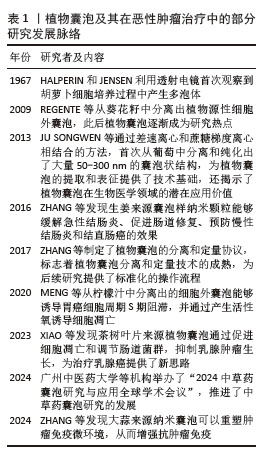
Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (7): 1828-1838.doi: 10.12307/2026.066
Previous Articles Next Articles
Plant-derived vesicles and malignant tumor therapy: cross-species communication and modulation of host cell responses
Huang Jiawen, Pan Zhiyi, Xue Wenjun, Lian Yuanpei, Xu Jianda
- 1Department of Orthopedics, Changzhou Hospital Affiliated to Nanjing University of Chinese Medicine, Changzhou Hospital of Traditional Chinese Medicine, Changzhou 213003, Jiangsu Province, China
-
Received:2025-01-26Revised:2025-05-18Accepted:2025-06-20Online:2026-03-08Published:2025-08-21 -
Contact:徐建达,博士,副主任医师,硕士生导师,南京中医药大学常州附属医院,常州市中医医院骨科,江苏省常州市 213003 -
About author:Huang Jiawen, Master candidate, Department of Orthopedics, Changzhou Hospital Affiliated to Nanjing University of Chinese Medicine, Changzhou Hospital of Traditional Chinese Medicine, Changzhou 213003, Jiangsu Province, China -
Supported by:National Natural Science Foundation of China, No. 12372305 (to XJD); Jiangsu Provincial Administration of Traditional Chinese Medicine Project, No. YB20200053 (to XJD)
CLC Number:
Cite this article
Huang Jiawen, Pan Zhiyi, Xue Wenjun, Lian Yuanpei, Xu Jianda. Plant-derived vesicles and malignant tumor therapy: cross-species communication and modulation of host cell responses[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1828-1838.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
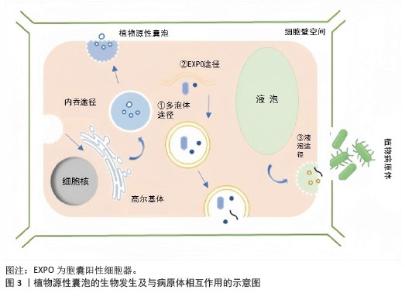
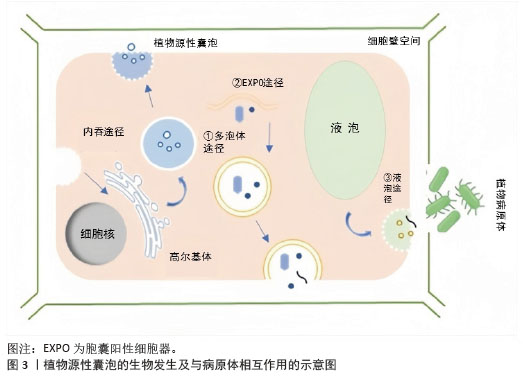
2.1.1 植物源性囊泡的生物合成 植物源性囊泡是由细胞向细胞外空间释放的含有细胞溶胶的脂质双分子层封闭的囊泡。在植物中,囊泡的主要功能是用作物质运输的保护隔室,它有利于植物的生长、发育、防御反应,同时也在植物与微生物的共生关系中发挥重要作用[20]。 植物源性囊泡在结构上与哺乳动物的外泌体具有相似性,其直径一般在30-150 nm之间[21]。植物源性囊泡的形成可能来源于多种细胞器,包括胞囊阳性细胞器、多泡体和液泡[22-23]。 多泡体是晚期核内体或泡前室,包含直径30-100 nm的腔内囊泡[24-25]。它们以内出芽的方式形成,并且受运输所需的内体分选复合体机制的调控[26]。在哺乳动物细胞中,多泡体与质膜结合后,会释放出直径在50-150 nm之间的外泌体[27]。1967年,科学家们通过透射电镜观察到胡萝卜细胞培养过程中产生多泡体,并与质膜融合,形成并释放具有膜结构的新囊泡到细胞外区域[28]。与哺乳动物的外泌体类似,植物细胞在受到病原体攻击时,会通过分泌多泡体作为防御反应,这些多泡体的形成和功能与动物来源的外泌体相似。 除了多泡体形成途径外,植物细胞还有其他形成囊泡的途径。例如在拟南芥和烟草细胞中存在一种双层膜的细胞器,它们能够与细胞的质膜相融合,进而释放出具有单层膜的囊泡,这些囊泡与细胞壁结合,形成胞囊阳性细胞器[29]。 此外,植物源性细胞外囊泡还可以通过液泡与质膜融合的方式形成。在特定的生物学情境下,液泡能够与质膜发生融合,将液泡内部的物质释放到细胞外环境中,这一过程可能与囊泡的形成密切相关[30]。 总的来说,植物细胞通过多种途径形成和释放囊泡,这些囊泡在植物的生长、防御和共生关系中扮演着重要角色,见图3。尽管植物源性囊泡的研究相对较少,但它们在功能上与哺乳动物的外泌体有许多相似之处。"
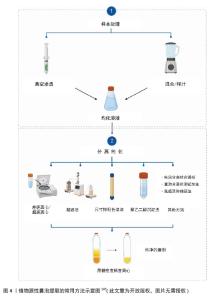
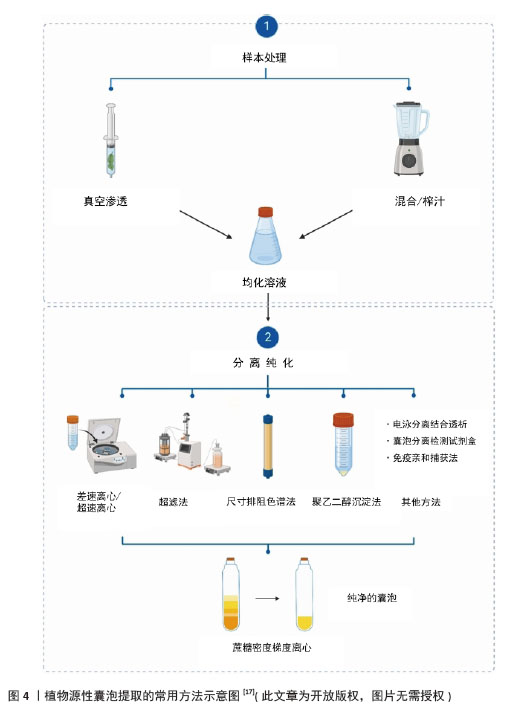
2.1.2 植物源性囊泡的制备 目前,从植物中提取囊泡的技术手段多种多样,包括超速离心联合蔗糖梯度密度离心法、超滤法、聚乙二醇沉淀法、尺寸排阻色谱法、电泳分离结合透析法、试剂盒、免疫亲和捕获法等,见图4。 (1)超速离心联合蔗糖梯度密度离心:在植物囊泡的分离过程中,超速离心是最常用的方法,然后再采用蔗糖梯度密度离心法纯化这些囊泡。基于不同的离心力和蔗糖梯度,可以分离出不同大小和密度的囊泡。 一般来说,新鲜提取的植物汁液要进行低速离心(小于10 000×g),以去除大颗粒,如纤维和其他植物碎屑,然后将上清液高速离心(约150 000×g) 1 h以上,促进纳米级颗粒的聚集,将这些颗粒重新悬浮于少量的磷酸盐缓冲液中,随后将均匀的悬浮液应用于蔗糖梯度溶液(8%/15%/30%/45%/60%)中,并在150 000×g的条件下离心约2 h[31]。基于高重力沉降和蔗糖梯度的不同速度,可以有效地去除其他复杂的蛋白质、DNA和RNA,从而获得高纯度的细胞外囊泡,例如有研究者用超速离心联合蔗糖梯度密度离心提取了天冬来源囊泡样纳米颗粒[32]。 在提取植物源性囊泡的方法中,超速离心结合蔗糖密度梯度离心被认为是“金标准”。然而,这一方法也有一些缺点,如操作繁琐、成本较高、耗时较长等[33]。 (2)超滤法:超滤法是分离植物源性囊泡的常用方法,其基本原理类似于传统的膜过滤,即根据纳米颗粒的大小或分子质量分离囊泡[34]。相较于超速离心,超滤技术的分离效率更高,并且不需要依赖复杂的仪器设备。但是,只通过颗粒大小或分子质量很难分离其他杂质(如脂蛋白等),也有可能堵塞设备影响超滤效率。因此,超滤法通常和其他分离技术联合使用,这样才能获得更高纯度的植物源性囊泡[35]。 (3)聚乙二醇沉淀法:聚乙二醇沉淀法是一种原始的、非特异性的技术。该方法与超速离心沉淀法在初期步骤相似,都通过低离心力去除植物汁液中的大颗粒杂质。通过在上清液中加入适量的聚乙二醇-6000,然后在4 ℃下摇匀过夜,次日再用低离心力离心30 min,用PBS重新悬浮。在对聚乙二醇沉淀法获得的细胞外囊泡进行表征时,发现Zeta电位值与超速离心法获得的囊泡相似,同时在RNA、蛋白质和脂质组成上也呈现出较高的相似性[36]。聚乙二醇沉淀法具有操作简便、成本较低且分离速度较快等优点,但也存在产率和纯度相对较低等缺陷。 (4)尺寸排阻色谱法:尺寸排阻色谱法也是先采用低速离心去除植物汁液中的大颗粒杂质,然后通过尺寸排阻色谱法进一步分离上清液。由于植物源性囊泡的粒径普遍大于杂质蛋白,所以囊泡会先于杂质蛋白通过色谱柱流出,而较小的杂质蛋白在色谱柱中保留时间较长,进而在不同时间收集到不同的组分,实现了植物源性囊泡与杂质蛋白的有效分离。尺寸排阻色谱法在分离植物源性囊泡时,通常能够获得更高的产率以及更完整的功能特征[37]。但此法制备过程繁琐、耗时长,且在分离大粒径杂质颗粒方面存在一定的局限性。 (5)电泳分离结合透析:电泳分离结合透析是一种创新的方法。在电场作用下,带电的蛋白质和RNA会向正负极移动,然后通过透析袋在电极两侧得到富集,同时植物源性囊泡因尺寸排除作用而留在透析袋中,从而实现分离。YANG等[38]采用电泳技术结合300 kD截止的透析袋来分离柠檬来源囊泡。这种方法节省时间,无需特殊设备。通过电泳分离结合透析法分离自柠檬的囊泡在尺寸和数量上与通过超速离心分离的囊泡相似。值得注意的是,电泳分离结合透析法不仅节省时间,而且不需要专门的设备,使它成为缺乏高端仪器设备的实验室的一个可行选择。但电泳分离结合透析法可能需要进一步的自动化和规模化改进,以提高其在更广泛应用中的适用性。 (6)细胞外囊泡分离检测试剂盒:市售的细胞外囊泡分离检测试剂盒为细胞外囊泡分离提供了一种简便易行的方法。但该方法分离效果较差,一些大小相似的蛋白质和各种杂质差异太大,无法有效分离,从而影响分离效果;每次分离的数量也可能很小,仅适于小规模应用[39]。 (7)免疫亲和捕获法:免疫亲和捕获法是一种利用特异性抗体捕获和分离带有特定表面标志物的细胞外囊泡的方法。该方法可以根据外泌体的大小、密度、表面电荷及其表面相关的蛋白质进行分离和纯化。有研究者已经使用Protein A珠子与自制的兔抗TET8抗体结合[40],捕获了TET8阳性的细胞外囊泡。免疫亲和捕获法具有高特异性、高纯度和广泛适应性等优点,但是该方法还存在产量较低、抗体成本较高的问题[41]。 由于每种方法都存在优点和局限性,见表2。研究人员将上述分离方法结合起来以期获得更高质量的植物源性囊泡。由于动物细胞和植物细胞之间的差异,如淀粉、纤维素和其他植物汁中的大分子成分,相同的分离方法在不同植物源性囊泡的提取中可能不太有效,因此,需要进一步开发从植物中分离囊泡的方法。适当的技术组合更有利于获得高纯度、高产率和高活性的植物源性囊泡。"
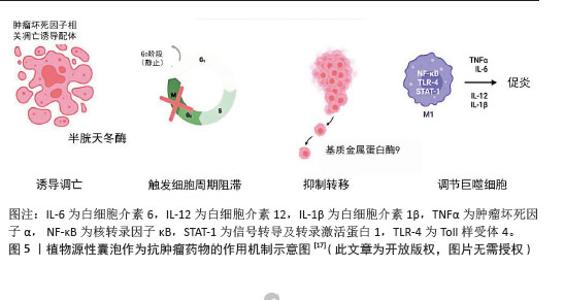
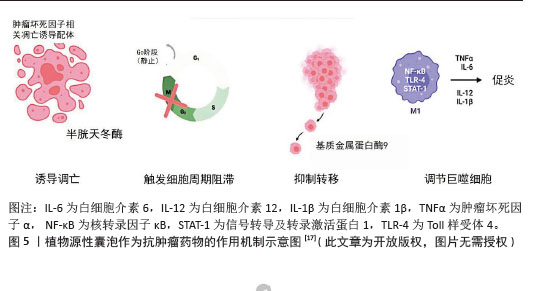
2.2.1 抗肿瘤药物 (1)抑制癌细胞的增殖、诱导癌细胞凋亡:植物源性囊泡自身具有较好的抗肿瘤特性,能够抑制癌细胞增殖,促进癌细胞凋亡,且不会对非癌细胞产生有害影响。柑橘、柠檬和葡萄中的囊泡样纳米颗粒可以不同程度地抑制A375(黑色素瘤)、A549(肺腺癌)和MCF-7(乳腺癌)细胞的增殖[42-43],且对非癌细胞(HaCat)没有伤害作用。从玉米中提取的囊泡样纳米颗粒显著减少了各种细胞系(如colon26、MDA-MB-231和Panc-1)的细胞数量,且不影响非癌细胞(NIH3T3、RAW264.7)的活力。在植物源性囊泡抗癌机制研究方面,目前已经取得了一定进展。天冬来源的囊泡样纳米颗粒具有与凋亡诱导途径相关的特异性抗肿瘤细胞增殖活性。天冬纳米囊泡对肝癌细胞(HepG2、Hep3B、SMMC-7721)具有显著的抑制作用,而对正常肝细胞(LO2)的毒性较低;同时它能够诱导肝癌细胞凋亡,调节凋亡相关的蛋白表达水平(如AIF、Bax和Bak的增加以及Caspase-9的激活)[32]。大蒜衍生的纳米颗粒对人类肾癌细胞(A498)和人类肺癌细胞(A549)显示出显著的抗增殖效果,通过Annexin V/PI染色实验证实,大蒜衍生的纳米颗粒能够诱导A498和A549癌细胞的凋亡,且这种凋亡效应随时间增加而增强[44]。 植物源性囊泡还表现出了较好的肿瘤组织靶向性。SASAKI等[45]在研究中发现,colon26荷瘤小鼠皮下注射玉米来源植物源性囊泡后,肿瘤生长受到抑制,小鼠器官未出现严重毒性。从茶花和茶叶中提取的纳米囊泡可以通过提高体内和体外的活性氧水平诱导4T1细胞凋亡。在小鼠模型中,茶花和茶叶中提取的纳米囊泡通过静脉注射和口服给药能够积累在乳腺肿瘤和肺转移部位,抑制肿瘤生长[46-47]。 目前,各种植物源性囊泡抑制癌细胞增殖、促进癌细胞凋亡的机制已经开展了较为广泛的研究,见图5。但相关复杂的生物学机制,仍有待于后续更为深入的研究。 (2)调节癌细胞周期:癌细胞周期可以分为4个:G1期(间隙1期)、S期(DNA合成期)、G2期(间隙2期)、M期(有丝分裂期)。植物源性囊泡携带生物活性分子,例如某些蛋白质或核酸,它们可以进入癌细胞,干扰癌细胞周期相关蛋白的表达或者活性。 葡萄柚来源纳米囊泡能够触发细胞周期在G2/M检查点的停滞[42]。研究人员通过实时定量反转录聚合酶链反应分析发现葡萄柚来源纳米囊泡能够下调CycB2(G2/M转换的关键调节因子)的表达,并且显著上调细胞周期抑制因子(p21)的表达,这有助于加强细胞周期的阻滞。通过流式细胞术分析,研究发现柠檬来源细胞外囊泡能够使3种胃癌细胞系(AGS、BGC-823和SGC-7901)的细胞周期在S期阻滞,而GADD45A蛋白表达上调,在柠檬来源细胞外囊泡介导的胃癌细胞S期阻滞和凋亡中发挥关键作用[38]。 植物源性囊泡通过多种机制来调节癌细胞周期,包括直接阻滞细胞周期的特定阶段和间接影响细胞内信号通路,这些发现为利用植物源性囊泡作为抗肿瘤药物提供了科学依据,同时也指出了未来研究的潜在方向。 (3)调节肿瘤微环境:肿瘤微环境是指肿瘤细胞生长和生存的局部环境,它影响着肿瘤的发展、侵袭、转移以及对治疗的响应。肿瘤微环境包括肿瘤细胞、免疫细胞(T细胞、B细胞、巨噬细胞等)、血管、信号分子等。 肿瘤相关巨噬细胞在肿瘤微环境中发挥着重要作用,M1型(抗肿瘤)和M2型(促肿瘤)之间的极化状态与肿瘤生长、血管生成和侵袭密切相关。有研究表明,人参来源纳米样颗粒能够通过Toll样受体4和髓样分化抗原88(MyD88)介导的信号传导[48],显著促进M2型巨噬细胞向M1型极化,并促进小鼠黑色素瘤细胞的凋亡,从而显著抑制荷瘤小鼠的黑色素瘤生长。 人参来源囊泡样纳米样颗粒还可以通过重新编程冷肿瘤微环境来增强免疫检查点抗体的效力。冷肿瘤微环境中效应T细胞浸润低,导致对免疫检查点抑制剂治疗反应弱。人参来源囊泡样纳米样颗粒和PD-1单克隆抗体的联合治疗策略能够将肿瘤微环境从冷转变为热,增强PD-1单克隆抗体的抗肿瘤效力[49]。 由此可见,植物源性囊泡在肿瘤相关巨噬细胞的极化状态和冷热肿瘤转化方面显示出巨大潜力,为免疫治疗提供新的策略。随着对肿瘤微环境的深入理解和人参来源囊泡样纳米样颗粒等新型治疗策略的开发,有望在未来实现对恶性肿瘤更精准、更有效的治疗。"
