Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (22): 5814-5831.doi: 10.12307/2026.179
Previous Articles Next Articles
Role of fibrosis in tissue injury repair
Li Feihong1, Wang Linrong1, 2, 3, Cheng Leping1, 2, 3
- 1Translational Medicine Research Center, Guangxi Medical University; Key Laboratory of Longevity and Aging-related Diseases, Ministry of Education, Nanning 530021, Guangxi Zhuang Autonomous Region, China; 2Institute of Neuroscience, Basic Medical College, Guangxi Medical University; Guangxi Key Laboratory of Brain Sciences, Key Laboratory of Basic Research on Brain Function and Brain Diseases (Guangxi Medical University), Health Commission of Guangxi Zhuang Autonomous Region, Nanning 530021, Guangxi Zhuang Autonomous Region, China; 3Collaborative Innovation Centre of Regenerative Medicine and Medical BioResource Development and Application Co-constructed by the Province and Ministry, Guangxi Key Laboratory of Regenerative Medicine, Nanning 530021, Guangxi Zhuang Autonomous Region, China
-
Received:2025-06-07Accepted:2025-08-12Online:2026-08-08Published:2025-12-27 -
Contact:Cheng Leping, MD, Professor, Master’s supervisor, Doctoral supervisor, Translational Medicine Research Center, Guangxi Medical University; Key Laboratory of Longevity and Aging-related Diseases, Ministry of Education, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Institute of Neuroscience, Basic Medical College, Guangxi Medical University; Guangxi Key Laboratory of Brain Sciences, Key Laboratory of Basic Research on Brain Function and Brain Diseases (Guangxi Medical University), Health Commission of Guangxi Zhuang Autonomous Region, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Collaborative Innovation Centre of Regenerative Medicine and Medical BioResource Development and Application Co-constructed by the Province and Ministry, Guangxi Key Laboratory of Regenerative Medicine, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
About author:Li Feihong, MS candidate, Translational Medicine Research Center, Guangxi Medical University; Key Laboratory of Longevity and Aging-related Diseases, Ministry of Education, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 32070976 (to CLP); Guangxi Science and Technology Base and Talent Special Project, No. AD21075052 (to CLP)
CLC Number:
