Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (18): 4627-4637.doi: 10.12307/2026.713
Previous Articles Next Articles
High-intensity interval training improves the function of exosomes derived from endothelial progenitor cells in spontaneously hypertensive rats
Lu Anran1, Wang Chenyu2, Zhang Yan3, Huang Huasheng3
- 1Zhengzhou Health College, Zhengzhou 450064, Henan Province, China; 2Zhengzhou University of Aeronautics, Zhengzhou 450046, Henan Province, China; 3Guangxi University of Chinese Medicine, Nanning 530021, Guangxi Zhuang Autonomous Region, China
-
Received:2025-05-19Accepted:2025-09-06Online:2026-06-28Published:2025-12-04 -
Contact:Huang Huasheng, MS, Associate professor, Guangxi University of Chinese Medicine, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
About author:Lu Anran, MS, Lecturer, Zhengzhou Health College, Zhengzhou 450064, Henan Province, China -
Supported by:Guangxi Education Science “14th Five-Year Plan” Key Project, No. 2023ZJY535 (to ZY); Henan Province Science and Technology Research Project, No. 232102321125 (to WCY)
CLC Number:
Cite this article
Lu Anran, Wang Chenyu, Zhang Yan, Huang Huasheng. High-intensity interval training improves the function of exosomes derived from endothelial progenitor cells in spontaneously hypertensive rats[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4627-4637.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
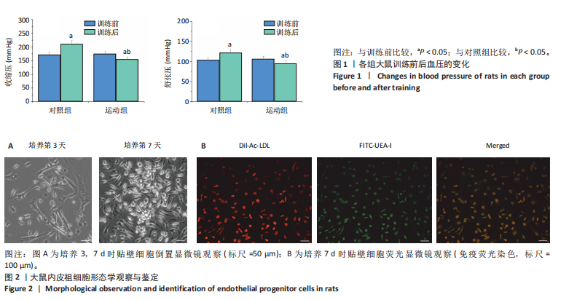
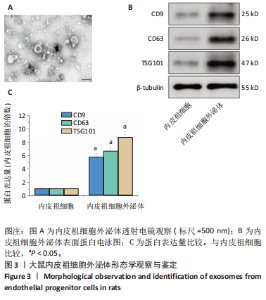
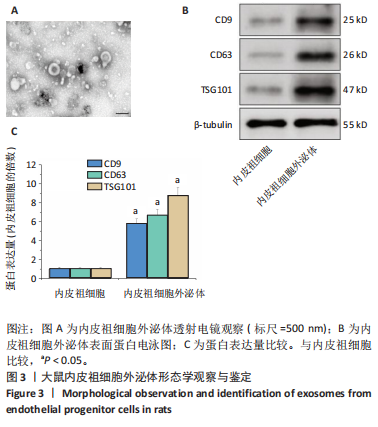
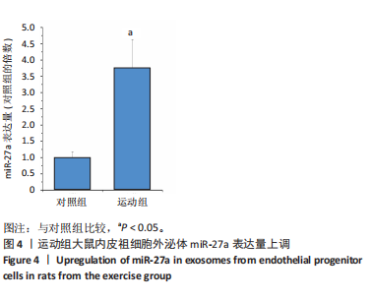
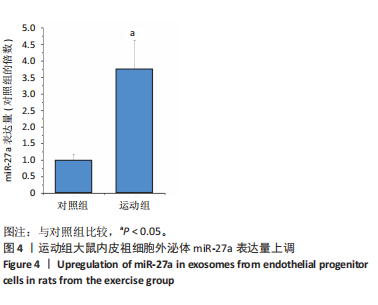
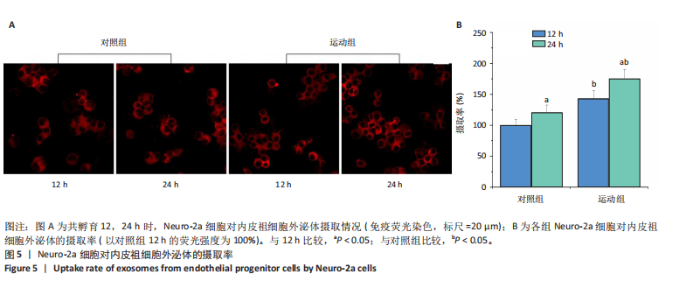
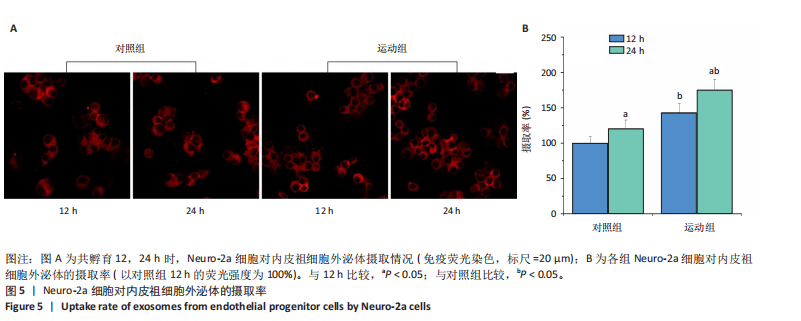
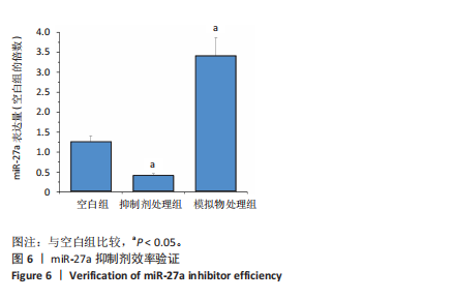
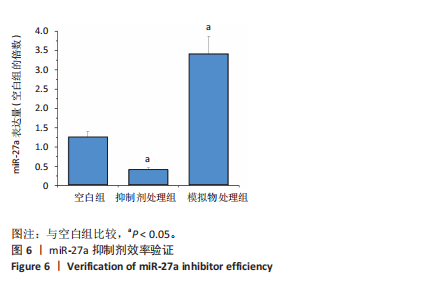
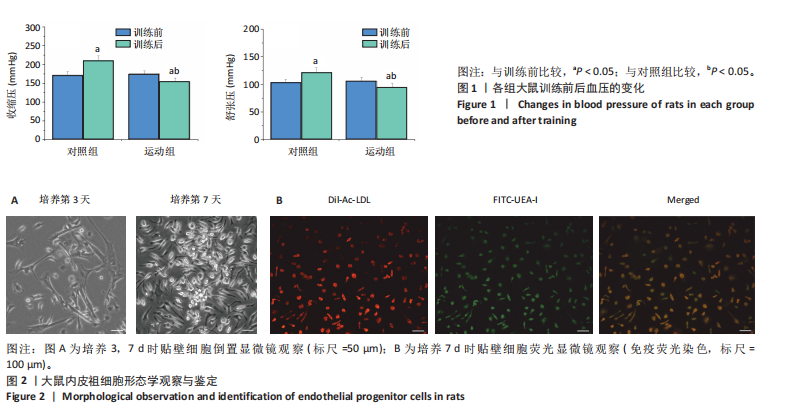
2.1 各组大鼠训练前后血压的变化 训练前,两组大鼠血压水平比较均无统计学差异(P > 0.05)。与训练前比较,运动组训练后收缩压和舒张压降低(P < 0.05),而对照组收缩压和舒张压升高(P < 0.05);与对照组比较,运动组收缩压和舒张压下降(P < 0.05),见图1。 2.2 内皮祖细胞形态学观察与鉴定 倒置显微镜观察发现,培养3 d时培养细胞呈鹅卵石样,形态为梭状或不规则样,7 d时形成细胞集落,见图2A。荧光显微镜观察发现,培养7 d时Dil-Ac-LDL呈红色荧光,FITC-UEA-I呈绿色荧光,双阳性细胞(红色和绿色荧光重叠,呈现黄色)即为内皮祖细胞,见图2B。 2.3 内皮祖细胞外泌体形态学观察与鉴定 透射电镜观察显示,内皮祖细胞外泌体形态为杯形或圆形膜囊泡结构,见图3A。免疫印迹结果显示,内皮祖细胞外泌体高表达特异性外泌体表面蛋白CD9、CD63和TSG101,各蛋白表达量均显著高于内皮祖细胞,见图3B,C。 2.4 内皮祖细胞外泌体miR-27a表达量 与对照组比较,运动组内皮祖细胞外泌体miR-27a表达量上调(P < 0.05),见图4。 2.5 细胞实验结果 2.5.1 Neuro-2a细胞对内皮祖细胞外泌体摄取率 免疫荧光显示,PKH26标记的内皮祖细胞外泌体呈现红色荧光,见图5A。与12 h比较,共孵育24 h后Neuro-2a细胞对两组内皮祖细胞外泌体摄取率均升高(P < 0.05);与对照组比较,共孵育12,24 h后Neuro-2a细胞对运动组内皮祖细胞外泌体摄取率显著升高(P < 0.05),见图5B。"
