Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (23): 5954-5963.doi: 10.12307/2026.304
Previous Articles Next Articles
Exercise interventions regulate thyroid hormones: effects on the liver, skeleton, muscle, heart, and brain
Cheng Yang1, 2, Huang Qingqiang1, Bu Shumin1, Yi Yue2
- 1School of Kinesiology and Health, Capital University of Physical Education and Sports, Beijing 100191, China; 2School of Life Science, Beijing Institute of Technology, Beijing 100081, China
-
Received:2025-04-24Accepted:2025-05-16Online:2026-08-18Published:2025-12-30 -
Contact:Bu Shumin, PhD, Professor, School of Kinesiology and Health, Capital University of Physical Education and Sports, Beijing 100191, China Co-corresponding author: Yi Yue, PhD, Associate researcher, School of Life Science, Beijing Institute of Technology, Beijing 100081, China -
About author:Cheng Yang, MS candidate, School of Kinesiology and Health, Capital University of Physical Education and Sports, Beijing 100191, China; School of Life Science, Beijing Institute of Technology, Beijing 100081, China -
Supported by:a grant from the Emerging Interdisciplinary Platform for Medicine and Engineering in Sports (EIPMES), No. 11000024210200089230-XM001 (to BSM [project participant]); Beijing Institute of Technology Research Fund Program for Young Scholars, No. 3160012222114 (to YY); Fundamental Research Funds for the Central Universities, No. 2024CX06051 (to YY)
CLC Number:
Cite this article
Cheng Yang, Huang Qingqiang, Bu Shumin, Yi Yue. Exercise interventions regulate thyroid hormones: effects on the liver, skeleton, muscle, heart, and brain[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 5954-5963.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

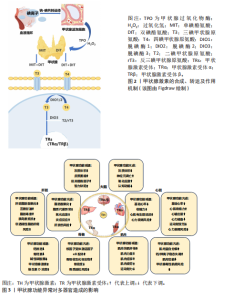
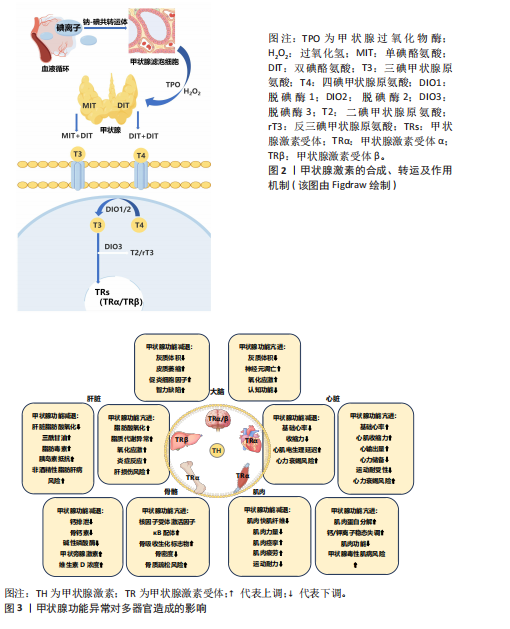
2.1 甲状腺激素合成、代谢及其运动调节作用 甲状腺激素包括三碘甲状腺原氨酸和四碘甲状腺原氨酸,是由全身最大内分泌器官甲状腺分泌并合成的含碘酪氨酸衍生物,在所有机体组织的代谢、生长和发育过程中发挥重要调控作用。甲状腺激素的早期研究历程与发展可以追溯至19世纪碘元素的发现,随着检测方法手段的不断创新以及相关实验数据的不断积累,逐步为后续甲状腺激素分子作用机制奠定了坚实基础。甲状腺激素的合成主要在甲状腺滤泡细胞内完成,涉及碘的摄取、氧化、甲状腺球蛋白的合成与碘化以及甲状腺激素的储存与释放(图2)。首先,甲状腺滤泡细胞基底膜上的钠-碘共转运体通过主动转运机制将血液中的碘离子浓缩并摄入细胞内,随后顶膜上的PENDRIN通道将碘离子转运至滤泡腔[4]。在甲状腺过氧化物酶和过氧化氢共同作用下,碘离子被氧化为高活性的单原子碘,与此同时,在滤泡细胞内甲状腺球蛋白经内质网和高尔基体加工后被分泌至滤泡腔。甲状腺过氧化物酶催化单原子碘与甲状腺球蛋白中的酪氨酸残基结合,形成单碘酪氨酸和双碘酪氨酸;一个双碘酪氨酸与一个单碘酪氨酸偶联生成三碘甲状腺原氨酸,而2个双碘酪氨酸分子偶联形成四碘甲状腺原氨酸,生成的三碘甲状腺原氨酸和四碘甲状腺原氨酸与甲状腺球蛋白结合,储存在滤泡腔的胶质中[5]。在下丘脑-垂体-甲状腺轴的经典调控机制中,下丘脑分泌促甲状腺激素释放激素,刺激垂体分泌促甲状腺激素。促甲状腺激素与甲状腺滤泡细胞表面受体结合后,促进甲状腺球蛋白的内化,形成内吞小泡,后者与溶酶体融合,在溶酶体酶的作用下降解甲状腺球蛋白,进而在滤泡细胞的基底膜释放出游离的三碘甲状腺原氨酸和四碘甲状腺原氨酸。血液中三碘甲状腺原氨酸和四碘甲状腺原氨酸的水平通过负反馈机制调节下丘脑和垂体分泌的促甲状腺激素释放激素与促甲状腺激素,从而实现对甲状腺激素合成与分泌的精确调控[6]。脱碘酶在甲状腺激素的活性与代谢调控中扮演着至关重要的角色。脱碘酶1和脱碘酶2是甲状腺激素代谢过程中的2种重要脱碘酶,它们通过将甲状腺分泌的四碘甲状腺原氨酸脱碘转化为生物活性更强的三碘甲状腺原氨酸,对维持体内甲状腺激素的稳态起关键作用。具体而言,脱碘酶1主要分布于肝脏和甲状腺,是循环三碘甲状腺原氨酸的主要来源;而脱碘酶2广泛存在于骨骼、肌肉、大脑等特定组织中,能够为这些局部组织提供细胞内活性三碘甲状腺原氨酸[7]。相反,脱碘酶3的作用则是将活性三碘甲状腺原氨酸转化为无活性的二碘甲状腺原氨酸或将四碘甲状腺原氨酸转化为反三碘甲状腺原氨酸,降低甲状腺激素的活性。尤其在胚胎发育和组织修复过程中,脱碘酶3通过限制三碘甲状腺原氨酸的作用避免发育中的组织器官暴露于不适当的三碘甲状腺原氨酸水平,防止过量甲状腺激素引发代谢紊乱[8]。甲状腺激素的早期研究历程与发展,见表2。"
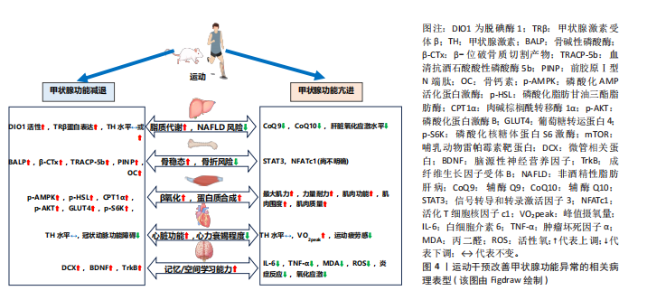
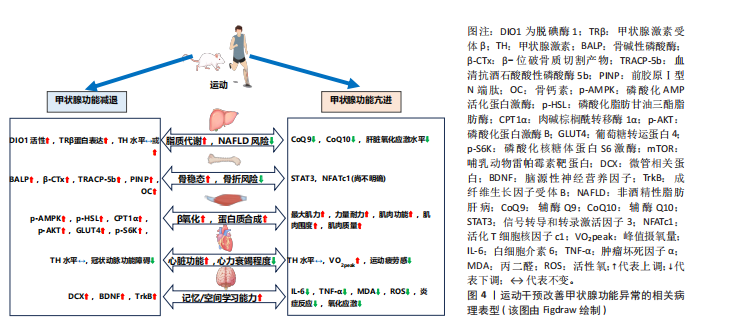
