Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (22): 5782-5791.doi: 10.12307/2026.236
Previous Articles Next Articles
Virtual reality therapy on neuropathic pain following spinal cord injury
Du Xinyu1, Zhao Donglin1, Zhang Shuyang1, Li Shihao2, Xing Zheng2, Chu Xiaolei2, Li Qi2
- 1College of Exercise & Health, Tianjin University of Sport, Tianjin 301617, China; 2Department of Rehabilitation, Tianjin Hospital, Tianjin 300211, China
-
Received:2025-09-10Accepted:2025-10-21Online:2026-08-08Published:2025-12-27 -
Contact:Li Qi, MS, Associate chief physician, Department of Rehabilitation, Tianjin Hospital, Tianjin 300211, China -
About author:Du Xinyu, Master candidate, College of Exercise & Health, Tianjin University of Sport, Tianjin 301617, China -
Supported by:“Biological and Information Fusion (BT and IT Fusion)” Key Special Project of the National Key Research & Development Program, No. 2023YFF1205200 (to XZ); Tianjin Natural Science Foundation General Project, No. 22JCYBJC00210 (to LQ); Tianjin Natural Science Foundation General Project, No. 22JCYBJC00220 (to CXL)
CLC Number:
Cite this article
Du Xinyu, Zhao Donglin, Zhang Shuyang, Li Shihao, Xing Zheng, Chu Xiaolei, Li Qi. Virtual reality therapy on neuropathic pain following spinal cord injury[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5782-5791.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
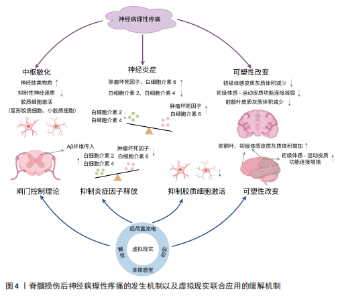
2.1 脊髓损伤后神经病理性疼痛的发生机制 2.1.1 疼痛通路与中枢敏化 疼痛调控涉及“自下而上”的痛觉处理(上行疼痛感觉传入通路)和“自上而下”的内源性疼痛调控(下行疼痛抑制系统和易化系统)[18]。当痛觉感受器受刺激时,Aδ纤维和C纤维会将不同类型的疼痛信号经背根神经传入脊髓背角进行初步处理,然后经上行通路传递到中继站丘脑,最终到达大脑疼痛相关区域进行加工、处理、分析,产生疼痛的感觉,随后激活下行疼痛调控系统,该系统包括抑制和易化系统两部分,具有双向调节功能[19]。下行疼痛抑制系统通过分泌去甲肾上腺素、5-羟色胺、多巴胺和内源性阿片类物质等抑制性神经递质,阻碍脊髓背角内感觉神经细胞间信息传递以及向丘脑传递信号,从而减轻疼痛[20]。下行疼痛易化系统则会释放谷氨酸等兴奋性神经递质,增强脊髓背角神经元的兴奋性,促进疼痛信号的传递和放大[21],二者之间的平衡决定了最终的疼痛体验。 脊髓损伤后,上述平衡被打破以及其他原因通常会导致中枢敏化现象的发生[22-23]。中枢敏化是中枢神经系统对疼痛信号的异常增强和持久敏感状态,被认为是导致神经病理性疼痛最重要的中枢机制,主要特征为神经元兴奋性增加[23]。神经递质失衡、胶质细胞激活、炎症因子释放等都会引起中枢敏化[24],导致神经病理性疼痛的发生,见图4。 神经递质失衡与中枢敏化发生密切相关,脊髓损伤后初级传入末梢释放的神经肽类物质显著增加,这些物质与受体结合后,通过激活腺苷酸环化酶而增加脊髓背角中谷氨酸的释放,持续激活兴奋性N-甲基-D-天冬氨酸受体,这一过程可视作中枢性敏化的启动因素,此时,抑制性神经递质γ-氨基丁酸和甘氨酸表达会降低[25]。兴奋性神经递质谷氨酸释放增加,抑制性神经递质释放减少,二者比例失衡,使脊髓背角中神经元过度激活,对外部刺激的反应性增强,导致中枢敏化。此时脊髓对疼痛信号的响应变得更加敏感,疼痛感知增强,诱发神经病理性疼痛。 胶质细胞激活、炎症因子释放同样是引起中枢敏化的关键机制。脊髓损伤促使局部、远端星形胶质细胞和小胶质细胞激活[26],它们会产生各种促炎因子和神经毒性剂,包括肿瘤坏死因子和白细胞介素6等[27],这些促炎因子同样会诱导神经元过度兴奋,导致神经病理性疼痛的发生[24]。 2.1.2 疼痛矩阵与大脑可塑性改变 国际疼痛研究协会将疼痛定义为:“一种与实际或潜在的组织损伤相关或类似的不愉快的感觉和情感体验”。疼痛的感觉和情感状态之间存在一种相互关系,不良的情感体验会加剧疼痛现象,并使感觉和情感之间的恶性循环永久化[28]。疼痛的感觉和情感体验由不同脑区处理,MELZACK[29]提出了“疼痛矩阵”的概念,在这个概念中,与疼痛相关的大脑区域形成了一个相互联系的网络,共同编码疼痛的感觉和情感体验,包括丘脑、岛叶、躯体感觉皮质、前额叶皮质、前扣带回、杏仁核、臂旁核等脑区。丘脑、岛叶、躯体感觉皮质主要负责处理疼痛的感觉辨别方面;前额叶皮质、前扣带皮质、杏仁核、臂旁核则负责处理疼痛的情感维度。这些大脑区域协作编码疼痛的感觉和情感体验,构成大脑中的疼痛 “信号”。当疼痛矩阵中某一区域功能减弱时,会导致对疼痛的编"
