突软骨细胞增殖即Ⅰ型胶原、Ⅱ型胶原基因表达的作用,对髁突软骨的修复和再生提供积极作用。LUO等[47]研究发现,人脱落乳牙牙髓干细胞来源外泌体明显降低了髁突软骨细胞中炎症因子的表达,其机制可能是:人脱落乳牙牙髓干细胞来源外泌体中富集的miR-100-5p直接靶向作用于哺乳动物雷帕霉素靶蛋白3’非翻译区域,抑制哺乳动物雷帕霉素靶蛋白表达,从而抑制炎症因子的表达。ZHANG等[48]在动物实验中证实,人胚胎干细胞来源外泌体能减轻大鼠关节疼痛、修复损伤的关节软骨、维持关节稳态。综上所述,干细胞来源外泌体可能是间充质干细胞有前途的替代方案。然而,目前只有有限的证据证明间充质干细胞来源外泌体在颞下颌关节再生中的有效性,还需要更多的研究进一步验证。新型生物制剂治疗颞下颌关节骨关节炎的研究汇总,见表2。 2.4 多种药物联合注射治疗 2.4.1 透明质酸与其他药物联合透明质酸与类固醇类药物:类固醇类药物与透明质酸联合注射可作为传统治疗的替代疗法,这种方法充分利用了类固醇药物的强抗炎作用和透明质酸的黏弹性特性。SINGH等[49]比较关节腔内注射透明质酸联合曲安奈德与单独注射透明质酸或曲安奈德治疗颞下颌关节骨关节炎的效果,发现联合用药在改善关节区疼痛和弹响方面优于单独用药,但在改善下颌功能运动方面两者没有显著差异。 透明质酸与非类固醇类抗炎药物:大量临床试验表明,透明质酸能缓解颞下颌关节骨关节炎的症状,并且具有良好的长期稳定性[50],而非类固醇类抗炎药早期疗效好,多用于短期缓解关节区疼痛、改善张口度[51],但远期疗效较差,二者联合关节腔内注射有取长补短、协同增效的作用。ZHU等[52]将透明质酸与帕瑞昔布联合负载聚乳酸-羟基乙醇微球内,体外实验发现微球内药物持续释放时间可达28 d,体内实验发现微球显著降低了关节内炎症因子的水平。 透明质酸与浓缩生长因子:透明质酸具有润滑关节、抑制炎症、为软骨修复提供适宜环境的作用。浓缩生长因子富含多种生长因子,能促进软骨细胞增殖、软骨修复,调节细胞代"

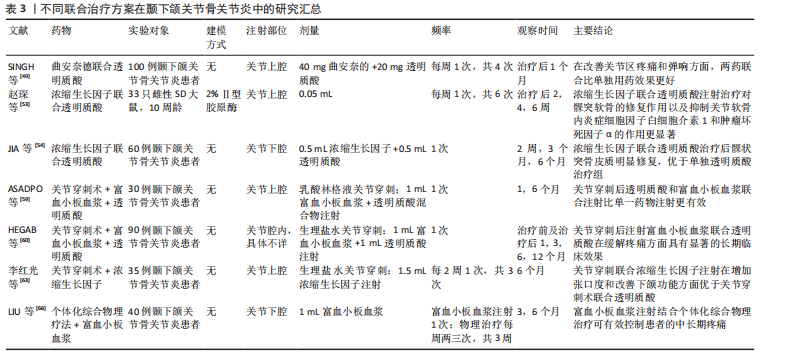
谢。透明质酸为浓缩生长因子发挥作用提供稳定基础,而浓缩生长因子则弥补透明质酸在软骨再生方面的不足,二者联合具有协同增效的作用。赵琛等[53]通过动物实验证实,透明质酸联合浓缩生长因子注射治疗能够促进髁突软骨的修复,降低关节软骨内炎性细胞因子白细胞介素1和肿瘤坏死因子α的水平,治疗效果优于单独注射透明质酸或浓缩生长因子。JIA等[54]在临床试验中也得出相似的结论:与单独注射透明质酸组相比,透明质酸联合浓缩生长因子治疗组在关节疼痛、开口度、关节弹响以及下颌功能运动方面均有显著改善,并且观察到了髁突皮质骨的形成,表明联合治疗的效果更佳。 透明质酸与臭氧:臭氧通过高效抗炎止痛为透明质酸发挥作用创造良好条件,透明质酸则持续润滑关节、促进软骨细胞增殖与基质合成以修复软骨,它们协同调节关节微环境,从抗炎、修复、改善循环等多方面共同作用,显著提升对颞下颌关节骨关节炎的治疗功效。透明质酸联合臭氧在治疗膝骨关节炎中确实展现出了较好的治疗效果[55],然而,关于透明质酸联合臭氧在颞下颌关节骨关节炎治疗方面的研究相对较少。目前,这种联合治疗在长期随访中显示出更优异的临床疗效,但仍需要开展更多的研究来证明其在颞下颌关节骨关节炎治疗中的实用价值和长期效果。 2.4.2 富血小板血浆与其他药物联合 富血小板血浆与透明质酸:随着对自体生长因子研究的不断深入,人们发现富血小板血浆与透明质酸在骨关节治疗中能够产生协同效应[56],这种协同作用的机制在于:富血小板血浆和透明质酸能够通过化学键和离子键相互作用形成稳定性强、生物相容性高的黏弹性三维支架,此支架能够延长富血小板血浆的作用时间,提高其存储模量,并调控生长因子的释放[57],还能在细胞周围形成分子屏障,改善关节内环境,维持软骨的生理功能,进而获得比单独注射更好的软骨重建效果[58]。ASADPOUR等[59]研究发现,在增大张口度和缓解疼痛方面,富血小板血浆联合透明质酸治疗的效果优于单独使用富血小板血浆或透明质酸治疗。还有研究表明,富血小板血浆联合透明质酸治疗组较透明质酸单独治疗组在长期疗效上具有更明显的优势[60]。ATTIA等[61]比较了富血小板血浆联合透明质酸与类固醇药物联合透明质酸治疗颞下颌关节紊乱病的效果,研究发现富血小板血浆联合透明质酸在控制疼痛方面具有更好的长期效果,术后6个月时仍显示出明显的疼痛控制效果。关于富血小板血浆与透明质酸联合治疗的临床研究存在样本量较少、随访时间较短的问题,这限制了对其长期临床效果的有效评估;此外,研究中患者年龄和性别构成比的不同也可能对试验结果产生影响[62]。因此,为了更准确地评估富血小板血浆与透明质酸联合治疗的长期效果,需要开展更多样本量更大、随访时间更长的研究。 2.4.3 药物注射与其他疗法联合治疗 关节腔注射药物联合关节穿刺术:关节穿刺术也称为关节灌洗术,是一种通过向关节腔内注入生理盐水、乳酸林格溶液或其他特定溶液,随后将这些液体连同关节内的炎性物质、软骨碎屑等致病因素一起引流出体外的医疗程序。关节穿刺术的主要作用是清除关节内的炎性物质,但它并不能改变关节的微结构。为了促进组织的愈合,可以在关节穿刺术后补充具有愈合和再生特性的生物或非生物制剂,这将有助于组织的修复和再生。近年来,研究者进行了关节穿刺术后联合不同药物注射的效果比较,获得了较好的临床效果。李红光等[63]发现关节穿刺术联合浓缩生长因子注射在增加张口度和改善下颌功能方面优于关节穿刺术联合透明质酸注射,并且两组部分病例有髁突骨质修复改建的表现,但需进一步扩大样本量进行验证。LI等[64]系统综述显示,关节穿刺术联合富血小板血浆在减轻患者疼痛、改善下颌功能运动方面优于关节穿刺术联合透明质酸,并且其长期疗效更佳。目前关于关节穿刺术联合透明质酸、富血小板血浆的临床研究较多,报道的术后并发症极少,大多数是术后关节周围组织的肿胀且通常是暂时的,这证明了此联合治疗方案的有效性和安全性[65]。未来应关注关节穿刺术与其他药物的联合应用,寻找更加有效的临床组合治疗方案。 关节腔内注射药物联合个体化综合物理治疗:个体化的综合物理治疗主要包括健康教育、物理因子治疗(超短波、超声波)、手法治疗(按摩、推拿)、颞下颌关节运动训练等。