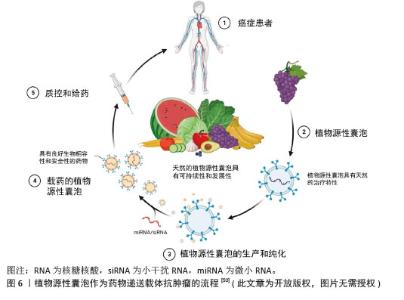
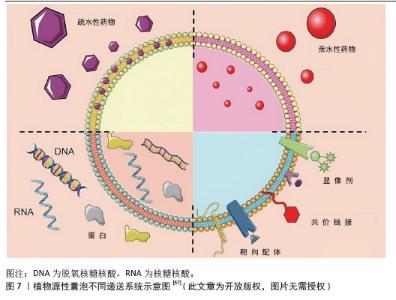
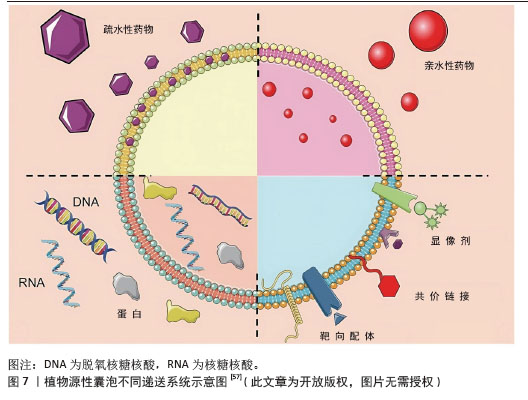
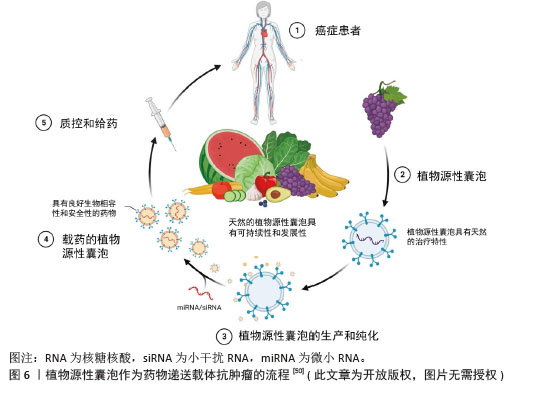
2.2.2 植物源性囊泡作为药物递送的载体 (1)植物源性囊泡作为药物递送载体的优点:植物源性囊泡拥有脂质双分子层结构,可以作为小分子药物和核酸的天然载体[50],其内含多种蛋白质、RNA和其他药理活性分子,能够将亲水药物封装在空腔中,并将疏水药物加载到脂质双层中[51],从而实现药物的高效递送,见图6。作为药物递送载体,其优点主要包括:在生物相容性方面:植物源性囊泡表现出卓越的特性,且它们无毒、免疫原性低,这些特性使得植物源性囊泡可以通过口服途径进入体内被肠道吸收,然后转运到身体的不同部位[52]。有研究表明,植物源性囊泡具有抵抗胃内强酸环境和肠道内活性蛋白酶的能力[53]。 在药理效果和靶向性方面:外泌体具有良好的转运和信号传导能力,药物和分子经过修饰后可以靶向到靶细胞和器官[54]。一些外泌体囊泡容易被溶酶体识别和杀死,而植物源性囊泡不受动物界生物体的识别,这使得它们在跨物种药物递送中具有更好的药理效果和靶向性。 在药物分布特性方面:植物源性囊泡可以穿过血脑屏障,但不能穿过胎盘屏障[55]。它能够将药物输送到孕妇体内而不影响胎儿,这为孕妇提供了一种潜在的药物治疗途径。 动物源性细胞外囊泡在药物递送中存在安全性和伦理问题,且在大规模生产方面面临不可持续性和不可扩展性的挑战[56-57],而植物源性囊泡则完全避免了这些问题。它们的提取过程简单,且能够实现大规模生产,为药物递送领域提供了一种全新的、可持续的解决方案。"
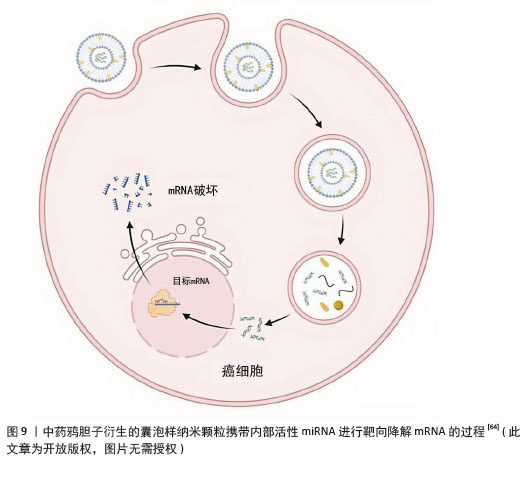
结直肠癌:植物来源囊泡样纳米颗粒可以发挥抗炎、调节免疫反应、抑制细胞增殖、促进细胞凋亡等作用,促进肠道修复。生姜及其有效成分具有抗炎和抗癌活性的作用[59],源自生姜的细胞外囊泡能诱导巨噬细胞表达血红素氧合酶1和白细胞介素10,从而发挥抗炎作用[60]。生姜来源囊泡样纳米颗粒与外泌体的结构和组成相似,含有大量的脂质、少量的蛋白质、约125种miRNAs,以及大量的生姜生物活性成分(6-姜醇和6-生姜酚)。最近, YAN等[61]研究发现生姜来源囊泡样纳米颗粒作为一种新型的天然纳米载体,能够有效地将活性miRNAs传递到结肠,促进肠上皮细胞的生存和增殖,同时减少促炎细胞因子(肿瘤坏死因子α、白细胞介素6和白细胞介素1β)、增加抗炎细胞因子(白细胞介素10和白细胞介素22)在结肠炎模型中的表达,通过多种机制显示出缓解急性结肠炎、增强肠道修复、预防慢性结肠炎和结直肠癌的效果。 目前,生姜来源囊泡样纳米颗粒的生物活性和药理作用正在不断被研究,其在治疗炎性肠病和结直肠癌中的机制也在逐渐明确,为未来基于天然纳米颗粒的药物开发开辟了新的道路。 三阴性乳腺癌:三阴性乳腺癌是由于雌激素受体、孕激素受体和人表皮生长因子受体2缺乏而导致的一种恶性程度高、临床预后差的恶性肿 瘤[62]。目前大多数治疗方案如肿瘤切除和化疗,都存在明显的毒性、化疗耐药和低敏感性等不良反应[63]。而植物源性囊泡具有良好的生物相容性和递送效率,可以在不需要其他额外材料的情况下将miRNA递送到哺乳动物细胞中。 中药鸦胆子衍生的囊泡样纳米颗粒能够传递10种功能性miRNAs到4T1细胞中,见图9。鸦胆子衍生的囊泡样纳米颗粒通过调节PI3K/Akt/mTOR信号通路,促进活性氧/Caspase介导的凋亡,显著抑制4T1细胞的生长和转移[64]。 鸦胆子衍生的囊泡样纳米颗粒作为一种高效的活性miRNA递送纳米平台发挥抑制乳腺癌生长、转移和血管生成的疗效,拓宽了植物源性细胞外囊泡的研究领域,并为开发药用植物纳米新制剂与安全有效的三阴性乳腺癌治疗策略提供参考。"
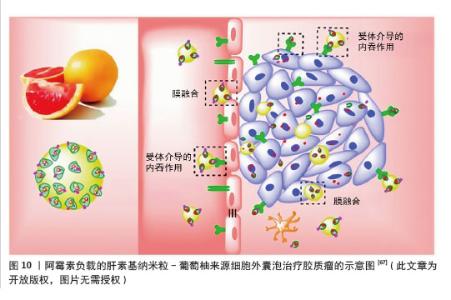
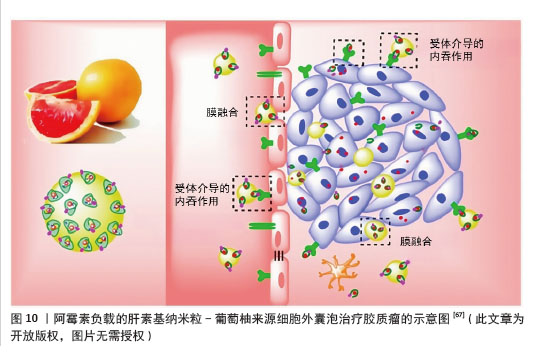
(3)工程化植物源性囊泡作为药物递送的载体:工程化植物源性囊泡是指通过先进的生物工程技术对植物源性囊泡进行改造,包括在其表面修饰药物或抗体等分子。经过改造和优化的植物源性囊泡在药物递送、生物活性物质运输等方面的性能都会有显著提升。在恶性肿瘤的治疗领域,工程化植物源性囊泡有望成为一种有前景的新型治疗工具。 胶质瘤:胶质瘤是中枢神经系统肿瘤中侵袭性和致死率极高的肿瘤[65]。胶质瘤治疗中,由于血脑屏障/血脑肿瘤屏障的存在和肿瘤渗透不足,系统化疗的纳米颗粒药物递送系统面临巨大挑战[66]。NIU等[67]将阿霉素负载的肝素基纳米粒(DNs)“补丁”到葡萄柚来源细胞外囊泡(EVs)表面,以实现高效药物递送和增强抗胶质瘤效果,通过整合细胞外囊泡和肝素基纳米粒的优势,即通过αvβ3受体介导的转运和膜融合绕过血脑屏障/血脑肿瘤屏障,以及高药物载荷能力和胶质瘤靶向能力,显著提高了胶质瘤治疗的递送效率和效果,见图10。这种工程化植物囊泡为药物递送平台设计提供了新的方法,该系统克服了传统纳米药物递送系统的局限性,为开发更有效的脑肿瘤治疗方案提供了新的思路。随着进一步的研究和优化,这种基于工程化植物囊泡的递送系统有望实现更精准的药物递送,提高治疗效果,减少不良反应,为胶质瘤患者带来新的希望。"
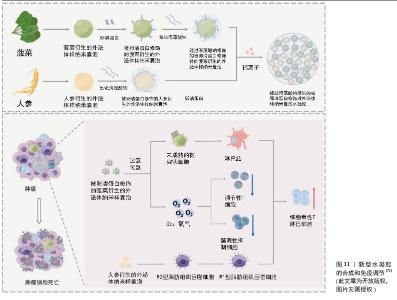
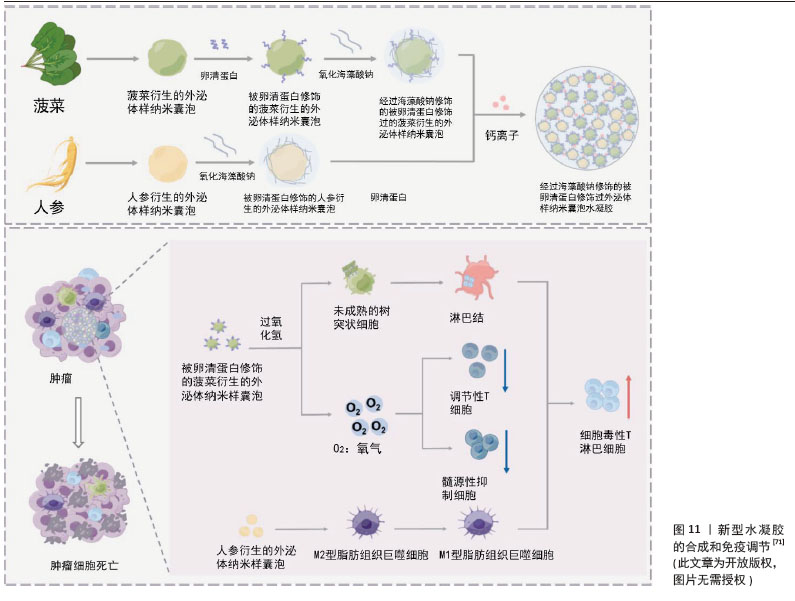
卵巢癌:卵巢癌是妇科恶性肿瘤中致死率最高的疾病[68],尽管目前采用包括手术在内的多种治疗手段,但复发率仍然很高[69] 。XIAO等[70]设计了一种柠檬来源细胞外囊泡的仿生药物递送系统,通过在柠檬来源细胞外囊泡表面修饰肝素-cRGD,然后装载多柔比星来制备HRED(肝素-cRGD-细胞外囊泡-多柔比星)。HRED通过内吞触发的能量耗竭和ATP产生减少,有效抑制了P-糖蛋白的外排功能,在体内外均显示出对多柔比星耐药卵巢癌的优异抗增殖能力,有效克服了卵巢癌的多药耐药。 由此可见,基于柠檬来源的细胞外囊泡纳米药物HRED为克服癌症多药耐药性提供了一种有效的新策略。随着对植物囊泡表面修饰及内部载药的深入研究和开发,癌症治疗领域将会新的突破,同时也为患者提供了更安全、更有效的治疗选择。 (4) 植物源性囊泡与其他载体协同递送药物:植物源性囊泡除了直接作为载体递送药物以外,也可以与其他载体(如与水凝胶、金属-有机骨架纳米粒子、中空纳米球等)一起发挥协同作用。有研究者用人参衍生的外泌体样纳米囊泡(ginseng-derived exosomelike nanovesicles,GDEN)和菠菜衍生的外泌体样纳米囊泡(ginseng-derived exosomelike nanovesicles,SDEN)构建了一种水凝胶[71]。他们将SDEN与马来酰亚胺修饰的OVA257-264肽(OVA-MaI)结合,形成SDEN@OVA,再通过氧化海藻酸钠与GDEN和SDEN@OVA进行化学修饰,使其成为凝胶剂(O-GS@OVA)。