码能力下降,促使疼痛信号的产生和放大。 脊髓损伤不仅会导致脊髓水平的中枢敏化现象,还会引起大脑的异常可塑性改变[8]。这种异常改变不仅涉及感觉运动皮质,还涉及位于疼痛矩阵中处理情感和认知的前额叶皮质[30],降低了大脑对疼痛的编码能力,最终导致脊髓损伤后神经病理性疼痛的发生,见图4。 感觉和运动皮质的可塑性改变主要呈现以下特征:初级体感皮质灰质体积减小,反映了神经元树突复杂性降低和突触连接减少,这会影响正常的感觉信息处理。JUTZELER等[31]在脊髓损伤患者的研究中观察到初级体感皮质灰质体积减小,且这一变化与疼痛程度呈负相关关系;初级运动皮质活性下降[7,32],初级运动皮质与导水管周围灰质-延髓头端腹内侧-脊髓背角构成的下行疼痛抑制通路密切相关[33],对初级运动皮质进行刺激可增强同侧导水管周围灰质神经元的放电活性,同时减弱γ-氨基丁酸能中间神经元对导水管周围灰质投射神经元的抑制作用,从而激活下行疼痛抑制通路[33-34]。这种激活促进了内源性阿片类物质释放,能有效抑制疼痛信号传递,最终降低痛觉感知[34],而这一机制在脊髓损伤后效能下降;初级体感皮质-初级运动皮质兴奋-抑制失衡,导致初级运动皮质神经元的活动降低,进而减弱疼痛下行抑制系统的能力,促使疼痛的发生[32];静息态功能连接研究表明,脊髓损伤会导致初级体感皮质与初级运动皮质功能连接减弱,而与其他疼痛相关脑区的连接增强,这种异常连接模式变化使得更容易激活疼痛网络[35],从而诱发神经病理性疼痛。上述改变破坏了感觉反馈与运动意图的匹配,导致感觉-运动整合环路异常,疼痛通路进一步被激活,这是脊髓损伤后神经病理性疼痛的关键发病机制[36]。 脊髓损伤还会引起前额叶皮质的可塑性改变,前额叶皮质位于疼痛矩阵的内侧上升通路,处理疼痛的情感维度,同时前额叶皮质纤维束可以向下投射至导水管周围灰质[30],发挥疼痛抑制作用。导水管周围灰质是疼痛下行抑制系统的重要组成部分,在疼痛下行调控通路和疼痛缓解中起重要作用,是诸多高级痛觉中枢下行传递至脑干的交汇点,通过向脊髓投射抑制性信号,促进阿片类物质的释放[37],实现对疼痛的调控。神经影像学研究显示,脊髓损伤后神经病理性疼痛患者前额叶区域灰质体积减小[38],这些变化直接影响患者的疼痛认知评价和情绪调节能力,导致疼痛灾难化思维增加、痛苦无助感增强以及疼痛应对策略不当。 2.2 虚拟现实应用于脊髓损伤后神经病理性疼痛的历史研究进展 虚拟现实应用主要经历了从基础2D显示、2D与神经调控融合、3D立体显示到交互式体验,最终至多感官整合的演进历程,见图5。MOSLEY[10]最早将虚拟现实应用于脊髓损伤后神经病理性疼痛患者中,采用2D屏幕式虚拟行走干预,首次验证了虚拟现实对脊髓损伤后神经病理性疼痛患者的镇痛效果。随后为进一步提高疗效,SOLER等[39]将2D屏幕式虚拟行走干预与非侵入性神经调控技术经颅直流电刺激联合应用于脊髓损伤后神经病理性疼痛患者中,用于判断二者联合干预是否更具协同效应,结果显示联合干预的效果比单独使用任何一种干预更好、更持久。随后POZEG等[40]成功开发出沉浸感更强的3D头戴式虚拟现实干预设备,该装置完全阻隔了使用者对外部物理环境的视觉感知,使感官系统被虚拟环境全方位包围,从而实现最高程度的存在与沉浸体验。TROST等[41]在3D虚拟现实干预基础上,研究了一种新型交互式虚拟步行疗法,作为视觉反馈与幻觉步行疗法的延伸。其中,虚拟步行干预仍建立在先前的研究基础上,但允许脊髓损伤后神经病理性疼痛患者自主控制虚拟步态,与完全沉浸式的虚拟环境进行交互,结果显示患者疼痛强度明显减弱。如今更注重虚拟现实应用的多感官整合,例如视觉与本体感觉刺激的联合应用,SABALETTE等[42]将虚拟现实干预与肌腱振动刺激结合用于脊髓损伤后神经病理性疼痛患者,结果显示组合干预效果最佳,干预后疼痛显著减轻。"
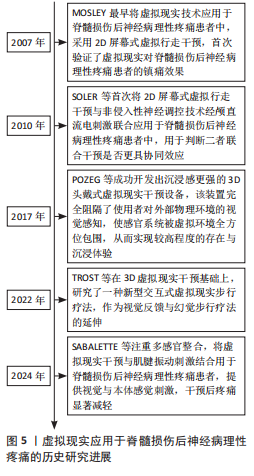
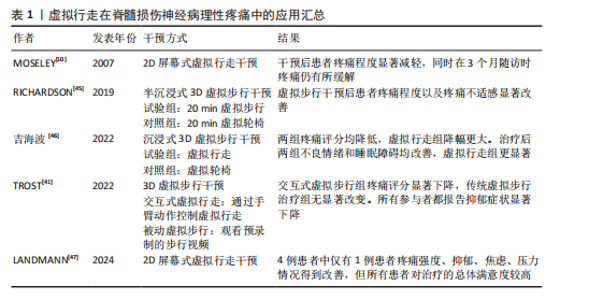
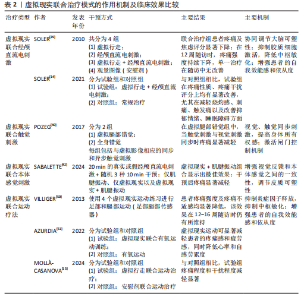
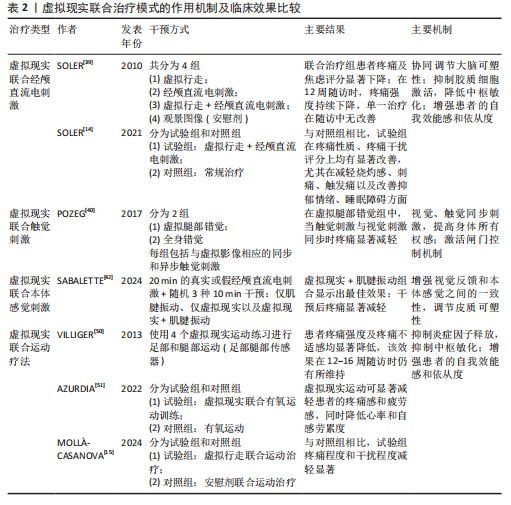
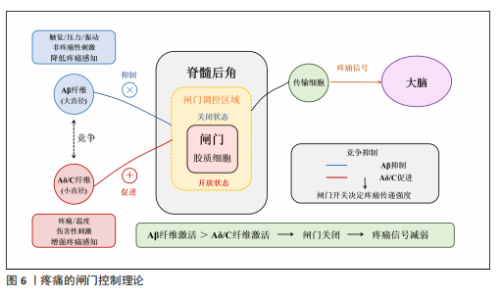
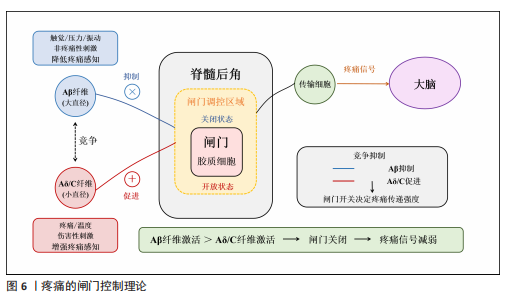
2.3 虚拟现实在脊髓损伤后神经病理性疼痛中的单独应用及缓解机制 虚拟现实借助各类显示设备以及高级动作追踪系统来提供视觉、听觉、触觉以及交互式反馈,这样的多感官干预可有效分散患者对于疼痛的注意力,并且有助于调节异常的疼痛网络,推动大脑可塑性重组,改善异常的中枢痛觉处理机制,缓解疼痛体验[43]。部分临床研究已证实了虚拟现实显著的镇痛效果[10,14,39,44],为脊髓损伤后神经病理性疼痛的非药物管理提供了新方向。 虚拟行走视觉错觉是目前应用最广泛的单模态虚拟现实干预策略。脊髓损伤导致初级体感皮质与初级运动皮质可塑性改变,视觉反馈和本体感觉之间存在差异,引发、加重疼痛[8]。视觉反馈疗法通过视觉错觉恢复感觉输入,以针对与传入神经阻滞相关的皮质破坏,是治疗脊髓损伤后神经病理性疼痛和其他神经病症的一种很有前景的干预措施[10]。过去研究表明,镜像疗法视觉反馈显示出较强的治疗效果,虚拟现实行走与镜像疗法相似,同属视觉反馈但更具优势[39],对坐起受限以及双侧受累的患者更具兼容性,同时灵活性强,更具定制潜力。 虚拟行走干预涉及在受试者进行运动想象期间给予相应的视觉反馈。DIERS等[44]研究显示运动想象期间给予与预期运动相匹配的视觉反馈,可同时激活初级运动皮质,增强初级感觉皮质与初级运动皮质之间的功能连接,二者之间功能连接的增强有助于恢复扰乱的感觉运动环路,重建错配的传入传出反馈,缓解疼痛。与此同时,初级运动皮质的激活后可有效激活内源性下行疼痛抑制系统,通过释放内啡肽和去甲肾上腺素等抑制性神经递质,减少中枢敏化的发生,在脊髓水平抑制痛觉传导[33]。 MOSELEY[10]最早将虚拟现实应用于脊髓损伤后神经病理性疼痛的治疗中,通过2D屏幕式虚拟行走干预验证了虚拟现实的镇痛效果。如今,虚拟现实技术不断进步,相较2D屏幕式虚拟现实干预,3D沉浸式以及交互式虚拟现实被更多应用于治疗中。虚拟行走在脊髓损伤后神经病理性疼痛中的具体应用情况,见表1[10,41,45-47]。 2.4 虚拟现实在脊髓损伤后神经病理性疼痛中的联合应用及缓解机制 基于虚拟现实单独应用的良好效果,越来越多的研究开始不断探索虚拟现实与其他治疗方法的联合应用,以充分发挥综合治疗的优势,达到更好的治疗效果。 2.4.1 虚拟现实联合经颅直流电刺激 虚拟现实与经颅直流电刺激的联合应用可以通过调节大脑可塑性,降低中枢敏化,增强患者的自我效能感和依从度,产生协同镇痛效应[11,48]。经颅直流电刺激是一种非侵入性皮质脑刺激,有助于恢复递质平衡、减少胶质细胞激活和炎症因子释放[11,48],在抑制中枢敏化、缓解疼痛方面发挥了重要作用。但经颅直流电刺激属于被动干预方式,缺乏患者的主动参与,这会降低患者的自我效能感,而虚拟现实则可通过丰富的感官输入激活感知处理网络,使患者主动参与虚拟环境并对其进行控制,这有助于增强患者的自我效能感和依从度,提高治疗效果[49]。同时虚拟现实的加入,在调节大脑可塑性方面也显示出一定优势,二者联合既有利于降低中枢敏化又有助于调节大脑可塑性,具有较高的治疗潜力。 支持虚拟行走和经颅直流电刺激联合干预减轻脊髓损伤后神经病理性疼痛的研究相对较多,这也是目前最常见的多模态虚拟现实干预方案,多项研究证实了二者联合在疼痛缓解效果上的显著优势,如表2所示[14-15,39-40,42,50-51]。 2.4.2 虚拟现实联合触觉刺激 虚拟现实联合触觉刺激可通过激活闸门控制理论、提高身体所有权感来缓解疼痛。MELZACK等[52]最早提出了疼痛的闸门控制理论,指出触觉刺激(Aβ纤维)会直接激活感觉传入通路,与疼痛信号(Aδ纤维C纤维)在脊髓和丘脑水平竞争处理资源,这抑制或减弱了疼痛信号的传递,减少了疼痛处理资源,从而减轻疼痛,见图6。同"
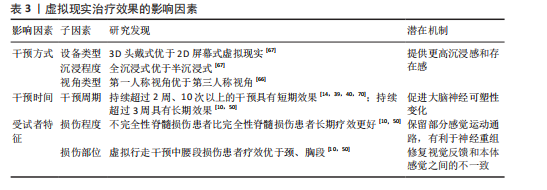