临床上,医生会根据患者的具体情况给予相应的物理治疗,然而,物理治疗常常面临诸如就诊次数多、疗效显现慢、患者依从性不高等问题。与之相对,结合关节腔内药物注射可以在短期内改善关节症状、缩短临床治疗周期,并延长术后疗效。LIU等[66]对个体化综合物理治疗联合富血小板血浆注射的临床疗效进行了评估,发现术后3个月和6个月时,接受联合治疗的患者在疼痛减轻和开口度改善方面的效果均优于仅接受富血小板血浆注射的患者。宋瑜等[67]对比了关节腔内注射透明质酸并配合颞下颌关节功能训练(对照组)与仅进行关节内注射透明质酸(观察组)的临床疗效,结果显示对照组的总有效率为91.1%,观察组的总有效率为74.1%,这一结果证实了配合术后功能训练可以更有效地改善临床症状。因此,联合个体化综合物理治疗对患者的症状改善具有一定的帮助作用,但这与医生方案的制定、患者患病程度及不良习惯是否纠正等存在密切关联。联合治疗方案在颞下颌关节骨关节炎中的研究汇总,见表3。"
| [1] HORTON CP. Treatment of arthritic temporomandibular joints by intra-articular injection of hydrocortisone. Oral Surg Oral Med Oral Pathol. 1953;6(7):826-829. [2] KOPP S, CARLSSON GE, HARALDSON T, et al. Long-term effect of intra-articular injections of sodium hyaluronate and corticosteroid on temporomandibular joint arthritis. J Oral Maxillofac Surg. 1987;45(11):929-935. [3] YOUNG CM, SHIELS WE 2ND, COLEY BD, et al. Ultrasound-guided corticosteroid injection therapy for juvenile idiopathic arthritis: 12-year care experience. Pediatr Radiol. 2012;42(12):1481-1489. [4] KÜTÜK N, BAŞ B, SOYLU E, et al. Effect of platelet-rich plasma on fibrocartilage, cartilage, and bone repair in temporomandibular joint. J Oral Maxillofac Surg. 2014;72(2):277-284. [5] ABUBAKR N, SALEM Z, ALI Z, et al. Comparative evaluation of the early effects of the low-level laser therapy versus intra-articular steroids on temporomandibular joint acute osteoarthritis in rats: A histochemical, molecular and imaging evaluation. Dent Med Probl. 2018;55(4):359-366. [6] RESNICK CM, VAKILIAN PM, KABAN LB, et al. Quantifying the Effect of Temporomandibular Joint Intra-Articular Steroid Injection on Synovial Enhancement in Juvenile Idiopathic Arthritis. J Oral Maxillofac Surg. 2016;74(12): 2363-2369. [7] MCCRUM C. Therapeutic Review of Methylprednisolone Acetate Intra-Articular Injection in the Management of Osteoarthritis of the Knee - Part 1: Clinical Effectiveness. Musculoskeletal Care. 2017; 15(1):79-88. [8] 张露珠,何冬梅,杨驰.关节腔内反复激素注射诱发颞下颌关节骨关节炎1例报告及文献复习[J].中国实用口腔科杂志,2019,12(2):125-128. [9] GAGÉ J, GALLUCCI A, ARNAUD M, et al. [Temporomandibular joint arthropathy in situ steroid injection]. Rev Stomatol Chir Maxillofac Chir Orale. 2016;117(4):298-301. [10] DERWICH M, MITUS-KENIG M, PAWLOWSKA E. Orally Administered NSAIDs-General Characteristics and Usage in the Treatment of Temporomandibular Joint Osteoarthritis-A Narrative Review. Pharmaceuticals (Basel). 2021;14(3):219. [11] BI RY, DING Y, GAN YH. Non-steroidal Anti-inflammatory Drugs Attenuate Hyperalgesia and Block Upregulation of Trigeminal Ganglionic Sodium Channel 1.7 after Induction of Temporomandibular Joint Inflammation in Rats. Chin J Dent Res. 2016; 19(1):35-42. [12] OUANOUNOU A, GOLDBERG M, HAAS DA. Pharmacotherapy in Temporomandibular Disorders: A Review. J Can Dent Assoc. 