该O-GS@OVA水凝胶可以诱导全身免疫反应,调节肿瘤微环境中的免疫抑制细胞,并诱导长期免疫记忆,有效抑制肿瘤生长和转移,见图11。此外,已经有研究表明外泌体包被金属-有机骨架纳米粒子[72],有助于靶向递送而不会过早泄露。此机制已经在哺乳动物外泌体中得到证明,也为研究植物源性囊泡提供了新的探索机会。 另外有研究发现,工程化外泌体还可以与载药的中空纳米粒子一起发挥协同作用。有研究者将光敏剂吲哚青绿(Indocyanine Green,ICG)负载到中空二氧化锰(MnO2)纳米球(ICG@MnO2)中,然后包被在PD-L1单克隆抗体重编程的外泌体中,构建成为 ICG@MnO2@Exoanti-PD-L1纳米递药系统[73]。该递药系统偶联PD-L1抗体有效调节肿瘤微环境以对抗非小细胞肺癌。 由此可见,植物源性囊泡与外泌体结构相似,工程化植物源性囊泡与中空纳米粒子的协同载药研究是一个具有潜力的领域,相信在不久的将来也能转化为实际的治疗方案,为患者带来更有效、更安全的治疗选择。"
| [1] HUANG R, ZHU J, FAN R, et al. Extracellular vesicle-based drug delivery systems in cancer. Extracell Vesicle. 2024;4:100053. [2] BRAY F, LAVERSANNE M, SUNG H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263. [3] LIU D, LU M, LI J, et al. The patterns and timing of recurrence after curative resection for gastric cancer in China. World J Surg Oncol. 2016;14(1):305. [4] HESKETH PJ, KRIS MG, BASCH E, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35(28):3240-3261. [5] SHANTHANNA H, LADHA KS, KEHLET H, et al. Perioperative Opioid Administration. Anesthesiology. 2021;134(4):645-659. [6] BATISTA IA, MACHADO JC, MELO SA. Advances in exosomes utilization for clinical applications in cancer. Trends Cancer. 2024; 10(10):947-968. [7] ZHONGYU X, WEI X, HONGMEI Z, et al. Review of pre-metastatic niches induced by osteosarcoma-derived extracellular vesicles in lung metastasis: A potential opportunity for diagnosis and intervention. Biomed Pharmacother. 2024;178:117203. [8] WANG M, WANG Y, TIAN X, et al. Diagnostic and predictive value of liquid biopsy-derived exosome miR-21 for breast cancer: a systematic review and meta-analysis. Expert Rev Mol Diagn. 2023;23(4):315-324. [9] BAKHSH T, ALHAZMI S, FARSI A, et al. Molecular detection of exosomal miRNAs of blood serum for prognosis of colorectal cancer. Sci Rep. 2024;14(1):8902. [10] PU X, DING G, WU M, et al. Elevated expression of exosomal microRNA-21 as a potential biomarker for the early diagnosis of pancreatic cancer using a tethered cationic lipoplex nanoparticle biochip. Oncol Lett. 2020;19(3):2062-2070. [11] NARAUSKAITĖ D, VYDMANTAITĖ G, RUSTEIKAITĖ J, et al. Extracellular Vesicles in Skin Wound Healing. Pharmaceuticals (Basel). 2021;14(8):811. [12] KIM DK, RHEE WJ. Antioxidative Effects of Carrot-Derived Nanovesicles in Cardiomyoblast and Neuroblastoma Cells. Pharmaceutics. 2021;13(8):1203. [13] JU S, MU J, DOKLAND T, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013;21(7):1345-1357. [14] PRADO N, ALCHÉ JDE D, CASADO-VELA J, et al. Nanovesicles are secreted during pollen germination and pollen tube growth: a possible role in fertilization. Mol Plant. 2014;7(3):573-577. [15] REGENTE M, PINEDO M, SAN CLEMENTE H, et al. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J Exp Bot. 2017;68(20): 5485-5495. [16] ZHAO Z, YU S, LI M, et al. Isolation of Exosome-Like Nanoparticles and Analysis of MicroRNAs Derived from Coconut Water Based on Small RNA High-Throughput Sequencing. J Agric Food Chem. 2018; 66(11):2749-2757. [17] LY NP, HAN HS, KIM M, et al. Plant-derived nanovesicles: Current understanding and applications for cancer therapy. Bioact Mater. 