时通过虚拟现实可以实现视觉、触觉的同步输入,二者输入的协调性有助于提高身体所有权感[53]。研究显示,更强的身体所有权感与疼痛减轻密切相关[40]。 HOFFMAN等[54]在一项随机交叉试验中发现,添加触觉反馈可以帮助虚拟现实更有效地减轻疼痛,同时也增加了化身的具体化,最后预测了触觉反馈联合虚拟现实在慢性疼痛中的应用,指出这项应用可能在未来的研究中提高治疗慢性疼痛的有效性。POZEG等[40]成功将虚拟现实应用于脊髓损伤后神经病理性疼痛患者中,具体结果见表2。 2.4.3 虚拟现实联合本体感觉刺激 虚拟现实联合本体感觉刺激与虚拟行走具备相似的潜在机制,都有助于增强视觉反馈和本体感觉之间的一致性,增强感觉运动皮质兴奋性,调节大脑可塑性[55],从而缓解疼痛。肌腱振动是一种常用的本体感觉刺激方式,通过诱导肌腱伸长,诱发无痛运动感知[56],即当股四头肌受到振动时,在无实际运动的情况下会产生膝关节屈曲的运动感知,此时给予与动作感知相应的视觉反馈,有助于增强感觉运动区域的募集,增强大脑可塑性调节。 SABALETTE等[42]研究显示,虚拟行走与步态式肌腱振动的组合干预在缓解脊髓损伤后神经病理性疼痛方面效果显著,如表2所示。先前类似的干预手段多用于神经损伤患者的运动功能恢复方面[55],而这项研究则证实了此方案在疼痛治疗领域的潜力。 2.4.4 虚拟现实联合运动疗法 虚拟现实联合运动治疗能有效抑制炎症因子释放、恢复大脑皮质灰质体积,同时还能提高患者训练的积极性,既能减轻疼痛又能改善运动障碍。运动疗法虽能缓解疼痛[57],但传统重复训练的枯燥感容易降低患者的依从性,进而影响治疗效果。虚拟现实能提供游戏化设计同时还能根据不同患者进行个性化调整。将虚拟现实与运动训练联合,提高了训练的趣味性,提升了患者训练的积极性,有利于增强治疗效果。除此之外,虚拟现实运动训练还可促使双侧感觉运动皮质和海马灰质体积增加[58],这一变化对大脑的异常可塑性起到了调节作用。近期研究还证实了虚拟现实运动干预在炎症因子调控方面的作用,干预后肿瘤坏死因子、白细胞介素6水平明显下降,白细胞介素2和白细胞介素4水平则有所升高[59],见图4。 虚拟现实联合运动疗法的应用基于运动功能障碍与神经性疼痛共有的皮质机制[50],在调节与疼痛相关的体感皮质的同时还能激活参与运动的皮质区域,既能减轻疼痛又能改善运动障碍,如表2所示。 除上述联合方式外,虚拟现实与正念冥想、催眠、呼吸以及放松疗法的联合干预在慢性疼痛管理中同样显示出较大优势[16,60-62],但尚缺少在脊髓损伤后神经病理性疼痛中的应用。疼痛是一种复杂的、多因素的主观体验,包括感觉辨别、情感动机和认知评估成分,MELZACK[29]提出“疼痛矩阵”的概念,与疼痛相关的大脑区域形成一个相互联系的网络,会共同编码疼痛的感觉和情感体验,情感成分在疼痛处理中同样至关重要。然而当前尚缺乏针对脊髓损伤后神经病理性疼痛患者疼痛情感维度的系统性虚拟现实干预策略,例如虚拟现实与正念、冥想的联合。初步证据表明,正念冥想可诱导前额叶皮质等情感调节区域的可塑性变化,增加灰质体积及皮质厚度[63],这有望逆转脊髓损伤导致的神经结构损害,提高疼痛的情感调节,帮助缓解疼痛。但正念冥想训练也存在一个主要问题,即难以长时间集中注意力,尤其是对于初学者来说,主要因为这是一个主观的内感受过程。因此,迫切需要一种有形的方法,能够提供身临其境的感官引导,以促进精神控制,虚拟现实的发展就提供了很好的辅助选择。虚拟现实催眠与虚拟现实呼吸同样属于较新的干预形式。催眠主要与转移注意力相关,催眠通过诱导解离状态来改变疼痛刺激的处理,帮助受试者将注意力从伤害性输入上转移开,从而缓解疼痛[64]。不同受试者解离和对催眠做出反应的能力以及从催眠镇痛中受益的能力存在很大差异。然而有研究显示,在催眠中添加虚拟现实可以减轻大量个体的疼痛,即使是催眠水平较低的受试者[65],这可能是由于它增强了注意力集中并提供了视觉刺激。虚拟现实呼吸训练诱发了沉浸式 3D外感受过程,增强了视觉听觉皮质的激活,帮助大脑将更多的资源分配给视觉或听觉任务,减少了初级体感皮质的疼痛处理功能,同样能够有效缓解疼痛[61]。一项最新研究还显示出虚拟现实引导下的放松疗法对疼痛强度和焦虑情绪的显著改善,该技术有利于将注意力转移到大脑上进而影响身体的自主神经平衡,通过调节交感神经和副交感神经系统,引导放松和疼痛减轻[62]。未来可积极探索虚拟现实与正念冥想、催眠、呼吸以及放松疗法联合干预在脊髓损伤后神经病理性疼痛中的应用效能。 2.5 虚拟现实治疗效果的影响因素分析 大部分研究显示出虚拟现实干预缓解脊髓损伤患者神经性疼痛的有效性,但也存在一些相互矛盾的结果,这可能与干预方式(干预设备、干预视角)、干预时间、受试者损伤程度相关。 (1)干预方式:存在感与沉浸感是虚拟现实治疗效果的关键因素,更高程度的沉浸感及存在感与更好的疼痛缓解效果直接相关[41]。干预设备以及干预视角的不同是影响二者的重要因素[66-67],3D设备、第一视角的干预往往会带来更强的沉浸感,增强治疗效果。AUSTIN等[67]在一项随机对照研究中比较了3D头戴式以及2D屏幕式虚拟现实干预对脊髓损伤后神经性疼痛患者疼痛强度的影响,结果显示与2D屏幕相比,3D干预后患者的疼痛强度以及存在感水平显著改善。该结果解释了RICHARDSON等[45]与吉海波等[46]研究结果不同的原因,二者都研究了第一视角虚拟行走与虚拟轮椅干预对脊髓损伤后神经病理性疼痛的影响,但结果却不一致,主要原因在于前者使用的为半沉浸式干预而后者则为全沉浸式3D头戴式虚拟现实干预,前者较低的沉浸水平与存在感或是影响治疗效果的关键。干预视角的差异也会影响治疗效果,第一人称视角通常能带来更强的存在感与沉浸感。ROOSINK等[68]与TROST等[41]均研究了交互式虚拟行走的治疗效果,结果显示前者疼痛强度无显著改变,而TROST等[41]研究干预后疼痛评分和疼痛干扰都显著下降。虽然两人都采用了3D头戴式虚拟现实设备,但ROOSINK等[68]的虚拟现实干预主要为第三视角,与TROST等[41]第一视角相比受试者沉浸式体验较低。 (2)干预时间:研究表明,适当延长单次干预时间和干预总周期有利于恢复大脑可塑性改变[69],从而缓解疼痛。总结现有研究指出,持续超过2周、10次的虚拟现实干预能有效缓解疼痛,但要达到长期效应则需至少3周的干预。OZKUL等[39,41,70]的研究均以2周干预为周期,结果均显示出疼痛强度显著下降。在探讨虚拟现实干预持续效应的5项研究中[10,39,41,50,70],MOSELEY[10]以及VILLIGER等[50]在干预后随访时发现疼痛强度仍有所缓解,而?ZKUL等[70]、SOLER等[39]、TROST等[41]仅在2周干预后即刻观察到疼痛的缓解效果而在随访时则未观察到。这可能与干预时长相关,MOSELEY[10]与VILLIGER等[50]的干预均在3周以上,更长的干预时间有助于更好地恢复大脑感觉和运动皮质的异常可塑性变化,从而达到长期止痛效果[69]。 (3)受试者损伤程度:基于相关文献分析表明,受试者损伤程度同样对脊髓损伤后神经病理性疼痛患者长期疗效的维持具有重要影响,可能是虚拟行走成功的最重要和最可靠的预测因素[10]。与MOSELEY[10]与VILLIGER等[50]研究相比,?ZKUL等[70]、SOLER等[39]、TROST等[41]研究未显示出虚拟行走对脊髓损伤后神经病理性疼痛缓解的维持效应。前两个学者的研究中所有参与者都为不完全性脊髓损伤,同时主要为胸腰段损伤,?ZKUL等[70]研究以及SOLER等[39]研究接近一半或更多患者患有完全性脊髓损伤,节段为颈椎、胸椎和腰椎损伤的异质性组合。在TROST等[41]的最新研究中这一趋势更加明显,基于沉浸度更高的3D第一视角的虚拟现实头盔对完全性脊髓损伤患者进行虚拟行走干预,但在2周随访中并未观察到疼痛评分的显著变化。上述研究显示,受试者损伤程度可能是虚拟现实干预效果的可靠预测因素,但该发现主要集中于虚拟步行干预中,这与CHI等[43]观点相似。腰椎损伤患者报告的疼痛发生率和疼痛强度较高,且主要发生在下肢[71]。虚拟行走可能对此类患者最有益,虚拟行走可以产生下肢运动的错觉,修复视觉反馈和本体感觉的不一致,增强初级体感皮质-初级运动皮质的功能连接,调节大脑可塑性从而缓解疼痛。不完全性损伤效果较好可能与保留了部分感觉运动通路,支持神经可塑性调整和新神经连接建立有关[72]。 虚拟现实治疗效果的影响因素,见表3。"