在甲状腺功能正常的情况下,急性运动可作为一种物理应激因素,通过激活交感神经系统及下丘脑-垂体-甲状腺轴影响甲状腺激素的动态变化[21]。但急性运动的时间动力学特征存在一定差异,以大鼠模型为例,基于75%最大摄氧量的有氧运动可诱导外周血三碘甲状腺原氨酸水平出现短暂峰值后快速回落,而四碘甲状腺原氨酸水平则逐步升高,促甲状腺激素水平略有波动;此外,该急性运动还特异性改变了垂体、甲状腺及肝脏等靶器官中脱碘酶的活性,提示局部组织对甲状腺激素的代谢调控可能独立于循环系统[21]。DA SILVA等[22]的研究进一步表明,抗阻运动后三碘甲状腺原氨酸水平可能因不同的运动方式而表现出不同的延迟效应,单组抗阻运动在前的情况下,三碘甲状腺原氨酸水平在运动后30 min显著升高,而多组抗阻运动则需要约120 min才能观察到三碘甲状腺原氨酸水平的显著升高。这些发现表明,急性运动对甲状腺激素水平的影响具有一定的时效性和个体化差异,并且不同运动方式可能导致不同的生理响应。在甲状腺功能正常状态下,关于长期运动是否可以直接影响甲状腺激素水平仍存在争议。研究表明,为期12周、每周3次、每次60 min的有氧训练能够显著改变运动员的甲状腺激素水平,可能有助于运动员应对压力并提高竞技水平[23]。然而,大多数长期运动方案的研究中并未观察到甲状腺激素水平的显著变化[24-26]。研究指出,长期运动可能主要通过改变脱碘酶活性或调节甲状腺激素受体的敏感性,从而间接影响甲状腺激素水平[27]。 2.2 运动干预调节甲状腺激素:对肝脏、骨骼、肌肉、心脏与大脑的影响 2.2.1 肝脏 甲状腺激素受体作为核内受体家族的重要成员,主要介导甲状腺激素的生物学效应。甲状腺激素受体包括甲状腺激素受体α和甲状腺激素受体β,其中甲状腺激素受体β在肝脏中具有较高的表达水平。甲状腺激素受体β信号通路在调节肝脏新生脂肪生成、脂肪酸β-氧化、线粒体自噬以及胆固醇生物合成等多个代谢过程中发挥重要作用[1]。甲状腺激素受体β不仅能够有效促进脂肪代谢,还能显著降低低密度脂蛋白、载脂蛋白B及脂蛋白(a)水平。带有甲状腺激素受体β基因显性负性突变的小鼠,表现出血清中游离脂肪酸和三酰甘油水平显著升高,并伴随肝脏脂肪变性,这与脂肪生成酶的表达增加及β-氧化活性的下降密切相关[28]。而甲状腺激素受体β激动剂能够显著增强脂肪酸氧化、抑制新生脂肪生成,改善胰岛素敏感性,并促进线粒体的生物合成[29]。 除了对脂肪代谢的调节作用,三碘甲状腺原氨酸还能刺激肝糖原分解、促进糖异生并抑制糖酵解。甲状腺功能减退症通常伴随脂肪代谢紊乱和肝脏脂肪酸氧化减少,导致三酰甘油和脂肪毒素在肝脏中积累,进而引发胰岛素抵抗,促进脂肪合成,增加非酒精性脂肪性肝病的发生风险[28](图3)。相反,甲状腺功能亢进症虽然会导致代谢紊乱并引发脂质代谢异常,但它在引起肝脏脂肪积聚方面的作用较为有限。数据显示,甲状腺功能亢进患者非酒精性脂肪肝的患病率为11.97%,明显低于甲状腺功能减退(35.7%-36.3%)及甲状腺功能正常人群(27.4%-33.1%),这可能表明甲状腺功能亢进本身并不是非酒精性脂肪性肝病的主要风险因素[30]。研究表明甲状腺功能亢进会显著增加肝脏的氧化应激水平,导致肝脏炎症标志物(如白细胞介素18和肿瘤坏死因子α)显著升高,这些变化将进一步增加肝损伤风险[31]。 现有研究表明,运动通过调节甲状腺激素水平及其受体表达促进脂肪酸氧化,从而有效调节肝脏脂肪代谢(图4)。例如,XIA等[32]的研究表明,为期4周的跑台运动干预(速度22 m/min,每次30 min,每周5次)中,雄性甲状腺功能减退小鼠的循环甲状腺激素水平未显著变化,但有效维持了肝脏中的脱碘酶1活性,该干预促进了四碘甲状腺原氨酸向三碘甲状腺原氨酸的转化,为肝脏脂质代谢维持了足够的甲状腺激素;此外,运动干预还显著逆转了高脂饮食小鼠中miR-146b的上调趋势,同时增强了甲状腺激素受体β蛋白的表达,进一步提高了肝脏脂肪代谢功能。非酒精性脂肪性肝炎是非酒精性脂肪性肝病向更严重肝病进展的重要阶段,其特点是在脂肪沉积的基础上伴随显著的炎症反应、肝细胞损伤甚至纤维化[29]。研究显示,8周有氧运动(12 m/min,每天1 h,每周5 次)显著减少了非酒精性脂肪性肝炎小鼠肝脏的脂质积聚、炎症浸润及纤维化进程。此外,运动干预小鼠肝组织中脱碘酶1表达水平显著增加,并且循环三碘甲状腺原氨酸和四碘甲状腺原氨酸水平亦显著升高,表明这一改善作用与肝脏甲状腺激素信号通路的激活以及循环甲状腺激素水平的升高密切相关[33]。VENDITTI等[34]的研究探讨了长期有氧运动对甲状腺功能亢进大鼠肝脏氧化应激水平的影响,结果表明长期有氧运动可能会减少抗氧化物质(如辅酶Q10)水平,提示长期运动可能降低了肝脏部位的氧化应激水平,进而减少了相应的抗氧化物质,但具体机制目前仍有待深入探索。 2.2.2 骨骼 甲状腺激素能够直接通过甲状腺激素受体α和甲状腺激素受体β影响骨骼的发育与代谢。定量RT-PCR研究显示,甲状腺激素受体α的表达水平至少比甲状腺激素受体β高出10倍,表明甲状腺激素受体α在骨骼中是三碘甲状腺原氨酸的主要介导者。三碘甲状腺原氨酸通过甲状腺激素受体α可直接调控成骨细胞和破骨细胞的活性。研究发现,甲状腺激素受体α缺失或功能异常可导致生长迟缓、骨龄延迟、骨矿化紊乱及骨密度降低[35]。同样,脱碘酶在维持骨稳态中发挥重要作用,脱碘酶2在初级成骨细胞中有所表达,而脱碘酶3在软骨细胞、成骨细胞和破骨细胞中均有分布。小鼠成骨细胞中敲除脱碘酶2基因后表现为骨矿化增加、股骨韧性降低和易骨折倾向,这与临床上甲状腺功能减退症在骨骼中的表现相符,提示脱碘酶2在维持成骨细胞正常功能中发挥关键作用[36]。两样本孟德尔随机化分析发现,甲状腺功能亢进(OR=1.08)和甲状腺功能减退(OR=1.18)均显著增加了骨质疏松的风险,进一步强调了维持甲状腺激素水平稳定对于骨骼健康的重要性[37]。 在甲状腺功能亢进状态下,过量的三碘甲状腺原氨酸通过甲状腺激素受体α上调成骨细胞中核因子受体激活因子κB配体的表达,进而增加骨吸收生化标志物,显著加速骨重建过程并缩短骨重建周期,导致骨矿化时间相对缩短,最终导致骨密度降低,长期作用下可能诱发骨质疏松症[38-39]。同样,甲状腺功能减退在骨骼健康中的作用也不容忽视,甲状腺功能减退患者的钙磷代谢紊乱,表现为尿钙排泄减少、血清骨钙素和碱性磷酸酶浓度下降、甲状旁腺激素和维生素D的浓度升高[35](图3)。另外,儿童期未及时治疗的甲状腺功能减退会导致生长迟缓,影响软骨内成骨,造成骨龄延迟,最终导致身材矮小[40]。另外动物实验结果表明,与甲状腺功能减退相比,甲状腺功能亢进小鼠的骨密度降低更为明显,表现为骨小梁骨密度显著降低54%、皮质骨厚度显著减少15%[41],这一结果提示无论是甲状腺功能减退或亢进均将对骨骼健康造成不利影响。"

目前的研究表明,运动可作为一种补充策略对甲状腺功能异常个体的骨骼健康起到重要改善作用。KIM等[42]研究指出,甲状腺切除个体若每日进行额外规律性运动可显著降低椎体骨折风险。动物研究表明,亚临床甲状腺功能减退大鼠经左旋甲状腺素及维生素D3补充可显著改善骨密度,而在此基础上,额外的有氧运动能够进一步改善骨吸收与骨形成标志物(如碱性磷酸酶、前胶原Ⅰ型N端肽、血清抗酒石酸酸性磷酸酶5b和β-位破骨质切割产物),使得骨密度改善效果更为显著[43]。目前运动干预改善甲状腺功能亢进个体骨骼健康的研究较为有限,最近的研究表明,信号转导和转录激活因子3在维持骨稳态中发挥关键作用,因此可能对改善甲状腺功能亢进个体的骨骼健康具有重要作用。机械应力可显著增加骨髓间充质干细胞中信号转导和转录激活因子3的活性,促进成骨细胞的分化和骨形成[44]。信号转导和转录激活因子3还可调控活化T细胞核因子c1转录因子进一步控制破骨细胞分化,维护骨稳态[45]。而运动所产生的机械应力是信号转导和转录激活因子3的主要调节因素,并且甲状腺激素作为信号转导和转录激活因子3的上游信号,能够通过运动调节,促进信号转导和转录激活因子3的磷酸化,发挥双重调节作用[44,46](图4)。值得注意的是,运动改善骨骼健康的主要机制是通过机械应力激活多个经典通路,如Wnt/β-连环蛋白信号通路、丝裂原激活蛋白激酶和磷脂酰肌醇3激酶/蛋白激酶B信号通路等[47],而运动对甲状腺激素的调节进一步改善骨骼健康的作用机制仍需深入探讨。 2.2.3 肌肉 肌纤维类型按收缩速度和ATP生成速率由小到大依次排列为:Ⅰ型、Ⅱa型、Ⅱx型和Ⅱb型。甲状腺激素受体的改变与小鼠及人类的肌肉功能障碍密切相关,而甲状腺激素受体α是骨骼肌中主要的甲状腺激素受体[48]。在肌肉中,甲状腺激素的主要作用方式是通过三碘甲状腺原氨酸依赖的核受体(主要为甲状腺激素受体α)介导基因转录,进而调节一系列与肌肉功能相关的转录调控蛋白表达,如刺激肌浆网钙ATP酶、解偶联蛋白3、葡萄糖转运蛋白4、细胞质苹果酸酶和肌肉甘油-3-磷酸脱氢酶表达,同时抑制慢收缩肌球蛋白表达,使得肌纤维向Ⅱ型(快速酵解型)转变[1,49]。另外,三碘甲状腺原氨酸还可促进卫星细胞的分化、加速损伤后的肌肉再生[50]。值得注意的是,在骨骼肌中已鉴定出脱碘酶2和脱碘酶3,它们能够独立于循环甲状腺激素水平,特异性地调节肌细胞内的甲状腺激素水平[50]。研究表明,脱碘酶2缺失将导致甲状腺激素信号不足,影响卫星细胞分化为肌纤维,损害肌肉修复与再生[51]。 无论是甲状腺功能亢进还是减退都将对正常肌肉功能产生不利影响[52](图3)。甲状腺功能亢进时,过量的甲状腺激素可加速肌肉蛋白分解,导致钙和钾离子稳态失调,并伴随多种肌肉疾病,如甲状腺毒性肌病和甲状腺毒性周期性麻痹等,使得肌肉无力、肌肉功能下降[53]。