2022;22:365-383. [18] KAHROBA H, DAVATGARAN-TAGHIPOUR Y. Exosomal Nrf2: From anti-oxidant and anti-inflammation response to wound healing and tissue regeneration in aged-related diseases. Biochimie. 2020; 171-172:103-109. [19] ŞAHIN F, KOÇAK P, GÜNEŞ MY, et al. In Vitro Wound Healing Activity of Wheat-Derived Nanovesicles. Appl Biochem Biotechnol. 2019;188(2):381-394. [20] TIMMS K, DAY A, MCLAUGHLIN J, et al. Plant Extracellular Vesicles Influence Trophoblast Differentiation. Reprod Sci. 2019;26: 352A-353A. [21] JIANG D, LI Z, LIU H, et al. Plant exosome-like nanovesicles derived from sesame leaves as carriers for luteolin delivery: Molecular docking, stability and bioactivity. Food Chem. 2024;438: 137963. [22] WANG J, DING Y, WANG J, et al. EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. Plant Cell. 2010;22(12): 4009-4030. [23] ROBINSON DG, DING Y, JIANG L. Unconventional protein secretion in plants: a critical assessment. Protoplasma. 2016;253(1):31-43. [24] TSE YC, MO B, HILLMER S, et al. Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell. 2004;16(3): 672-693. [25] ALENQUER M, AMORIM MJ. Exosome Biogenesis, Regulation, and Function in Viral Infection. Viruses. 2015;7(9): 5066-5083. [26] GAO C, ZHUANG X, SHEN J, et al. Plant ESCRT Complexes: Moving Beyond Endosomal Sorting. Trends Plant Sci. 2017; 22(11):986-998. [27] MATHIEU M, MARTIN-JAULAR L, LAVIEU G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9-17. [28] LI X, BAO H, WANG Z, et al. Biogenesis and Function of Multivesicular Bodies in Plant Immunity. Front Plant Sci. 2018;9:979. [29] ZHOU Q, MA K, HU H, et al. Extracellular vesicles: Their functions in plant-pathogen interactions. Mol Plant Pathol. 2022;23(6):760-771. [30] CUI Y, GAO J, HE Y, et al. Plant extracellular vesicles. Protoplasma. 2020;257(1):3-12. [31] YANG C, ZHANG M, MERLIN D. Advances in Plant-derived Edible Nanoparticle-based lipid Nano-drug Delivery Systems as Therapeutic Nanomedicines. J Mater Chem B. 2018;6(9):1312-1321. [32] ZHANG L, HE F, GAO L, et al. Engineering Exosome-Like Nanovesicles Derived from Asparagus cochinchinensis Can Inhibit the Proliferation of Hepatocellular Carcinoma Cells with Better Safety Profile. Int J Nanomedicine. 2021;16:1575-1586. [33] ZHANG M, VIENNOIS E, XU C, et al. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers. 2016;4(2):e1134415. [34] LEE R, KO HJ, KIM K, et al. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J Extracell Vesicles. 2019;9(1):1703480. [35] LIGA A, VLIEGENTHART AD, OOSTHUYZEN W, et al. Exosome isolation: a microfluidic road-map. Lab Chip. 2015;15(11):2388-2394. [36] KALARIKKAL SP, PRASAD D, KASIAPPAN R, et al. A cost-effective polyethylene glycol-based method for the isolation of functional edible nanoparticles from ginger rhizomes. Sci Rep. 2020;10(1):4456. [37] KHAN F, SHARIQ M, ASIF M, et al. Green Nanotechnology: Plant-Mediated Nanoparticle Synthesis and Application. Nanomaterials (Basel). 2022;12(4):673. [38] YANG M, LIU X, LUO Q, et al. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J Nanobiotechnology. 