| [1] HUA R, ZHAO C, XU Z, et al. ROS-responsive nanoparticle delivery of ferroptosis inhibitor prodrug to facilitate mesenchymal stem cell-mediated spinal cord injury repair. Bioact Mater. 2024;38:438-454. [2] HUNT C, MOMAN R, PETERSON A, et al. Prevalence of chronic pain after spinal cord injury: a systematic review and meta-analysis. Reg Anesth Pain Med. 2021;46: 328-336. [3] STAROSTA AJ, BOMBARDIER CH, KAHLIA F, et al. Feasibility of Brief, Hypnotic Enhanced Cognitive Therapy for SCI-related Pain During Inpatient Rehabilitation. Arch Phys Med Rehabil. 2024;105:1-9. [4] COLLOCA L, LUDMAN T, BOUHASSIRA D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. [5] AURUCCI GV, PREATONI G, DAMIANI A, et al. Brain-Computer Interface to Deliver Individualized Multisensory Intervention for Neuropathic Pain. Neurotherapeutics. 2023;20:1316-1329. [6] 王玲,陈楠.心理意象治疗脊髓损伤后神经性疼痛:神经影像学研究进展[J].中国医学影像技术,2023,39(4):602-605. [7] 陈莉,雷静,尤浩军.脊髓损伤后病理性疼痛发生机制及治疗研究进展[J].中国疼痛医学杂志,2022,28(11):843-848. [8] CALDERONE A, CARDILE D, DE LUCA R, et al. Brain Plasticity in Patients with Spinal Cord Injuries: A Systematic Review. Int J Mol Sci. 2024;25(4):2224. [9] ROSNER J, DE ANDRADE DC, DAVIS KD, et al. Central neuropathic pain. Nat Rev Dis Primers. 2023;9:73. [10] MOSELEY LG. Using visual illusion to reduce at-level neuropathic pain in paraplegia. Pain. 2007;130:294-298. [11] TAN M, FENG Z, CHEN H, et al. Transcranial direct current stimulation regulates phenotypic transformation of microglia to relieve neuropathic pain induced by spinal cord injury. Front Behav Neurosci. 2023;17:1147693. [12] RUIMONTE-CRESPO J, PLAZA-MANZANO G, DÍAZ-ARRIBAS MJ, et al. Aerobic Exercise and Neuropathic Pain: Insights from Animal Models and Implications for Human Therapy. Biomedicines. 2023;11(12):3174. [13] HUANG Q, LIN J, HAN R, et al. Using Virtual Reality Exposure Therapy in Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Value Health. 2022;25:288-301. [14] SOLER D, MORIÑA D, KUMRU H, et al. Transcranial Direct Current Stimulation and Visual Illusion Effect According to Sensory Phenotypes in Patients With Spinal Cord Injury and Neuropathic Pain. J Pain. 2021;22:86-96. [15] MOLLÀ-CASANOVA S, MUÑOZ-GÓMEZ E, AGUILAR-RODRÍGUEZ M, et al. Effectiveness of virtual-walking intervention combined with exercise on improving pain and function in incomplete spinal cord injury: a feasibility study. Spinal Cord Ser Cases. 2024;10:64. [16] MA J, ZHAO D, XU N, et al. The effectiveness of immersive virtual reality (VR) based mindfulness training on improvement mental-health in adults: A narrative systematic review. Explore (NY). 2023;19:310-318. [17] 张舒扬,杜心愉,赵冬临,等.虚拟现实技术在周围神经损伤功能恢复中的应用[J].中国组织工程研究,2025,29(21):4593-4601. [18] KYATHANAHALLY SP, AZZARITO M, ROSNER J, et al. Microstructural plasticity in nociceptive pathways after spinal cord injury. J Neurol Neurosurg Psychiatry. 