甲状腺功能减退时,由于循环中三碘甲状腺原氨酸水平降低,导致肌肉中快肌纤维减少、肌肉力量下降、肌肉痉挛和僵硬[54],还会引起肌细胞线粒体功能减退,导致能量供应不足,表现为肌肉疲劳和运动耐力下降[55]。 关于运动改善甲状腺功能异常所引起的肌肉损害,当前研究较为有限(图4)。动物研究表明,相较于甲状腺功能亢进,电刺激模拟运动干预可以显著增加甲状腺功能减退兔子的磷酸化AMP活化蛋白激酶、磷酸化脂肪甘油三酯脂肪酶、肉碱棕榈酰转移酶1α、磷酸化蛋白激酶B、葡萄糖转运蛋白4和磷酸化核糖体蛋白S6激酶水平,同时激活哺乳动物雷帕霉素靶蛋白信号通路,使得肌肉β-氧化及蛋白质合成增加[56]。在人体研究中,患有甲状腺功能减退症的个体通常在休息和运动时表现出不良的骨骼肌症状,如肌肉疼痛和肌肉疲劳,尤其是在剧烈运动或使用降脂药物的情况下还可能会诱发横纹肌溶解症[57-58]。因此,目前甲状腺功能减退所导致的肌肉损害治疗策略主要是通过外源性补充甲状腺激素[59]。而甲状腺功能亢进的研究显示,运动有助于促进甲状腺功能亢进患者的肌肉功能恢复,并增加肌肉质量。例如,AHMAD等[60]研究通过对16名甲状腺功能亢进个体经过每周2次、共16周的抗阻运动干预后,受试者最大肌力和力量耐力显著改善,肌肉围度也显著增加。这些研究表明运动在改善甲状腺功能障碍所引起的肌肉损害方面具有一定的潜力,但具体机制尚未完全阐明,不同类型甲状腺功能障碍所导致的肌肉损害对运动干预的反应可能存在差异。 2.2.4 心脏 甲状腺激素受体α在心肌中广泛分布。通过与甲状腺激素受体α结合,甲状腺激素不仅能够增强心肌的收缩力和舒张能力,还能通过甲状腺激素受体α调节心肌细胞的能量代谢,促进糖类和脂肪酸的代谢。具体而言,甲状腺激素对心肌收缩力和舒张能力的调节主要通过上调肌球蛋白重链6和下调肌球蛋白重链7实现;此外,甲状腺激素还通过转录调控钾通道和钠/钙离子交换蛋白的表达维持心脏的正常收缩功能。甲状腺激素通过促进肌肉肉碱棕榈酰转移酶Ⅰ、丙酮酸脱氢酶激酶2和磷酸化AMP活化蛋白激酶等基因表达优先促进脂肪酸代谢,从而增强心肌能量供应[1,61]。甲状腺激素受体α缺失会导致载脂蛋白E缺陷小鼠的心脏功能和运动能力显著降低[62]。以上机制表明,甲状腺激素在心肌收缩、代谢调节及能量供应等多个方面中起着至关重要的作用。 甲状腺功能障碍将对心脏的各项生理功能产生直接影响,包括收缩性、收缩压、舒张压、心率、心脏质量、射血分数和心输出量,进而增加心律失常、心力衰竭等心血管疾病的风险[63](图3)。甲状腺功能亢进时,基础心率和心肌收缩力增加,显著提高心输出量并降低心力储备,这将导致运动不耐受。研究表明,相较于健康个体,甲状腺功能亢进患者在6 min步行测试中的运动距离、呼吸肌耐力显著下降,同时生活质量也受到显著影响[64];此外,甲状腺功能亢进症可能会引起心律失常、心脏肥大和血容量增加,这些变化最终可能导致心力衰竭[65]。甲状腺功能减退主要的心脏功能障碍表现为左心室舒张功能受损(心肌放松延迟和早期心室充盈受限),随着症状加重同样可能导致心力衰竭的发生[66]。另一方面,甲状腺功能减退会降低心肌细胞中三碘甲状腺原氨酸蛋白表达,导致心脏收缩力和心率降低、心脏电刺激传导延迟,从而影响心脏整体功能[61]。 目前的研究表明,运动对心脏功能的改善可能通过甲状腺激素实现(图4)。ADAMOPOULOS等[67]的研究表明,经过12周中等强度有氧运动后,心力衰竭患者的甲状腺激素信号通路,包括三碘甲状腺原氨酸和甲状腺激素受体α均得到增强,同时蛋白激酶B上调而JNK受到抑制,这些变化共同促进了心肌生长,进而改善了心脏功能。GAYNULLINA等[68]的研究显示,尽管8周有氧运动没有改变甲状腺功能减退大鼠的甲状腺激素水平,但有助于缓解甲状腺功能减退所引起的冠状动脉功能障碍,提示运动改善冠状动脉内皮功能障碍可能独立于甲状腺激素水平的调节。类似的是,CUTOVIC等[69]的研究表明,运动干预可以改善甲状腺功能亢进患者的心血管功能,使得峰值摄氧量提高、运动疲劳感下降,而对于甲状腺激素的改善并不显著。更具启示意义的是,TEIXEIRA等[70]发现10周有氧运动干预可使心肌梗死大鼠产生与三碘甲状腺原氨酸替代治疗相似的心脏功能改善,表现为梗死面积减小、射血分数提高、舒张期后壁厚度增加,同样三碘甲状腺原氨酸、四碘甲状腺原氨酸和促甲状腺激素水平未发生改变。这些研究表明运动改善心脏功能的主要途径并非直接改善甲状腺激素水平,因为大多数研究未发现甲状腺激素水平的变化。因此,运动可能通过其他途径,例如调节局部组织中脱碘酶的活性或改变甲状腺激素受体敏感性,从而实现对甲状腺激素的间接调控。 2.2.5 大脑 甲状腺激素广泛参与神经系统的多个生理过程,包括神经发生、神经元与胶质细胞的分化和迁移以及突触和髓鞘的生成,因而对大脑发育至关重要[71]。甲状腺激素受体α主要分布在海马、大脑皮质和小脑等区域,直接参与神经系统发育与功能调节,而甲状腺激素受体β主要表达于下丘脑和垂体[72]。脱碘酶通过局部调控大脑内的甲状腺激素水平对神经系统的正常发育和功能维持至关重要,其中脱碘酶2主要在星形胶质细胞和塔尼细胞中表达,而脱碘酶3则主要在神经元中表达。脱碘酶2基因敲除小鼠血清三碘甲状腺原氨酸水平正常,但大脑中的三碘甲状腺原氨酸水平显著降低,表明局部脱碘作用减少[7]。脱碘酶3基因敲除小鼠表现出小脑过早分化、运动功能受损以及因神经内分泌异常而导致攻击性增强、焦虑减少和抑郁样行为降低[8]。 研究显示,甲状腺功能减退患者的脑干、小脑、海马、额叶、扣带回、岛叶等脑区灰质体积均有减小,并伴随记忆力减退、执行功能受损、情绪波动、注意力集中困难和运动协调不足等症状[73](图3)。海马被认为是对甲状腺激素高度敏感的大脑结构,近期研究发现,甲状腺功能减退大鼠中海马区域的甲状腺激素缺乏与海马体积减少、β-淀粉样蛋白水平升高、微管相关蛋白过度磷酸化、促炎细胞因子产生以及空间学习和记忆障碍等一系列病理变化密切相关[74]。另外在胎儿及出生后发育期间,甲状腺激素缺乏可能导致大脑额叶和顶叶皮质萎缩,进而导致髓鞘形成异常和神经功能受损,最终将引发智力缺陷和运动功能障碍[73]。而甲状腺功能亢进的精神表现为焦虑、情绪不稳定、抑郁和躁狂[75]。研究表明,甲状腺功能亢进可引起神经元凋亡和氧化应激增加,从而导致认知功能障碍[76]。与健康对照组相比,成人甲状腺功能亢进患者除了在多个脑区(如海马、左侧颞极、杏仁体和脑沟)出现灰质体积减少,而且在多个脑区之间的功能连接性也发生改变,导致记忆、执行功能、视空间能力、运动功能及神经心理学测试表现下降[77]。 目前的研究表明,适度的运动干预能够显著改善甲状腺功能减退所带来的认知功能损害(图4)。SHIN等[78]研究通过持续4周的有氧运动(每天1次,每次30 min)探讨运动干预对甲状腺功能减退大鼠幼崽短期记忆和空间学习能力的影响,结果显示运动干预使小鼠血清三碘甲状腺原氨酸和四碘甲状腺原氨酸水平恢复至正常,同时显著提高了微管结合蛋白、海马中脑源性神经营养因子和成纤维生长因子受体B表达,从而增强神经发生、抑制神经凋亡,促进神经可塑性,进而改善了小鼠短期记忆和空间学习能力。RASHIDY-POUR等[79]研究表明,3周的有氧运动(每天1次,每次30 min)缓解了甲状腺功能减退大鼠的学习记忆缺陷,并且海马中的脑源性神经营养因子水平显著增加。因此,适度运动可通过促进甲状腺激素水平恢复以及上调神经可塑性相关因子表达,从而显著改善甲状腺功能减退所造成的认知功能损害。有研究指出,运动干预还可能通过减少脑内白细胞介素6和肿瘤坏死因子α水平、降低丙二醛和活性氧水平,进而减缓脑炎症和氧化应激水平,从而有助于改善焦虑和抑郁,这对于甲状腺功能亢进患者的认知功能损害具有重要作用[80-81]。 综上所述,甲状腺激素作为调控机体代谢、生长与发育的核心内分泌激素,其研究历经百年探索,从19世纪碘元素的发现到分子机制的解析,逐步揭示了它在多器官中的核心作用。甲状腺激素的合成与代谢受下丘脑-垂体-甲状腺轴调控,并依赖脱碘酶活性和甲状腺激素受体敏感性的局部调节,形成循环与组织特异性代谢的双重调节网络。近年的研究表明,急性运动通过应激反应诱导甲状腺激素的瞬时波动,而长期运动则主要通过调节脱碘酶活性或甲状腺激素受体敏感性而间接调节甲状腺激素水平。运动干预有潜力逆转甲状腺功能异常导致的多器官病理表型,如代谢相关脂肪性肝病、骨稳态失衡、肌肉功能下降、心脏功能衰退和认知功能障碍等。尽管现有证据揭示了运动对甲状腺激素的调节效应,但其分子机制和对多器官的调节作用仍需深入解析。"