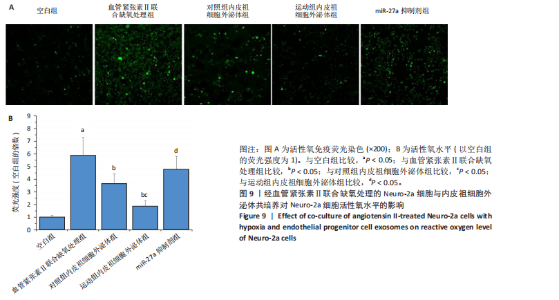
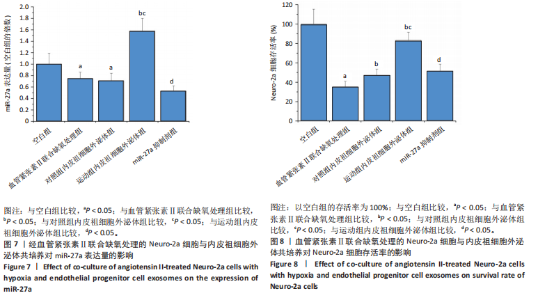
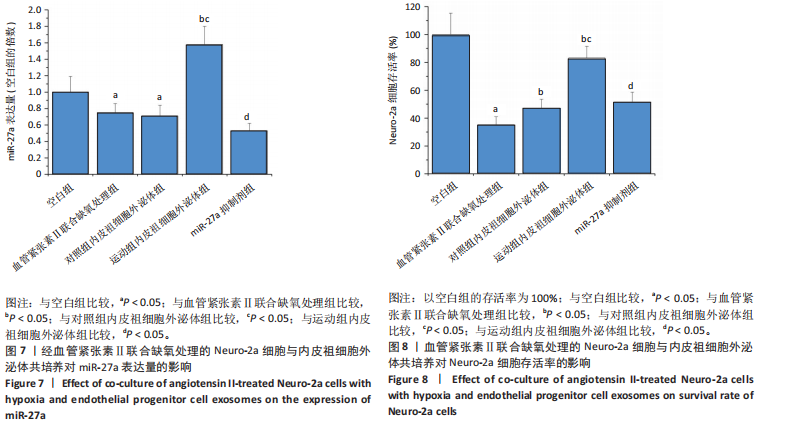
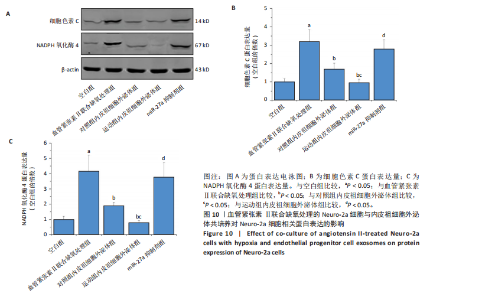
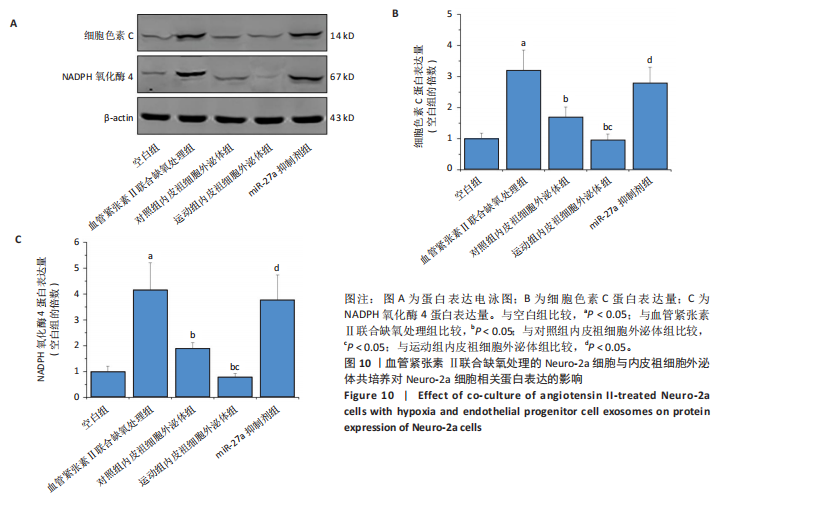
2.5.4 Neuro-2a细胞与内皮祖细胞外泌体共培养对Neuro-2a细胞存活率的影响 与空白组比较,血管紧张素Ⅱ联合缺氧处理后Neuro-2a细胞存活率下降(P < 0.05);与血管紧张素Ⅱ联合缺氧处理比较,添加对照组和运动组内皮祖细胞外泌体后Neuro-2a 细胞存活率均升高(P < 0.05);与添加对照组内皮祖细胞外泌体比较,添加运动组内皮祖细胞外泌体后Neuro-2a细胞存活率升高(P < 0.05);与添加运动组内皮祖细胞外泌体比较,使用miR-27a抑制剂后Neuro-2a细胞存活率降低(P < 0.05),见图8。 2.5.5 Neuro-2a细胞与内皮祖细胞外泌体共培养对Neuro-2a细胞活性氧水平的影响 免疫荧光观察显示,活性氧呈现绿色荧光。见图9A。与空白组比较,血管紧张素Ⅱ联合缺氧处理后Neuro-2a细胞活性氧水平升高(P < 0.05);与血管紧张素Ⅱ联合缺氧处理比较,添加对照组和运动组内皮祖细胞外泌体后Neuro-2a细胞活性氧水平均下降(P < 0.05);与添加对照组内皮祖细胞外泌体比较,添加运动组内皮祖细胞外泌体后Neuro-2a细胞活性氧水平降低(P < 0.05);与添加运动组内皮祖细胞外泌体比较,使用miR-27a抑制剂后Neuro-2a细胞活性氧水平升高(P < 0.05),见图9B。 2.5.6 Neuro-2a细胞与内皮祖细胞外泌体共培养对Neuro-2a细胞相关蛋白表达的影响 与空白组比较,血管紧张素Ⅱ联合缺氧处理后Neuro-2a细胞中细胞色素C和NADPH氧化酶4蛋白表达上调(P < 0.05);与血管紧张素Ⅱ联合缺氧处理比较,添加对照组和运动组内皮祖细胞外泌体后Neuro-2a细胞中细胞色素C和NADPH氧化酶4蛋白表达均下调(P < 0.05);与添加对照组内皮祖细胞外泌体比较,添加运动组内皮祖细胞外泌体后Neuro-2a细胞中细胞色素C和NADPH氧化酶4蛋白表达降低(P < 0.05);与添加运动组内皮祖细胞外泌体比较,使用miR-27a抑制剂后Neuro-2a细胞中细胞色素C和NADPH氧化酶4蛋白表达升高(P < 0.05),见图10。"

| [1] POTTER T, TANNOUS J, VAHIDY FS. A Contemporary review of epidemiology, risk factors, etiology, and outcomes of premature stroke. Curr Atheroscler Rep. 2022;24(12):939-948. [2] MALIK AN, TARIQ H, AFRIDI A, et al. Technological advancements in stroke rehabilitation. J Pak Med Assoc. 2022;72(8):1672-1674. [3] KIM Y, SHARP S, HWANG S, et al. Exercise and incidence of myocardial infarction, stroke, hypertension, type 2 diabetes and site-specific cancers: prospective cohort study of 257 854 adults in South Korea. BMJ Open. 2019;9(3):e025590. [4] BAKKER EA, SUI X, BRELLENTHIN AG, et al. Physical activity and fitness for the prevention of hypertension. Curr Opin Cardiol. 