2021;92:863-871. [19] OSSIPOV MH, MORIMURA K, PORRECA F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care. 2014;8:143-151. [20] FRANÇOIS A, LOW SA, SYPEK EI, et al. A Brainstem-Spinal Cord Inhibitory Circuit for Mechanical Pain Modulation by GABA and Enkephalins. Neuron. 2017;93(4):822-839.e6. [21] CHEN T, TANIGUCHI W, CHEN QY, et al. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nat Commun. 2018;9:1886. [22] LÜTOLF R, ROSNER J, CURT A, et al. Indicators of central sensitization in chronic neuropathic pain after spinal cord injury. Eur J Pain. 2022;26:2162-2175. [23] MA YC, KANG ZB, SHI YQ, et al. The Complexity of Neuropathic Pain and Central Sensitization: Exploring Mechanisms and Therapeutic Prospects. J Integr Neurosci. 2024;23:89. [24] FINNERUP NB, BAASTRUP C. Spinal cord injury pain: mechanisms and management. Curr Pain Headache Rep. 2012;16:207-216. [25] 贾延劼,李燕飞,陈雪梅.脊髓损伤后神经病理性疼痛的研究现状[J].内科理论与实践,2017,12(2):92-96. [26] SONG Y, XUE T, GUO S, et al. Inhibition of aquaporin-4 and its subcellular localization attenuates below-level central neuropathic pain by regulating astrocyte activation in a rat spinal cord injury model. Neurotherapeutics. 2024;21:e00306. [27] 阮婷婷,翁明奇,吴璨,等.认知行为疗法和虚拟现实疗法干预神经性疼痛的作用及机制[J].生物化学与生物物理进展, 2023,50(10):2396-2405. [28] YAO D, CHEN Y, CHEN G. The role of pain modulation pathway and related brain regions in pain. Rev Neurosci. 2023;34:899-914. [29] MELZACK R. From the gate to the neuromatrix. Pain. 1999;Suppl 6: S121-s126. [30] HUANG J, GADOTTI VM, CHEN L, et al. A neuronal circuit for activating descending modulation of neuropathic pain. Nat Neurosci. 2019;22:1659-1668. [31] JUTZELER CR, HUBER E, CALLAGHAN MF, et al. Association of pain and CNS structural changes after spinal cord injury. Sci Rep. 2016;6:18534. [32] WANG F, TIAN ZC, DING H, et al. A sensory-motor-sensory circuit underlies antinociception ignited by primary motor cortex in mice. Neuron. 2025;113(12):1947-1968.e7. [33] 邱义,马炜玮,张会娟,等.经颅电刺激镇痛研究的现状及展望[J].生物化学与生物物理进展,2024,51(5):1119-1133. [34] XIE L, WU H, CHEN Q, et al. Divergent modulation of pain and anxiety by GABAergic neurons in the ventrolateral periaqueductal gray and dorsal raphe. Neuropsychopharmacology. 2023;48: 1509-1519. [35] ONI-ORISAN A, KAUSHAL M, LI W, et al. Alterations in Cortical Sensorimotor Connectivity following Complete Cervical Spinal Cord Injury: A Prospective Resting-State fMRI Study. PLoS One. 2016;11: e0150351. [36] HARRIS AJ. Cortical origin of pathological pain. Lancet. 1999;354:1464-1466. [37] 严传婷,杜宜楠,韩静,等.神经递质和调质参与导水管周围灰质痛觉调控的研究进展[J].生物化学与生物物理进展, 2021,48(2):158-170. [38] 李雪静,陈楠.脊髓损伤后神经性疼痛患者大脑结构和功能变化的MRI研究进展[J].中国医学影像技术,2018,34(12):1889-1892. [39] SOLER MD, KUMRU H, PELAYO R, et al. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain. 2010;133:2565-2577. [40] POZEG P, PALLUEL E, RONCHI R, et al. Virtual reality improves embodiment and neuropathic pain caused by spinal cord injury. Neurology. 2017;89:1894-1903. [41] TROST Z, ANAM M, SEWARD J, et al. Immersive interactive virtual walking reduces neuropathic pain in spinal cord injury: findings from a preliminary investigation of feasibility and clinical efficacy. Pain. 2022;163:350-361. [42] SABALETTE P, DUBÉ N, MÉNARD P, et al. Immediate effect of alone and combined virtual reality, gait-like muscle vibration and transcranial direct current stimulation on neuropathic pain after spinal cord injury: a pilot study. Spinal Cord Ser Cases. 