2020; 18(1):100. [39] DAD HA, GU TW, ZHU AQ, et al. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol Ther. 2021;29(1):13-31. [40] HE B, CAI Q, QIAO L, et al. RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nat Plants. 2021;7(3):342-352. [41] HOSHINO A, KIM HS, BOJMAR L, et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell. 2020; 182(4):1044-1061.e18. [42] STANLY C, ALFIERI M, AMBROSONE A, et al. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells. 2020;9(12):2722. [43] YANG LY, LIANG GW, CAI BJ, et al. Enhanced tumor self-targeting of lemon-derived extracellular vesicles by embedding homotypic cancer cell membranes for efficient drug delivery. J Nanobiotechnology. 2025;23(1):74. [44] ÖZKAN İ, KOÇAK P, YILDIRIM M, et al. Garlic (Allium sativum)-derived SEVs inhibit cancer cell proliferation and induce caspase mediated apoptosis. Sci Rep. 2021; 11(1):14773. [45] SASAKI D, KUSAMORI K, TAKAYAMA Y, et al. Development of nanoparticles derived from corn as mass producible bionanoparticles with anticancer activity. Sci Rep. 2021;11(1):22818. [46] CHEN Q, LI Q, LIANG Y, et al. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm Sin B. 2022;12(2):907-923. [47] CHEN Q, ZU M, GONG H, et al. Tea leaf-derived exosome-like nanotherapeutics retard breast tumor growth by pro-apoptosis and microbiota modulation. J Nanobiotechnology. 2023;21(1):6. [48] CAO M, YAN H, HAN X, et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunother Cancer. 2019; 7(1):326. [49] HAN X, WEI Q, LV Y, et al. Ginseng-derived nanoparticles potentiate immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Mol Ther. 2022;30(1): 327-340. [50] ALZAHRANI FA, KHAN MI, KAMELI N, et al. Plant-Derived Extracellular Vesicles and Their Exciting Potential as the Future of Next-Generation Drug Delivery. Biomolecules. 2023;13(5):839. [51] FANG Z, LIU K. Plant-derived extracellular vesicles as oral drug delivery carriers. J Control Release. 2022;350:389-400. [52] MAN F, MENG C, LIU Y, et al. The Study of Ginger-Derived Extracellular Vesicles as a Natural Nanoscale Drug Carrier and Their Intestinal Absorption in Rats. AAPS PharmSciTech. 2021;22(6):206. [53] NEMIDKANAM V, CHAICHANAWONGSAROJ N. Characterizing Kaempferia parviflora extracellular vesicles, a nanomedicine candidate. PLoS One. 2022;17(1):e0262884. [54] KIM J, KIM M, HAN H, et al. Dual-delivery of exosome inhibitor and immune-activating gene via lipid nano-assemblies for tumor immune evasion inhibition. J Control Release. 2025;381:113569. [55] LI Y, CAI T, LIU H, et al. Exosome-shuttled miR-126 mediates ethanol-induced disruption of neural crest cell-placode cell interaction by targeting SDF1. Toxicol Sci. 2023;195(2):184-201. [56] ZHANG C, DENG R, ZHANG G, et al. Therapeutic Effect of Exosomes Derived From Stem Cells in Spinal Cord Injury: A Systematic Review Based on Animal Studies. Front Neurol. 2022;13:847444. [57] LUAN X, SANSANAPHONGPRICHA K, MYERS I, et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38(6):754-763. [58] KARAMANIDOU T, TSOUKNIDAS A. Plant-Derived Extracellular Vesicles as Therapeutic Nanocarriers. Int J Mol Sci. 2021;23(1):191. [59] MATACCHIONE G, BORGONETTI V, RAMINI D, et al. Zingiber officinale Roscoe Rhizome Extract Exerts Senomorphic and Anti-Inflammatory Activities on Human Endothelial Cells. Biology (Basel). 