2017;83:h7. [13] XU C, GU K, YASEN Y, et al. Efficacy and Safety of Celecoxib Therapy in Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore). 2016;95(20):e3585. [14] SU SC, TANIMOTO K, TANNE Y, et al. Celecoxib exerts protective effects on extracellular matrix metabolism of mandibular condylar chondrocytes under excessive mechanical stress. Osteoarthritis Cartilage. 2014;22(6):845-851. [15] ZHONG Y, ZHOU Y, DING R, et al. Intra-articular treatment of temporomandibular joint osteoarthritis by injecting actively-loaded meloxicam liposomes with dual-functions of anti-inflammation and lubrication. Mater Today Bio. 2023;19: 100573. [16] 仇雯颉,董凡,王宇光.新型二硫化钼纳米载体对颞下颌关节骨关节炎的治疗作用[J].新技术新工艺,2023,426(6):53-57. [17] LAMANA SMS, NAPIMOGA MH, NASCIMENTO APC, et al. The anti-inflammatory effect of tramadol in the temporomandibular joint of rats. Eur J Pharmacol. 2017;807:82-90. [18] ZIEGLER CM, WIECHNIK J, MÜHLING J. Analgesic effects of intra-articular morphine in patients with temporomandibular joint disorders: a prospective, double-blind, placebo-controlled clinical trial. J Oral Maxillofac Surg. 2010;68(3):622-627. [19] 傅开元.颞下颌关节及口颌面疼痛的治疗[J].中国实用口腔科杂志,2009,2(3): 139-143. [20] 贺春波,王丹,杨淑佳,等.新型阿片类镇痛药的研发进展[J].中国药房,2024, 35(17):2176-2180. [21] WANG XW, FANG W, LI YJ, et al. Synovial fluid levels of VEGF and FGF-2 before and after intra-articular injection of hyaluronic acid in patients with temporomandibular disorders: a short-term study. Br J Oral Maxillofac Surg. 2021;59(1):64-69. [22] GUO H, FANG W, LI Y, et al. Up-regulation of proteoglycan 4 in temporomandibular osteoarthritic synovial cells by hyaluronic acid. J Oral Pathol Med. 2015;44(8):622-627. [23] PARLAWAR AN, MUNDADA BP. Enhancing Pain Relief in Temporomandibular Joint Arthrocentesis: Platelet-Rich Plasma and Hyaluronic Acid Synergy. Cureus. 2023;15(9):e45646. [24] FERREIRA N, MASTERSON D, LOPES DE LIMA R, et al. Efficacy of viscosupplementation with hyaluronic acid in temporomandibular disorders: A systematic review. J Craniomaxillofac Surg. 2018;46(11):1943-1952. [25] DE SIRE A, MAROTTA N, FERRILLO M, et al. Oxygen-Ozone Therapy for Reducing Pro-Inflammatory Cytokines Serum Levels in Musculoskeletal and Temporomandibular Disorders: A Comprehensive Review. Int J Mol Sci. 2022;23(5):2528. [26] 买斯吐热木·黑力力,张婉霞,尼加提·努尔穆罕默德,等.关节腔注射医用臭氧对早期颞下颌骨关节炎模型大鼠髁突组织学的影响[J].中国组织工程研究,2024, 28(4):505-509. [27] 鲁彩霞,张思敏,艾合麦提尼格阿依,等.医用臭氧注射疗程对大鼠颞下颌关节骨关节炎及其疼痛作用的影响[J].口腔医学,2024,44(5):362-368. [28] 鲁彩霞,张思敏,艾合麦提尼格阿依,等.医用臭氧可缓解颞下颌关节骨关节炎的疼痛[J].