2024;10:83. [43] CHI B, CHAU B, YEO E, et al. Virtual reality for spinal cord injury-associated neuropathic pain: Systematic review. Ann Phys Rehabil Med. 2019;62:49-57. [44] DIERS M, CHRISTMANN C, KOEPPE C, et al. Mirrored, imagined and executed movements differentially activate sensorimotor cortex in amputees with and without phantom limb pain. Pain. 2010; 149:296-304. [45] RICHARDSON EJ, MCKINLEY EC, RAHMAN A, et al. Effects of virtual walking on spinal cord injury-related neuropathic pain: A randomized, controlled trial. Rehabil Psychol. 2019;64:13-24. [46] 吉海波,李永奎,邢叔星.虚拟行走对脊髓损伤相关神经病理性疼痛的影响[J].颈腰痛杂志,2022,43(3):398-400. [47] LANDMANN G, AERNI M, ABÄCHERLI R, et al. Virtual walking therapy in neuropathic spinal cord injury pain: a feasibility study. Spinal Cord Ser Cases. 2024;10:53. [48] AKCAY G, NEMUTLU SAMUR D, DERIN N. Transcranial direct current stimulation alleviates nociceptive behavior in male rats with neuropathic pain by regulating oxidative stress and reducing neuroinflammation. J Neurosci Res. 2023; 101:1457-1470. [49] ANG JY, LEONG EL, CHAN HK, et al. Health-related quality of life of Malaysian patients with chronic non-malignant pain and its associated factors: a cross-sectional study. BMC Musculoskelet Disord. 2022;23:400. [50] VILLIGER M, BOHLI D, KIPER D, et al. Virtual reality-augmented neurorehabilitation improves motor function and reduces neuropathic pain in patients with incomplete spinal cord injury. Neurorehabil Neural Repair. 2013;27:675-683. [51] AZURDIA D, ACUÑA SM, NARASAKI-JARA M, et al. Effects of Virtual Reality-Based Aerobic Exercise on Perceptions of Pain and Fatigue in Individuals with Spinal Cord Injury. Games Health J. 2022;11:236-241. [52] MELZACK R, WALL PD. Pain mechanisms: a new theory. Science. 1965;150:971-979. [53] O’KANE SH, CHANCEL M, EHRSSON HH. Hierarchical and dynamic relationships between body part ownership and full-body ownership. Cognition. 2024;246:105697. [54] HOFFMAN HG, FONTENOT MR, GARCIA-PALACIOS A, et al. Adding tactile feedback increases avatar ownership and makes virtual reality more effective at reducing pain in a randomized crossover study. Sci Rep. 2023;13:7915. [55] ADHAM A, BESSAGUET H, STRUBER L, et al. Distinct and additive effects of visual and vibratory feedback for motor rehabilitation: an EEG study in healthy subjects. J Neuroeng Rehabil. 2024;21:158. [56] ADHAM A, LE BT, BONNAL J, et al. Neural basis of lower-limb visual feedback therapy: an EEG study in healthy subjects. J Neuroeng Rehabil. 2024;21:114. [57] 王彩霞,黄媛馨,王林.运动疗法在慢性疼痛中的研究进展[J].中国疼痛医学杂志,2024,30(4):296-301. [58] SHEN J, GU X, FU J, et al. Virtual reality-induced motor function of the upper extremity and brain activation in stroke: study protocol for a randomized controlled trial. Front Neurol. 