2023;12(3):438. [60] ZHANG JJ, NI P, SONG Y, et al. Effective protective mechanisms of HO-1 in diabetic complications: a narrative review. Cell Death Discov. 2024;10(1):433. [61] YAN L, CAO Y, HOU L, et al. Ginger exosome-like nanoparticle-derived miRNA therapeutics: A strategic inhibitor of intestinal inflammation. J Adv Res. 2025; 69:1-15. [62] GHOSH A, CHAUBAL R, DAS C, et al. Genomic hallmarks of endocrine therapy resistance in ER/PR+HER2- breast tumours. Commun Biol. 2025;8(1):207. [63] BARDIA A, MAYER IA, VAHDAT LT, et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med. 2019;380(8):741-751. [64] YAN G, XIAO Q, ZHAO J, et al. Brucea javanica derived exosome-like nanovesicles deliver miRNAs for cancer therapy. J Control Release. 2024;367:425-440. [65] PINEL S, THOMAS N, BOURA C, et al. Approaches to physical stimulation of metallic nanoparticles for glioblastoma treatment. Adv Drug Deliv Rev. 2019;138: 344-357. [66] FENG J, LEPETRE-MOUELHI S, GAUTIER A, et al. A new painkiller nanomedicine to bypass the blood-brain barrier and the use of morphine. Sci Adv. 2019;5(2):eaau5148. [67] NIU W, XIAO Q, WANG X, et al. A Biomimetic Drug Delivery System by Integrating Grapefruit Extracellular Vesicles and Doxorubicin-Loaded Heparin-Based Nanoparticles for Glioma Therapy. Nano Lett. 2021;21(3):1484-1492. [68] TRABERT B, TWOROGER SS, O’BRIEN KM, et al. The Risk of Ovarian Cancer Increases with an Increase in the Lifetime Number of Ovulatory Cycles: An Analysis from the Ovarian Cancer Cohort Consortium (OC3). Cancer Res. 2020;80(5):1210-1218. [69] SARIVALASIS A, MOROTTI M, MULVEY A, et al. Cell therapies in ovarian cancer. Ther Adv Med Oncol. 2021;13:17588359211008399. [70] XIAO Q, ZHAO W, WU C, et al. Lemon-Derived Extracellular Vesicles Nanodrugs Enable to Efficiently Overcome Cancer Multidrug Resistance by Endocytosis-Triggered Energy Dissipation and Energy Production Reduction. Adv Sci (Weinh). 2022;9(20):e2105274. [71] YANG L, ZHANG D, LU D, et al. Plant-Derived Exosome-Like Nanovesicles-Created injectable hydrogel for augmented cancer immunotherapy. Chem Eng J. 2024; 491:152032. [72] PALAKURTHI SS, SHAH B, KAPRE S, et al. A comprehensive review of challenges and advances in exosome-based drug delivery systems. Nanoscale Adv. 2024;6(23): 5803-5826. [73] GUO J, ZHAO W, XIAO X, et al. Reprogramming exosomes for immunity-remodeled photodynamic therapy against non-small cell lung cancer. Bioact Mater. 2024;39:206-223. |
| [1] | Liu Dawei, Cui Yingying, Wang Fanghui, Wang Zixuan, Chen Yuhan, Li Yourui, Zhang Ronghe. Epigallocatechin gallate-mediated bidirectional regulation of reactive oxygen species and its application in nanomaterials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2101-2112. |
| [2] | Song Puzhen, Ma Hebin, Chen Hongguang, Zhang Yadong. Effect of bone marrow mesenchymal stem cell-derived exosomes combined with transforming growth factor beta 1 on macrophages [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1616-1623. |