| [1] SINHA RA, YEN PM. Metabolic Messengers: Thyroid Hormones. Nat Metab. 2024;6(4): 639-650. [2] LI H, CHEN C, CHEN Y, et al. High prevalence of metabolic diseases, liver steatosis and fibrosis among Chinese psychiatric patients. BMC Psychiatry. 2023;23(1):206. [3] CLEMENTE-SUÁREZ VJ, MARTÍN-RODRÍGUEZ A, REDONDO-FLÓREZ L, et al. New Insights and Potential Therapeutic Interventions in Metabolic Diseases. Int J Mol Sci. 2023; 24(13):10672. [4] MENDOZA-LEÓN MJ, MANGALAM AK, REGALDIZ A, et al. Gut microbiota short-chain fatty acids and their impact on the host thyroid function and diseases. Front Endocrinol (Lausanne). 2023;14:1192216. [5] THANAS C, ZIROS PG, CHARTOUMPEKIS DV, et al. The Keap1/Nrf2 Signaling Pathway in the Thyroid-2020 Update. Antioxidants (Basel). 2020;9(11):1082. [6] DE LUCA R, DAVIS PJ, LIN HY, et al. Thyroid Hormones Interaction With Immune Response, Inflammation and Non-thyroidal Illness Syndrome. Front Cell Dev Biol. 2020; 8:614030. [7] SABATINO L, VASSALLE C, DEL SEPPIA C, et al. Deiodinases and the Three Types of Thyroid Hormone Deiodination Reactions. Endocrinol Metab (Seoul). 2021;36(5):952-964. [8] HERNANDEZ A, MARTINEZ ME, NG L, et al. Thyroid Hormone Deiodinases: Dynamic Switches in Developmental Transitions. Endocrinology. 2021;162(8):bqab091. [9] BASSETT JH, WILLIAMS GR. Role of Thyroid Hormones in Skeletal Development and Bone Maintenance. Endocr Rev. 2016; 37(2):135-187. [10] PEARCE EN, ZIMMERMANN MB. The Prevention of Iodine Deficiency: A History. Thyroid. 2023;33(2):143-149. [11] ORD WM. On Myxœdema, a term proposed to be applied to an essential condition in the “Cretinoid” Affection occasionally observed in Middle-aged Women. Med Chir Trans. 1878;61:57-78.5. [12] BEATTY W. A Case of Myxoedeam Successfully Treated by Massage and Hypodermic Injections of the Thyroid Gland of a Sheep. Br Med J. 1892;1(1628):544-545. [13] KENDALL EC. Landmark article, June 19, 1915. The isolation in crystalline form of the compound containing iodin, which occurs in the thyroid. Its chemical nature and physiologic activity. By E.C. Kendall. JAMA. 1983;250(15):2045-2046. [14] AUB JC, BAUER W, HEATH C, et al. STUDIES OF CALCIUM AND PHOSPHORUS METABOLISM: III. The Effects of the Thyroid Hormone and Thyroid Disease. J Clin Invest. 1929;7(1):97-137. [15] LASHOF JC, BONDY PK, STERLING K, et al. Effect of muscular exercise on circulating thyroid hormone. Proc Soc Exp Biol Med. 1954;86(2):233-235. [16] RHODES BA. Effect of exercise on the thyroid gland. Nature. 1967;216(5118):917-918. [17] GHARIB H, RYAN RJ, MAYBERRY WE, et al. Radioimmunoassay for triiodothyronine (T 3 ): I. Affinity and specificity of the antibody for T 3. J Clin Endocrinol Metab. 1971;33(3):509-516. [18] OPPENHEIMER JH, KOERNER D, SCHWARTZ HL, et al. Specific nuclear triiodothyronine binding sites in rat liver and kidney. J Clin Endocrinol Metab. 1972;35(2):330-333. [19] STORY JA, GRIFFITH DR. Effect of exercise on thyroid hormone secretion rate in aging rats. Horm Metab Res. 1974;6(5):403-406. [20] HACKNEY AC, GULLEDGE T. Thyroid hormone responses during an 8-hour period following aerobic and anaerobic exercise. Physiol Res. 1994;43(1):1-5. [21] FORTUNATO RS, IGNÁCIO DL, PADRON AS, et al. The effect of acute exercise session on thyroid hormone economy in rats. J Endocrinol. 2008;198(2):347-353. [22] DA SILVA JMP, SILVA GCE, DA CONCEIÇÃO RR, et al. Influence of Resistance Training Exercise Order on Acute Thyroid Hormone Responses. Int J Exerc Sci. 2022;15(2):760-770. [23] ERDOĞAN R. Effects of Endurance Workouts on Thyroid Hormone Metabolism and Biochemical Markers In Athletes. Brain Broad Research in Artificial Intelligence and Neuroscience. 