2023;14:1094617. [59] NAMBI G, ALGHADIER M, KASHOO FZ, et al. Effects of Virtual Reality Exercises versus Isokinetic Exercises in comparison with Conventional Exercises on the Imaging Findings and Inflammatory Biomarker Changes in Soccer Players with Non-Specific Low Back Pain: A Randomized Controlled Trial. Int J Environ Res Public Health. 2022; 20(1):524. [60] ROUSSEAUX F, PANDA R, TOUSSAINT C, et al. Virtual reality hypnosis in the management of pain: Self-reported and neurophysiological measures in healthy subjects. Eur J Pain. 2023;27:148-162. [61] HU XS, BEARD K, SHERBEL MC, et al. Brain Mechanisms of Virtual Reality Breathing Versus Traditional Mindful Breathing in Pain Modulation: Observational Functional Near-infrared Spectroscopy Study. J Med Internet Res. 2021;23:e27298. [62] MA B, ZHANG L, JI Y, et al. The benefits and safety of a virtual reality intervention in patients suffering from acute and chronic pain: A pilot study. Digit Health. 2025;11:20552076241308703. [63] HÖLZEL BK, CARMODY J, VANGEL M, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011;191:36-43. [64] ROUSSEAUX F, BICEGO A, LEDOUX D, et al. Hypnosis Associated with 3D Immersive Virtual Reality Technology in the Management of Pain: A Review of the Literature. J Pain Res. 2020;13:1129-1138. [65] CARDINAL É, AUGIER P, GIGUÈRE É, et al. Combining Hypnosis and Virtual Reality: A Qualitative Investigation of User Experience During an Experimental Pain Study. J Clin Psychol Med Settings. 2025;32(2):336-346. [66] TROST Z, FRANCE C, ANAM M, et al. Virtual reality approaches to pain: toward a state of the science. Pain. 2021; 162:325-331. [67] AUSTIN PD, CRAIG A, MIDDLETON JW, et al. The short-term effects of head-mounted virtual-reality on neuropathic pain intensity in people with spinal cord injury pain: a randomised cross-over pilot study. Spinal Cord. 2021;59:738-746. [68] ROOSINK M, ROBITAILLE N, JACKSON PL, et al. Interactive virtual feedback improves gait motor imagery after spinal cord injury: An exploratory study. Restor Neurol Neurosci. 2016;34:227-235. [69] LAVER KE, LANGE B, GEORGE S, et al. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev. 2017;11: Cd008349. [70] ÖZKUL Ç, KILINÇ M, YILDIRIM SA, et al. Effects of visual illusion and transcutaneous electrical nerve stimulation on neuropathic pain in patients with spinal cord injury: A randomised controlled cross-over trial. J Back Musculoskelet Rehabil. 2015;28:709-719. [71] MODIRIAN E, PIROUZI P, SOROUSH M, et al. Chronic pain after spinal cord injury: results of a long-term study. Pain Med. 2010;11:1037-1043. [72] KUNER R, FLOR H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci. 2017;18:113. [73] SIMÓN-VICENTE L, RODRÍGUEZ-CANO S, DELGADO-BENITO V, et al. Cybersickness. A systematic literature review of adverse effects related to virtual reality. Neurologia (Engl Ed). 2024;39:701-709. |
| [1] | Yin Yongcheng, Zhao Xiangrui, Yang Zhijie, Li Zheng, Li Fang, Ning Bin. Effect and mechanism of peroxiredoxin 1 in microglial inflammation after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1106-1113. |
| [2] | Zheng Peng, Jia Xiaoning, Tao Jingwei, Fan Xiao. Tetramethylpyrazine improves iron metabolism disorders in a rat model of spinal cord injury via the Keap-1/Nrf2 signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 6134-6141. |