2018;33(4): 394-401. [5] XIA X, LI G, DONG Q, et al. Endothelial progenitor cells as an emerging cardiovascular risk factor in the field of food and nutrition research: advances and challenges. Crit Rev Food Sci Nutr. 2024;64(33):12166-12183. [6] KING TF, MCDERMOTT JH. Endothelial progenitor cells and cardiovascular disease. J Stem Cells. 2014;9(2):93-106. [7] LUO S, XIA W, CHEN C, et al. Endothelial progenitor cells and hypertension: current concepts and future implications. Clin Sci (Lond). 2016;130(22):2029-2042. [8] MARKETOU ME, KALYVA A, PARTHENAKIS FI, et al. Circulating endothelial progenitor cells in hypertensive patients with increased arterial stiffness. J Clin Hypertens (Greenwich). 2014;16(4):295-300. [9] CAVALCANTE SL, LOPES S, BOHN L, et al. Effects of exercise on endothelial progenitor cells in patients with cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Rev Port Cardiol (Engl Ed). 2019;38(11):817-827. [10] FERENTINOS P, TSAKIRIDES C, SWAINSON M, et al. The impact of different forms of exercise on circulating endothelial progenitor cells in cardiovascular and metabolic disease. Eur J Appl Physiol. 2022;122(4): 815-860. [11] MITSIOU G, TOKMAKIDIS SP, DINAS PC, et al. Endothelial progenitor cell mobilization based on exercise volume in patients with cardiovascular disease and healthy individuals: a systematic review and meta-analysis. Eur Heart J Open. 2022;2(6):e078. [12] DE SOUZA MESQUITA FO, GAMBASSI BB, DE OLIVEIRA SILVA M, et al. Effect of high-intensity interval training on exercise capacity, blood pressure, and autonomic responses in patients with hypertension: A systematic review and meta-analysis. Sports Health. 2023;15(4):571-578. [13] KRYLOVA SV, FENG D. The machinery of exosomes: Biogenesis, release, and uptake. Int J Mol Sci. 2023;24(2):e1337. [14] KIMIZ-GEBOLOGLU I, ONCEL SS. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533-543. [15] CHEN S, POLAKI V, BIHL JC, et al. Compromised endothelial progenitor cell exosomal communication with endothelial cells in hypertension ischemia conditions. Front Stroke. 2022;1:e1015463. [16] TE RIET L, VAN ESCH JH, ROKS AJ, et al. Hypertension: renin-angiotensin- aldosterone system alterations. Circ Res. 2015;116(6):960-975. [17] CHEN S, LI G, ZHANG W, et al. Ischemia-induced brain damage is enhanced in human renin and angiotensinogen double-transgenic mice. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1526-1531. [18] NI P, YANG L, LI F. Exercise-derived skeletal myogenic exosomes as mediators of intercellular crosstalk: a major player in health, disease, and exercise. J Physiol Biochem. 2023;79(3):501-510. [19] FRüHBEIS C, HELMIG S, TUG S, et al. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J Extracell Vesicles. 2015;4:e28239. [20] CHATURVEDI P, KALANI A, MEDINA I, et al. Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. J Cell Mol Med. 2015;19(9):2153-2161. [21] MA C, WANG J, LIU H, et al. Moderate exercise enhances endothelial progenitor cell exosomes release and function. Med Sci Sports Exerc. 2018;50(10):2024-2032. [22] YOON KJ, PARK S, KWAK SH, et al. Effects of voluntary running wheel exercise-induced extracellular vesicles on anxiety. Front Mol Neurosci. 2021;14:665800. [23] 袁国强,秦永生,彭朋.高强度间歇运动对自发性高血压模型大鼠病理性心脏肥大的影响及机制[J].中国组织工程研究,2020, 24(23):3708-3715. [24] ERKEN HA, ERKEN G, GENÇ O. Blood pressure measurement in freely moving rats by the tail cuff method. Clin Exp Hypertens. 2013;35(1): 11-15. [25] 卫肖艳,张莉,林明,等.大鼠骨髓源性内皮祖细胞的分离培养与鉴定[J].中国组织工程研究,2013,17(14):2570-2577. [26] ZHAO JL, ZHAO L, ZHAN QN, et al. BMSC-derived exosomes ameliorate peritoneal dialysis-associated peritoneal fibrosis via the miR-27a-3p/TP53 Pathway. Curr Med Sci. 2024;44(2):333-345. [27] ZHANG C, WANG J, MA X, et al. ACE2-EPC-EXs protect ageing ECs against hypoxia/reoxygenation-induced injury through the miR-18a/Nox2/ROS pathway. J Cell Mol Med. 2018;22(3):1873-1882. [28] 彭阿建,欧阳范馨,张熙,等.黄芪甲苷干预的EPC-Exos对高糖诱导损伤间充质干细胞向内皮分化的影响[J].世界科学技术-中医药现代化,2022,24(4):1593-1602. [29] KHATTAR KE, SAFI J, RODRIGUEZ AM, et al. Intercellular communication in the brain through tunneling nanotubes. Cancers (Basel). 2022;14(5): e1207. [30] MARAR C, STARICH B, WIRTZ D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. 2021;22(5): 560-570. [31] LI J, MA Y, MIAO XH, et al. Neovascularization and tissue regeneration by endothelial progenitor cells in ischemic stroke. Neurol Sci. 2021; 42(9):3585-3593. [32] WANG J, LIU H, CHEN S, et al. Moderate exercise has beneficial effects on mouse ischemic stroke by enhancing the functions of circulating endothelial progenitor cell-derived exosomes. Exp Neurol. 