| [3] | He Jiale, Huang Xi, Dong Hongfei, Chen Lang, Zhong Fangyu, Li Xianhui. Acellular dermal matrix combined with adipose-derived stem cell exosomes promotes burn wound healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1699-1710. |
| [4] | Xia Linfeng, Wang Lu, Long Qianfa, Tang Rongwu, Luo Haodong, Tang Yi, Zhong Jun, Liu Yang. Human umbilical cord mesenchymal stem cell-derived exosomes alleviate blood-brain barrier damage in mice with septic encephalopathy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1711-1719. |
| [5] | Chen Yulin, He Yingying, Hu Kai, Chen Zhifan, Nie Sha Meng Yanhui, Li Runzhen, Zhang Xiaoduo , Li Yuxi, Tang Yaoping. Effect and mechanism of exosome-like vesicles derived from Trichosanthes kirilowii Maxim. in preventing and treating atherosclerosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1768-1781. |
| [6] | Zhou Sirui, Xu Yukun, Zhao Kewei. Ideas and methods of anti-melanogenesis of Angelica dahurica extracellular vesicles [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1747-1754. |
| [7] | Han Teng, Ma Hong, Yang Ruoyi, Luo Yi, Li Chao. Oral squamous cell carcinoma-derived exosomal delivery of angiopoietin-2 is involved in tumor angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1755-1767. |
| [8] | Wang Baiyan, Yang Shu, Wang Yiming, Wu Mengqing, Xiao Yu, Guo Zixuan, Zhang Boyi, Feng Shuying. Exosome-delivered CRISPR/Cas system enables gene editing in target cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1839-1849. |
| [9] | Wang Zhenze, Liu Fende, Zhang Rui, Li Wujun. Mesenchymal stem cells in treatment of arteriosclerosis obliterans of lower extremities: systematic review and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1869-1876. |
| [10] | Liu Huan, Zeng Shaopeng, Chen Jun, He Linqian, Yang Ying, Zhang Jing. Aging-related dysregulation of glucose metabolism: crossroads of cancer and neurodegenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1527-1538. |
| [11] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [12] | Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Mechanism by which vascular endothelial growth factor A targets regulation of angiogenesis in the treatment of steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 671-679. |
| [13] | Yang Hu, Zheng Yu, Jia Chengming, Wang Tong, Zhang Guangfei, Ji Yaoyao. Immune microenvironment regulates bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 701-710. |
| [14] | Zhang Tingting, Li Yalong, Yue Haodi, Li Yanjun, Geng Xiwen, Zhang Yuwei, Liu Xiaozhuan. Protection of exosomes derived from bone marrow mesenchymal stem cells of different mouse ages on radiation-induced lung injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 1-9. |
| [15] | Wu Zhijing, Li Jiali, Zhang Jiaxin, Wang Tangrong, Zheng Yuzhou, Sun Zixuan. Alpha-ketoglutarate engineered small extracellular vesicles delay skin aging [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 120-129. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||