| [3] | Meng Zhuo, Zhao Renghao, Zhang Anqi, Hua Haotian, Wang Zicheng, Xu Yingtian, Tong Peijian. Literature visualization analysis of brain-computer interface applications in stroke rehabilitation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4802-4813. |
| [4] | Fu Yingxue, Wang Xianbin, Chen Xingyu, Wu Shuang. Hind limb muscle atrophy in rats with spinal cord injury: effects of different rehabilitation therapy strategies [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4357-4365. |
| [5] | Gao Shiai, Yu Zifu, Chen Jinhui, Cao Xinyan, Leng Xiaoxuan, Liu Xihua. Efficacy of non-invasive neuromodulation techniques on autism spectrum disorder: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2550-2559. |
| [6] | Fang Jun, Wei Wei, Xue Yating, Cui Chenlong, Wei Jiasheng, Shi Xiao, Yang Lijuan, Yang Baozhong. M2 macrophage-derived exosomes promote microglia M2-type polarization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5320-5327. |
| [7] | Zhang Shuyang, Du Xinyu, Zhao Donglin, Xing Zheng, Chu Xiaolei, Li Qi. Application of virtual reality technology in functional recovery of peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(21): 4593-4601. |
| [8] | Wang Qianliang, Chen Jianpeng, Wang Yuanbin, Yan Jun. Mechanism of circ05188 targeting miR-199a-5p involved in nociceptive hypersensitivity in a rat model of lumbar disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4230-4238. |
| [9] | Zhao Dun, Qi Lingchen, Xu Jinfan, Shao Min. Visual analysis of hotspots and frontiers in knee osteoarthritis pain field [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(15): 3280-3289. |
| [10] | Lyu Lijie, Yuan Yiming, Wang Yan, , Pei Fei, . Relationship between ferroptosis and peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(14): 3020-3026. |
| [11] | Jiang Yong, Guan Tianmin, Ci Yuan, Zhu Ye, Zhao Peng, Zheng Jiafa, Yang Tao, Zhang Guangyu. Application of mixed reality technology in vertebroplasty [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(30): 4812-4816. |
| [12] | Wang Shijuan, Chen Wensheng, Tang Hui. Effect of biotic amniotic membrane and corneal bandage lens on corneal wound repair after pterygium surgery [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(17): 2642-2646. |
| [13] | Liu Guangwei, Feng Minshan, Zhu Liguo, Yin Xunlu, Chen Zhuoxian. Dynamic analysis of structural changes in the lower cervical intervertebral foramen during rotation-traction manipulation by virtual reality technology [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1346-1351. |
| [14] | Hu Yue, Zhu Yuanliang, Wan Tenggang, Xu Fangyuan, Xu Zhangyu, Li Jiyang, Li Dan, Wang Jianxiong. Depression-like behavior characteristics of rats with neuropathic pain and expression of mGluR5 and NMDAR2B in the left dorsal agranular insular area [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(20): 3216-3223. |
| [15] | Zhao Feng, Fan Shaoqing, Cheng Xiaoyan, Li Xiaona, Li Changsheng, Ma Haojie. miR-132-3p targets and modulates Ptch1 to reduce neuropathic pain in mice with chronic constriction injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 230-236. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||