中国组织工程研究,2024, 28(27):4300-4305. [29] LI HP, SUN SF, FAN BT, et al. Prevention of adhesions in the temporomandibular joint by the use of chitosan membrane in goats. Br J Oral Maxillofac Surg. 2017;55(1):26-30. [30] DERWICH M, LASSMANN L, MACHUT K, et al. General Characteristics, Biomedical and Dental Application, and Usage of Chitosan in the Treatment of Temporomandibular Joint Disorders: A Narrative Review. Pharmaceutics. 2022; 14(2):305. [31] 张清彬,曹威,吴丽红,等.几丁糖对大鼠颞下颌关节滑膜炎的疗效研究[J].口腔颌面修复学杂志,2020,21(4):215-221+257. [32] 何洋,朱丹鹏.几丁糖治疗颞下颌关节骨性关节炎的临床研究[J].现代中西医结合杂志,2015,24(31):3471-3472. [33] COMBLAIN F, ROCASALBAS G, GAUTHIER S, et al. Chitosan: A promising polymer for cartilage repair and viscosupplementation. Biomed Mater Eng. 2017;28(s1):S209-S215. [34] LIU S, WU C, ZHANG Y. Transcriptomics analyses of IL-1β-stimulated rat chondrocytes in temporomandibular joint condyles and effect of platelet-rich plasma. Heliyon. 2024;10(4):e26739. [35] COSKUN U, CANDIRLI C, KERIMOGLU G, et al. Effect of platelet-rich plasma on temporomandibular joint cartilage wound healing: Experimental study in rabbits. J Craniomaxillofac Surg. 2019;47(2):357-364. [36] XIONG Y, GONG C, PENG X, et al. Efficacy and safety of platelet-rich plasma injections for the treatment of osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Front Med (Lausanne). 2023;10:1204144. [37] LIU SS, XU LL, LIU LK, et al. Platelet-rich plasma therapy for temporomandibular joint osteoarthritis: A randomized controlled trial. J Craniomaxillofac Surg. 2023;51(11):668-674. [38] WANG F, SUN Y, HE D, et al. Effect of Concentrated Growth Factors on the Repair of the Goat Temporomandibular Joint. J Oral Maxillofac Surg. 2017;75(3):498-507. [39] HADDAD C, ZOGHBI A, EL SKAFF E, et al. Platelet-rich plasma injections for the treatment of temporomandibular joint disorders: A systematic review. J Oral Rehabil. 2023;50(11):1330-1339. [40] WANG Y, ZHAO M, LI W, et al. BMSC-Derived Small Extracellular Vesicles Induce Cartilage Reconstruction of Temporomandibular Joint Osteoarthritis via Autotaxin-YAP Signaling Axis. Front Cell Dev Biol. 2021;9:656153. [41] JIANG Y, SHI J, DI W, et al. Mesenchymal Stem Cell-Based Therapies for Temporomandibular Joint Repair: A Systematic Review of Preclinical Studies. Cells. 2024;13(11):990. [42] FAYED HM, KHAIRY MA, ELDAHSHAN D, et al. Bone marrow aspirate concentrate - A novel approach to alter the course of temporomandibular joint osteoarthritis (a clinical study). J Stomatol Oral Maxillofac Surg. 2024;125(1):101644. [43] KIM H, YANG G, PARK J, et al. Therapeutic effect of mesenchymal stem cells derived from human umbilical cord in rabbit temporomandibular joint model of osteoarthritis. Sci Rep. 2019;9(1):13854. [44] KHAZENI S, GHAVIMI M, MESGARI-ABBASI M, et al. Therapeutic Effects of Mesenchymal Stem Cells Derived from Bone Marrow and Adipose Tissue in a Rat Model of Temporomandibular Osteoarthritis. J Oral Biosci. 