2020;11:136-146. [24] WINDER WW, GARHART SJ, PREMACHANDRA BN. Peripheral markers of thyroid status unaffected by endurance training in rats. Pflugers Arch. 1981;389(3):195-198. [25] BORZYKH AA, GAYNULLINA DK, SHVETSOVA AA, et al. Voluntary wheel exercise training affects locomotor muscle, but not the diaphragm in the rat. Front Physiol. 2022; 13:1003073. [26] TYLER J, PODARAS M, RICHARDSON B, et al. High intensity interval training exercise increases dopamine D2 levels and modulates brain dopamine signaling. Front Public Health. 2023;11:1257629. [27] BOLOTTA A, FILARDO G, ABRUZZO PM, et al. Skeletal Muscle Gene Expression in Long-Term Endurance and Resistance Trained Elderly. Int J Mol Sci. 2020;21(11):3988. [28] HATZIAGELAKI E, PASCHOU SA, SCHÖN M, et al. NAFLD and thyroid function: pathophysiological and therapeutic considerations. Trends Endocrinol Metab. 2022;33(11):755-768. [29] XIE Z, LI Y, CHENG L, et al. Potential therapeutic strategies for MASH: from preclinical to clinical development. Life Metab. 2024;3(5):loae029. [30] WANG B, WANG B, YANG Y, et al. Thyroid function and non-alcoholic fatty liver disease in hyperthyroidism patients. BMC Endocr Disord. 2021;21(1):27. [31] BAHTIYAR N, YOLDAŞ A, AYDEMIR B, et al. Influence of hyperthyroidism on hepatic antioxidants and cytokines Levels: An Experimental Study. Med Sci Discovery. 2020;7:439-444. [32] XIA SF, JIANG YY, QIU YY, et al. Role of diets and exercise in ameliorating obesity-related hepatic steatosis: Insights at the microRNA-dependent thyroid hormone synthesis and action. Life Sci. 2020;242:117182. [33] LIU Q, LI H, HE W, et al. Role of aerobic exercise in ameliorating NASH: Insights into the hepatic thyroid hormone signaling and circulating thyroid hormones. Front Endocrinol (Lausanne). 2022;13:1075986. [34] VENDITTI P, DE ROSA R, CALDARONE G, et al. Effect of prolonged exercise on oxidative damage and susceptibility to oxidants of rat tissues in severe hyperthyroidism. Arch Biochem Biophys. 2005;442(2):229-237. [35] TUCHENDLER D, BOLANOWSKI M. The influence of thyroid dysfunction on bone metabolism. Thyroid Res. 2014;7(1):12. [36] ZHU S, PANG Y, XU J, et al. Endocrine Regulation on Bone by Thyroid. Front Endocrinol (Lausanne). 2022;13:873820. [37] TIAN L, LU C, TENG W. Association between physical activity and thyroid function in American adults: a survey from the NHANES database. BMC Public Health. 2024;24(1):1277. [38] LADEMANN F, RIJNTJES E, KÖHRLE J, et al. Hyperthyroidism-driven bone loss depends on BMP receptor Bmpr1a expression in osteoblasts. Commun Biol. 2024;7(1):548. [39] APOSTU D, LUCACIU O, OLTEAN-DAN D, et al. The Influence of Thyroid Pathology on Osteoporosis and Fracture Risk: A Review. Diagnostics (Basel). 2020;10(3):149. [40] DYREK N, WIKAREK A, NIEMIEC M, et al. Selected musculoskeletal disorders in patients with thyroid dysfunction, diabetes, and obesity. Reumatologia. 2023;61(4):305-317. [41] TSOURDI E, RIJNTJES E, KÖHRLE J, et al. Hyperthyroidism and Hypothyroidism in Male Mice and Their Effects on Bone Mass, Bone Turnover, and the Wnt Inhibitors Sclerostin and Dickkopf-1. Endocrinology. 2015;156(10):3517-3527. [42] KIM J, HAN K, JUNG JH, et al. Physical activity and reduced risk of fracture in thyroid cancer patients after thyroidectomy - a nationwide cohort study. Front Endocrinol (Lausanne). 2023;14:1173781. [43] 温霜威,武青梅.有氧运动联合左旋甲状腺素与维生素D3改善亚临床甲减大鼠骨质疏松的作用[J]. 