2020;330: e113325. [33] CORREIA RR, BATISTA V, VERAS A, et al. High-intensity interval training attenuates the effects caused by arterial hypertension in the ventral prostate. Prostate. 2022;82(3):373-387. [34] 付常喜,马刚,李平,等.不同强度运动对自发性高血压大鼠肾脏纤维化的影响及作用机制研究[J].首都体育学院学报,2021, 33(6):638-648. [35] 张敏,彭朋,秦永生,等.不同负荷剂量高强度间歇训练对高血压肾病大鼠肾脏损伤的影响[J].山东体育学院学报,2020,36(6):54-64. [36] 白春宏,秦永生,薄海,等.不同运动方式对自发性高血压大鼠骨骼肌纤维类型与代谢的影响[J].中国康复医学杂志,2020,35(3): 294-300. [37] ZHENG D, HUO M, LI B, et al. The role of exosomes and exosomal microRNA in cardiovascular disease. Front Cell Dev Biol. 2020;8: e616161. [38] DARRAGH I, O’DRISCOLL L, EGAN B. Exercise training and circulating small extracellular vesicles: Appraisal of methodological approaches and current knowledge. Front Physiol. 2021;12:e738333. [39] CASTAñO C, MIRASIERRA M, VALLEJO M, et al. Delivery of muscle-derived exosomal miRNAs induced by HIIT improves insulin sensitivity through down-regulation of hepatic FoxO1 in mice. Proc Natl Acad Sci U S A. 2020;117(48):30335-30343. [40] XI T, JIN F, ZHU Y, et al. MiR-27a-3p protects against blood-brain barrier disruption and brain injury after intracerebral hemorrhage by targeting endothelial aquaporin-11. J Biol Chem. 2018;293(52):20041-20050. [41] CHEN Q, XU J, LI L, et al. MicroRNA-23a/b and microRNA-27a/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis. Cell Death Dis. 2014;5(3):e1132. [42] GU Q, WANG B, ZHANG XF, et al. Contribution of renin-angiotensin system to exercise-induced attenuation of aortic remodeling and improvement of endothelial function in spontaneously hypertensive rats. Cardiovasc Pathol. 2014;23(5):298-305. [43] HERGENHAHN L, PADUTSCH N, AZAWI S, et al. Cytogenomic characterization of murine neuroblastoma cell line Neuro-2a and its two derivatives Neuro-2a TR-alpha and Neuro-2a TR-beta. Cells. 2024;13(22):e1889. [44] XIA C, DAI Z, JIN Y, et al. Emerging antioxidant paradigm of mesenchymal stem cell-derived exosome therapy. Front Endocrinol (Lausanne). 2021;12:727-739. [45] ŞIŞLI HB, HAYAL TB, ŞENKAL S, et al. Apelin receptor signaling protects GT1-7 GnRH neurons against oxidative stress in vitro. Cell Mol Neurobiol. 