2024;66(4):107-115. [45] MA J, LI J, WEI S, et al. Delivery of dental pulp stem cells by an injectable ROS-responsive hydrogel promotes temporomandibular joint cartilage repair via enhancing anti-apoptosis and regulating microenvironment. J Tissue Eng. 2024;15:20417314241260436. [46] 邢超,徐灵巧,廖文婷,等.骨髓间充质干细胞来源的外泌体促进髁突软骨细胞再生的研究[J].中华口腔医学研究杂志(电子版),2021,15(4):207-214. [47] LUO P, JIANG C, JI P, et al. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res Ther. 2019;10(1):216. [48] ZHANG S, TEO KYW, CHUAH SJ, et al. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35-47. [49] SINGH J, BHARDWAJ B. Treatment of Temporomandibular Joint Arthritis with Triamcinolone Acetonide and Hyaluronic Acid Injection: An Observational Study. Indian J Otolaryngol Head Neck Surg. 2020; 72(4):403-410. [50] LIU Y, WU J, FEI W, et al. Is There a Difference in Intra-Articular Injections of Corticosteroids, Hyaluronate, or Placebo for Temporomandibular Osteoarthritis? J Oral Maxillofac Surg. 2018;76(3):504-514. [51] 肖朋,阿迪力·麦木提敏,古扎丽努尔·阿巴拜克力.美洛昔康联合羟氯喹缓解急性颞下颌关节骨关节炎的疗效初探[J].实用药物与临床,2023,26(3):216-219. [52] ZHU D, BAI H, XU W, et al. Hyaluronic Acid/Parecoxib-Loaded PLGA Microspheres for Therapy of Temporomandibular Disorders. Curr Drug Deliv. 2021;18(2):234-245. [53] 赵琛,姜杉杉,刘阳,等.液态浓缩生长因子联合透明质酸注射治疗对颞下颌关节骨关节炎的影响[J].口腔医学研究, 2023,39(4):362-369. [54] JIA XY, JING SL, SUN Y, et al. A randomized controlled clinical trial of concentrated growth factor combined with sodium hyaluronate in the treatment of temporomandibular joint osteoarthritis. BMC Oral Health. 2024;24(1):540. [55] GIOMBINI A, MENOTTI F, DI CESARE A, et al. Comparison between intrarticular injection of hyaluronic acid, oxygen ozone, and the combination of both in the treatment of knee osteoarthrosis. J Biol Regul Homeost Agents. 2016;30(2):621-625. [56] PARLAWAR AN, MUNDADA BP. Enhancing Pain Relief in Temporomandibular Joint Arthrocentesis: Platelet-Rich Plasma and Hyaluronic Acid Synergy. Cureus. 2023; 15(9):e45646. [57] ZHAO J, HUANG H, LIANG G, et al. Effects and safety of the combination of platelet-rich plasma (PRP) and hyaluronic acid (HA) in the treatment of knee osteoarthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2020;21(1):224. [58] SU K, BAI Y, WANG J, et al. Comparison of hyaluronic acid and PRP intra-articular injection with combined intra-articular and intraosseous PRP injections to treat patients with knee osteoarthritis. Clin Rheumatol. 