中国组织工程研究, 2020,24(26):4118-4124. [44] HUANG X, ZHU Y, SUN S, et al. Exercise maintains bone homeostasis by promoting osteogenesis through STAT3. Int J Biol Sci. 2023;19(7):2021-2033. [45] YANG Y, CHUNG MR, ZHOU S, et al. STAT3 controls osteoclast differentiation and bone homeostasis by regulating NFATc1 transcription. J Biol Chem. 2019; 294(42):15395-15407. [46] LIN HY, SHIH A, DAVIS FB, et al. Thyroid hormone promotes the phosphorylation of STAT3 and potentiates the action of epidermal growth factor in cultured cells. Biochem J. 1999;338(Pt 2)(Pt 2):427-432. [47] SUN Y, YUAN Y, WU W, et al. The effects of locomotion on bone marrow mesenchymal stem cell fate: insight into mechanical regulation and bone formation. Cell Biosci. 2021;11(1):88. [48] ZHOU J, GAUTHIER K, HO JP, et al. Thyroid Hormone Receptor α Regulates Autophagy, Mitochondrial Biogenesis, and Fatty Acid Use in Skeletal Muscle. Endocrinology. 2021;162(8):bqab112. [49] SALVATORE D, SIMONIDES WS, DENTICE M, et al. Thyroid hormones and skeletal muscle--new insights and potential implications. Nat Rev Endocrinol. 2014;10(4):206-214. [50] OGAWA-WONG A, CARMODY C, LE K, et al. Modulation of Deiodinase Types 2 and 3 during Skeletal Muscle Regeneration. Metabolites. 2022;12(7):612. [51] IGNACIO DL, SILVESTRE DH, ANNE-PALMER E, et al. Early Developmental Disruption of Type 2 Deiodinase Pathway in Mouse Skeletal Muscle Does Not Impair Muscle Function. Thyroid. 2017;27(4):577-586. [52] DE STEFANO MA, AMBROSIO R, PORCELLI T, et al. Thyroid Hormone Action in Muscle Atrophy. Metabolites. 2021;11(11):730. [53] SETOYAMA D, LEE HY, MOON JS, et al. Immunometabolic signatures predict recovery from thyrotoxic myopathy in patients with Graves’ disease. J Cachexia Sarcopenia Muscle. 2022;13(1):355-367. [54] BLOISE FF, OLIVEIRA TS, CORDEIRO A, et al. Thyroid Hormones Play Role in Sarcopenia and Myopathies. Front Physiol. 2018;9:560. [55] GONçALVES A, TOLENTINO CC, SOUZA FR, et al. The thyroid hormone receptor β-selective agonist GC-1 does not affect tolerance to exercise in hypothyroid rats. Arch Endocrinol Metab. 2015;59(2):141-147. [56] ZHOU J, PARKER DC, WHITE JP, et al. Thyroid Hormone Status Regulates Skeletal Muscle Response to Chronic Motor Nerve Stimulation. Front Physiol. 2019;10:1363. [57] JANJUA I, BASHIR T, HAQ MZU, et al. Severe Hypothyroidism Presenting With Rhabdomyolysis in a Young Patient. Cureus. 2021;13(3):e13993. [58] SALEHI N, AGOSTON E, MUNIR I, et al. Rhabdomyolysis in a Patient with Severe Hypothyroidism. Am J Case Rep. 2017;18: 912-918. [59] HANKE L, POETEN P, SPANKE L, et al. The Influence of Levothyroxine on Body Composition and Physical Performance in Subclinical Hypothyroidism. Horm Metab Res. 2023;55(1):51-58. [60] AHMAD AM, SERRY ZH, ABD ELGHAFFAR HA, et al. Effects of aerobic, resistance, and combined training on thyroid function and quality of life in hypothyroidism. A randomized controlled trial. Complement Ther Clin Pract. 2023;53:101795. [61] YAMAKAWA H, KATO TS, NOH JY, et al. Thyroid Hormone Plays an Important Role in Cardiac Function: From Bench to Bedside. Front Physiol. 2021;12:606931. [62] LIU KL, CANAPLE L, DEL CARMINE P, et al. Thyroid hormone receptor-α deletion decreases heart function and exercise performance in apolipoprotein E-deficient mice. Physiol Genomics. 2016;48(2):73-81. [63] ABDEL-MONEIM A, GABER AM, GOUDA S, et al. Relationship of thyroid dysfunction with cardiovascular diseases: updated review on heart failure progression. Hormones (Athens). 