2022;42(3):753-775. [46] DU ZD, YU S, QI Y, et al. NADPH oxidase inhibitor apocynin decreases mitochondrial dysfunction and apoptosis in the ventral cochlear nucleus of D-galactose-induced aging model in rats. Neurochem Int. 2019;124:31-40. [47] JUN Z, LEI W, CE F, et al. NADPH oxidase 4 facilitates progression of chondrosarcoma via generation of reactive oxygen species. Acta Biochim Pol. 2023;70(3):685-692. |
| [1] | Wang Zheng, Cheng Ji, Yu Jinlong, Liu Wenhong, Wang Zhaohong, Zhou Luxing. Progress and future perspectives on the application of hydrogel materials in stroke therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2081-2090. |
| [2] | Gao Feng, Zhang Jun, Yu Wenjun, Chanyu Yujing, Zhao Le, Hu Yuting, Wang Junhua, Liu Yongfu. Effects of wrist-hand orthosis on hand dysfunction in stroke patients: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2124-2131. |
| [3] | Song Puzhen, Ma Hebin, Chen Hongguang, Zhang Yadong. Effect of bone marrow mesenchymal stem cell-derived exosomes combined with transforming growth factor beta 1 on macrophages [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1616-1623. |
| [4] | He Jiale, Huang Xi, Dong Hongfei, Chen Lang, Zhong Fangyu, Li Xianhui. Acellular dermal matrix combined with adipose-derived stem cell exosomes promotes burn wound healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1699-1710. |
| [5] | Xia Linfeng, Wang Lu, Long Qianfa, Tang Rongwu, Luo Haodong, Tang Yi, Zhong Jun, Liu Yang. Human umbilical cord mesenchymal stem cell-derived exosomes alleviate blood-brain barrier damage in mice with septic encephalopathy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1711-1719. |
| [6] | Chen Yulin, He Yingying, Hu Kai, Chen Zhifan, Nie Sha Meng Yanhui, Li Runzhen, Zhang Xiaoduo , Li Yuxi, Tang Yaoping. Effect and mechanism of exosome-like vesicles derived from Trichosanthes kirilowii Maxim. in preventing and treating atherosclerosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1768-1781. |
| [7] | Han Teng, Ma Hong, Yang Ruoyi, Luo Yi, Li Chao. Oral squamous cell carcinoma-derived exosomal delivery of angiopoietin-2 is involved in tumor angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1755-1767. |