2018;37(5):1341-1350. [59] ASADPOUR N, SHOOSHTARI Z, KAZEMIAN M, et al. Combined Platelet-Rich Plasma and Hyaluronic Acid can Reduce Pain in Patients Undergoing Arthrocentesis for Temporomandibular Joint Osteoarthritis. J Oral Maxillofac Surg. 2022;80(9):1474-1485. [60] HEGAB AF, HAMEED HIAA, HASSANEEN AM, et al. Synergistic effect of platelet rich plasma with hyaluronic acid injection following arthrocentesis to reduce pain and improve function in TMJ osteoarthritis. J Stomatol Oral Maxillofac Surg. 2023; 124(1S):101340. [61] ATTIA AAMM, AWAD SS. Hyaluronic Acid and Platelet-Rich Plasma Mixture Versus Hyaluronic Acid and Corticosteroid in the Treatment of Temporomandibular Joint Internal Derangement: A Comparative Randomized Study. J Maxillofac Oral Surg. 2023;23(2):1-7. [62] CHĘCIŃSKI M, LUBECKA K, BLIŹNIAK F, et al. Hyaluronic Acid/Platelet-Rich Plasma Mixture Improves Temporomandibular Joint Biomechanics: A Systematic Review. Int J Mol Sci. 2024;25(17):9401. [63] 李红光,韩玮华,吴训,等.关节腔冲洗联合液态浓缩生长因子注射治疗单侧颞下颌关节骨关节炎的初步研究[J].北京大学学报(医学版),2024,56(2):338-344. [64] LI J, CHEN H. Intra-articular injection of platelet-rich plasma vs hyaluronic acid as an adjunct to TMJ arthrocentesis: A systematic review and meta-analysis. J Stomatol Oral Maxillofac Surg. 2024; 125(2):101676. [65] VAIRA LA, RAHO MT, SOMA D, et al. Complications and post-operative sequelae of temporomandibular joint arthrocentesis. Cranio. 2018;36(4):264-267. [66] LIU SS, XU LL, FAN S, et al. Effect of platelet-rich plasma injection combined with individualised comprehensive physical therapy on temporomandibular joint osteoarthritis: A prospective cohort study. J Oral Rehabil. 2022;49(2):150-159. [67] 宋瑜,廖学娟,王婷,等.透明质酸钠注射治疗颞下颌关节紊乱病184例随访分析[J].四川医学,2018,39(6):677-681. |
| [1] | Yang Zhijie, Zhao Rui, Yang Haolin, Li Xiaoyun, Li Yangbo, Huang Jiachun, Lin Yanping, Wan Lei, HuangHongxing. Postmenopausal osteoporosis: predictive values of muscle mass, grip strength, and appendicular skeletal muscle index [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1073-1080. |
| [2] | Zhang Qian, Huang Dongfeng. Weighted gene co-expression network analysis combined with machine learning to screen and validate biomarkers for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1096-1105. |
| [3] | Yin Yongcheng, Zhao Xiangrui, Yang Zhijie, Li Zheng, Li Fang, Ning Bin. Effect and mechanism of peroxiredoxin 1 in microglial inflammation after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1106-1113. |
| [4] | Zhang Jiuxuan, Zhang Jinnan, Sui Xiaofan, Pei Xiaxia, Wei Jianhong, Su Qiang, Li Tian. Effects of ammonia poisoning on cognitive behavior and hippocampal synaptic damage in mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1122-1128. |