2020;19(3):301-309. [64] YILMAZ F, BABAYEVA A, YETKIN İ, et al. Comparison of exercise capacity and physical activity in patients with hyperthyroidism and controls. J Bodyw Mov Ther. 2024;40:1752-1760. [65] BIONDI B. Mechanisms in endocrinology: Heart failure and thyroid dysfunction. Eur J Endocrinol. 2012;167(5):609-618. [66] KONAR KD, PILLAY S, SOOKDEV N. Myxedema ascites? A rare presentation of ascites in severe hypothyroidism: A case report and review. SAGE Open Med Case Rep. 2024;12:2050313X241282218. [67] ADAMOPOULOS S, GOUZIOUTA A, MANTZOURATOU P, et al. Thyroid hormone signalling is altered in response to physical training in patients with end-stage heart failure and mechanical assist devices: potential physiological consequences? Interact Cardiovasc Thorac Surg. 2013;17(4): 664-668. [68] GAYNULLINA DK, BORZYKH AA, SOFRONOVA SI, et al. Voluntary exercise training restores anticontractile effect of NO in coronary arteries of adult rats with antenatal/early postnatal hypothyroidism. Nitric Oxide. 2018;74:10-18. [69] CUTOVIC M, KONSTANTINOVIC L, STANKOVIC Z, et al. Structured exercise program improves functional capacity and delays relapse in euthyroid patients with Graves’ disease. Disabil Rehabil. 2012; 34(18):1511-1518. [70] TEIXEIRA RB, ZIMMER A, DE CASTRO AL, et al. Long-term T3 and T4 treatment as an alternative to aerobic exercise training in improving cardiac function post-myocardial infarction. Biomed Pharmacother. 2017;95: 965-973. [71] BAKSI S, PRADHAN A. Thyroid hormone: sex-dependent role in nervous system regulation and disease. Biol Sex Differ. 2021;12(1):25. [72] ALCAIDE MARTIN A, MAYERL S. Local Thyroid Hormone Action in Brain Development. Int J Mol Sci. 2023;24(15):12352. [73] SALAS-LUCIA F. Mapping Thyroid Hormone Action in the Human Brain. Thyroid. 2024; 34(7):815-826. [74] SABATINO L, LAPI D, DEL SEPPIA C. Factors and Mechanisms of Thyroid Hormone Activity in the Brain: Possible Role in Recovery and Protection. Biomolecules. 2024;14(2):198. [75] BILICHODU RANGAPPA S, SHARMA A, AVULA S, et al. The Intertwined Relationship Between an Overactive Thyroid and an Overactive Mind: A Case Report and Review of Literature. Cureus. 2023;15(12):e50748. [76] KHALEGHZADEH-AHANGAR H, TALEBI A, MOHSENI-MOGHADDAM P. Thyroid Disorders and Development of Cognitive Impairment: A Review Study. Neuroendocrinology. 2022;112(9):835-844. [77] KUMAR M, SINGH S, RANA P, et al. Brain functional connectivity in patients with hyperthyroidism after anti-thyroid treatment. J Neuroendocrinol. 2022;34(1):e13075. [78] SHIN MS, KO IG, KIM SE, et al. Treadmill exercise ameliorates symptoms of methimazole-induced hypothyroidism through enhancing neurogenesis and suppressing apoptosis in the hippocampus of rat pups. Int J Dev Neurosci. 2013;31(3): 214-223. [79] RASHIDY-POUR A, DERAFSHPOUR L, VAFAEI AA, et al. Effects of treadmill exercise and sex hormones on learning, memory and hippocampal brain-derived neurotrophic factor levels in transient congenital hypothyroid rats. Behav Pharmacol. 2020; 31(7):641-651. [80] DEBOER LB, POWERS MB, UTSCHIG AC, et al. Exploring exercise as an avenue for the treatment of anxiety disorders. Expert Rev Neurother. 2012;12(8):1011-1022. [81] IBRAHIM EM, MEGAHED AA, METWALLY AIK, et al. Neuroprotective And Therapeutic Effects of Exercise in Restraint Stress Induced Depression Like Behavior Albino Rat Model. Egyptian J Hosp Med 2023; 92(1):5558. |
| [1] | Haonan Yang, Zhengwei Yuan, Junpeng Xu, Zhiqi Mao, Jianning Zhang. Preliminary study on the mechanisms and efficacy of deep brain stimulation in treating depression [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(在线): 1-9. |