| [8] | Tao Daiju, Su Haiyu, Wang Yuqi, Shen Zhiqiang, He Bo . Construction and identification of stable PC12 cell lines with high/low expression of miR-122-5p [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1790-1799. |
| [9] | Huang Jiawen, Pan Zhiyi, Xue Wenjun, Lian Yuanpei, Xu Jianda. Plant-derived vesicles and malignant tumor therapy: cross-species communication and modulation of host cell responses [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1828-1838. |
| [10] | Wang Baiyan, Yang Shu, Wang Yiming, Wu Mengqing, Xiao Yu, Guo Zixuan, Zhang Boyi, Feng Shuying. Exosome-delivered CRISPR/Cas system enables gene editing in target cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1839-1849. |
| [11] | Wang Zhenze, Liu Fende, Zhang Rui, Li Wujun. Mesenchymal stem cells in treatment of arteriosclerosis obliterans of lower extremities: systematic review and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1869-1876. |
| [12] | Yang Yuanyuan, Zhou Shanshan, Cheng Xiaofei, Feng Luye, Tang Jiqin. Network meta-analysis of non-invasive brain stimulation in the treatment of lower limb motor dysfunction after stroke [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1008-1018. |
| [13] | Leng Xiaoxuan, Yu Zifu, Cao Xinyan, Gao Shiai, Chen Jinhui, Liu Xihua. Balance function and its influencing factors in patients with post-stroke hemiplegia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4611-4617. |
| [14] | Wang Xinyue, Li Hongli, Guo Chunhui, Chen Jibing, Yu Hua. Changes in the expression of six microRNAs in ovarian tissue from animal models of premature ovarian failure and in peripheral blood of patients with premature ovarian failure [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4675-4684. |
| [15] | Wu Xue, Zhang Linao, Luo Shifang, Liu Feifan, Wan Yan, Bai Yuanmei, Cao Julin, Xie Yuhuan, Guo Peixin. Dandeng Tongnao soft capsules against ischemic stroke: fingerprinting and network pharmacological analysis of efficacy and mechanism of action [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4517-4528. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||