| [5] | Sun Yajie, Zhao Xinchen, Bo Shuangling. Spatiotemporal expression of bone morphologic protein 7 in mouse kidney development [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1156-1161. |
| [6] | Li Haojing, Wang Xin, Song Chenglin, Zhang Shengnan, Chen Yunxin. Therapeutic efficacy of extracorporeal shock wave therapy in the upper trapezius muscle area combined with exercise control training in patients with chronic non-specific neck pain [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1162-1170. |
| [7] | Liu Yu, Lei Senlin, Zhou Jintao, Liu Hui, Li Xianhui. Mechanisms by which aerobic and resistance exercises improve obesity-related cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1171-1183. |
| [8] | Yu Huifen, Mo Licun, Cheng Leping. The position and role of 5-hydroxytryptamine in the repair of tissue injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1196-1206. |
| [9] | Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Advance in the mechanisms underlying miRNAs in steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1207-1214. |
| [10] | Wen Fan, Xiang Yang, Zhu Huan, Tuo Yanfang, Li Feng. Exercise improves microvascular function in patients with type 2 diabetes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1225-1235. |
| [11] | Liu Xinyue, Li Chunnian, Li Yizhuo, Xu Shifang. Regeneration and repair of oral alveolar bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1247-1259. |
| [12] | Leng Xiaoxuan, Zhao Yuxin, Liu Xihua. Effects of different neuromodulatory stimulation modalities on non-motor symptoms in Parkinson’s patients: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1282-1293. |
| [13] | Wen Xiaolong, Weng Xiquan, Feng Yao, Cao Wenyan, Liu Yuqian, Wang Haitao. Effects of inflammation on serum hepcidin and iron metabolism related parameters in patients with type 2 diabetes mellitus: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1294-1301. |
| [14] | Yang Zeyu, Zhi Liang, Wang Jia, Zhang Jingyi, Zhang Qingfang, Wang Yulong, Long Jianjun. A visualized analysis of research hotspots in high-frequency repetitive transcranial magnetic stimulation from the macroscopic perspective [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1320-1330. |
| [15] | Chen Yixian, Chen Chen, Lu Liheng, Tang Jinpeng, Yu Xiaowei. Triptolide in the treatment of osteoarthritis: network pharmacology analysis and animal model validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 805-815. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||