| [2] | Jiang Xianglong, Li Zhongshan, Che Tongtong. Application effects and mechanisms of low-frequency pulsed electromagnetic fields in muscle repair and growth [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2350-2360. |
| [3] | Xinjiang Branch of China Trauma Rescue & Treatment Association. Expert consensus on diagnosis and treatment of brucellar osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2403-2412. |
| [4] | Cheng Qisheng, Julaiti·Maitirouzi, Xiao Yang, Zhang Chenwei, Paerhati·Rexiti. Finite element analysis of novel variable-diameter screws in modified cortical bone trajectory of lumbar vertebrae [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2162-2171. |
| [5] | Sun Lei, Zhang Qi, Zhang Yu. Pro-osteoblastic effect of chlorogenic acid protein microsphere/polycaprolactone electrospinning membrane [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1877-1884. |
| [6] | Wu Yanting, Li Yu, Liao Jinfeng. Magnesium oxide nanoparticles regulate osteogenesis- and angiogenesis-related gene expressions to promote bone defect healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1885-1895. |
| [7] | Li Qingbin, Lin Jianhui, Huang Wenjie, Wang Mingshuang, Du Jiankai, Lao Yongqiang. Bone cement filling after enlarged curettage of giant cell tumor around the knee joint: a comparison of subchondral bone grafting and non-grafting [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1896-1902. |
| [8] | Jiang Xinghai, Song Yulin, Li Dejin, Shao Jianmin, Xu Junzhi, Liu Huakai, Wu Yingguo, Shen Yuehui, Feng Sicheng. Vascular endothelial growth factor 165 genes transfected into bone marrow mesenchymal stem cells to construct a vascularized amphiphilic peptide gel module [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1903-1911. |
| [9] | Liu Dawei, Cui Yingying, Wang Fanghui, Wang Zixuan, Chen Yuhan, Li Yourui, Zhang Ronghe. Epigallocatechin gallate-mediated bidirectional regulation of reactive oxygen species and its application in nanomaterials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2101-2112. |
| [10] | Fu Lyupeng, Yu Peng, Liang Guoyan, Chang Yunbing. Electroactive materials applied in spinal surgery [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2113-2123. |
| [11] | Lai Yu, Chen Yueping, Zhang Xiaoyun. Research hotspots and frontier trends of bioactive materials in treating bone infections [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2132-2144. |
| [12] | Zhou Hongli, Wang Xiaolong, Guo Rui, Yao Xuanxuan, Guo Ru, Zhou Xiongtao, He Xiangyi. Fabrication and characterization of nanohydroxyapatite/sodium alginate/polycaprolactone/alendronate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1962-1970. |
| [13] | Hu Xiongke, Liu Shaohua, Tan Qian, Liu Kun, Zhu Guanghui. Shikonin intervention with bone marrow mesenchymal stem cells improves microstructure of femur in aged mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1609-1615. |
| [14] | Yuan Xiaoshuang, Yang Xu, Yang Bo, Chen Xiaoxu, Tian Ting, Wang Feiqing, Li Yanju, Liu Yang, Yang Wenxiu. Effect of conditioned medium of diffuse large B-cell lymphoma cells on proliferation and apoptosis of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1632-1640. |
| [15] | Li Zhenyu, Zhang Siming, Bai Jiaxiang, Zhu Chen. Osthole improves osteogenic differentiation function of bone marrow mesenchymal stem cells under high-glucose conditions [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1641-1648. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||