Cite this article
Li Feihong, Wang Linrong, Cheng Leping. Role of fibrosis in tissue injury repair[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5814-5831.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

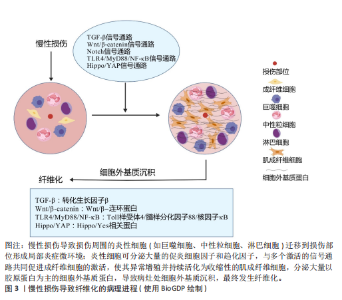
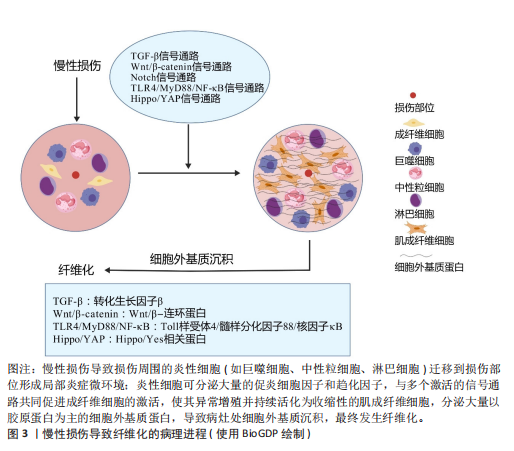
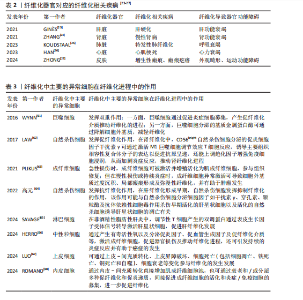
2.1 纤维化疾病的研究历程 见表1。 2.2 器官纤维化 纤维化的一个共同特征是成纤维细胞的持续活化和细胞外基质的过度沉积,有多个细胞和通路参与纤维化过程,见图3。目前研究较为深入的纤维化器官包括肝脏、肾脏、肺脏、心脏和皮肤,不同器官纤维化相关疾病见表2。 2.2.1 肝脏纤维化 肝纤维化由常见的3种导致慢性肝损伤的病因引起,分别是嗜肝病毒(主要是乙型和丙型肝炎病毒)的慢性感染、慢性中毒性损伤(主要是饮酒过量)以及代谢相关损伤,如非酒精性脂肪性肝病及进行性非酒精性脂肪性肝炎[7]。持续累积的严重肝损伤导致广泛的肝纤维化、肝功能障碍和窦状血管异常,最终导致肝硬化[42]。作为全球第11大死亡原因和第14大发病原因[43],肝硬化进一步发展导致肝细胞癌和肝脏代偿失调,包括腹水、肝性脑病和静脉曲张出血[44],由于再生肝结节取代正常的肝脏结构,最终导致肝功能衰竭[23]。 2.2.2 肾脏纤维化 慢性肾病是第九大死亡原因[45],而肾纤维化是几乎所有类型的慢性肾病的共同表现[46]。肾纤维化的主要病理特征为肾小管上皮-间充质细胞转化的形成、细胞外基质的过度沉积,伴随炎性细胞浸润及实质细胞的缺失[47],这些导致肾小球硬化和肾间质纤维化[11],最终肾功能衰竭[24]。 2.2.3 肺脏纤维化 肺纤维化是由多种病因引起的肺组织损伤,肺间质中胶原蛋白和其他细胞外基质的异常积累,导致肺组织硬化和肺功能下降的病理状态,主要影响肺间质[25]。其中,特发性肺纤维化是典型的进行性肺间质纤维化[12],是一种平均寿命为3-5年的致命疾病[48]。肺纤维化的主要致病机制包括上皮-间质转化、成纤维细胞大量增殖和细胞外基质过度沉积,形成纤维化灶[49],导致肺实质进行性硬化,气体交换能力逐渐减弱,肺功能持续下降[12]。肺纤维化患者表现为呼吸困难,生活质量不仅受到极"

大影响,最终还会引起呼吸衰竭并导致死亡[25]。 2.2.4 心脏纤维化 心肌纤维化是由于细胞外基质蛋白过度沉积导致心脏间质和/或血管周围空间的扩张[50],心室壁顺应性降低,心脏功能受到严重影响,最终导致心力衰竭[26]。心力衰竭是世界范围内日益增长的死亡率和发病率的原因[51]。心肌纤维化分为心肌梗死后局灶性纤维化瘢痕与弥漫性心肌纤维化[52]。心肌纤维化可由诸多因素刺激而诱发,包括心肌损伤、炎症、压力、代谢功能障碍、衰老或容量过载等。随着心脏僵硬度增加、顺应性降低、心脏收缩性降低,心脏代偿失调,最终发展为收缩期和/或舒张期心力衰竭[53]。由于心肌细胞增殖能力差[54],在心肌梗死的急性期,心肌细胞的大量丢失触发了一系列修复过程,成纤维细胞被激活为具有收缩能力的肌成纤维细胞,产生大量的细胞外基质,生成富含胶原蛋白的瘢痕来替代死亡的心肌细胞[26]。最初细胞外基质的沉积对于保持心室的结构完整性以防止心脏破裂至关重要[55],然而,瘢痕组织中成纤维细胞积累和重塑的增加可能会在成纤维细胞和残余心肌细胞之间产生新的偶联点,成纤维细胞膜电位的变化可引发心肌细胞的兴奋性改变及心律失常[56]。 2.2.5 皮肤纤维化 皮肤作为人体主要的外部屏障,是组织损伤的常见部位[57]。损伤后,皮肤会经历复杂的创伤修复反应,主要分为止血、炎症、增殖和重塑4个阶段,以促进伤口愈合,涉及多种细胞分泌不同的细胞因子和其他生物分子[27]。其中,合成和沉积新胶原以促进创面修复的主要是成纤维细胞及其活化的肌成纤维细胞[13]。通常情况下,创伤修复后会留下瘢痕,然而,若炎症和感染持续存在可能导致皮肤纤维化的发生,表现为异常的瘢痕形成,主要特征是肉芽组织中的成纤维细胞持续增殖,并伴随细胞外基质的过度沉积[58]。在皮肤纤维化过程中,结缔组织中常出现过度交联的胶原纤维基质和较低的细胞密度,这不仅导致皮肤生物力学性能受损(如皮肤变得僵硬、失去弹性),还与皮肤附件(如毛囊、汗腺等)的缺乏紧密相关,进而影响皮肤的正常功能,如感觉、调节和保护作用[13]。异常的瘢痕主要有增生性瘢痕与瘢痕疙瘩两种类型,增生性瘢痕只填满创面,不超过原发病灶的边界,瘢痕疙瘩则会扩大,超出原发病灶的边界[59],两者均呈突起和色素沉着,血管化和细胞增多,这些瘢痕常伴随疼痛、瘙痒和挛缩等功能性症状[60],并可能导致外观畸形和运动功能障碍,严重影响患者的生活质量及心理健康[27]。 2.3 纤维化中主要发生异常的细胞 见表3。 2.3.1 免疫细胞 巨噬细胞是介导炎症反应和纤维化疾病的关键细胞[69],它们包括组织常驻巨噬细胞和血液源性单核细胞源性巨噬细胞[70]。当组织受到炎症刺激后,巨噬细胞被招募到损伤部位并极化为经典激活的M1型和交替激活的M2型[71-72]。M1型巨噬细胞出现在炎症早期,具有促炎作用,可产生大量的促炎细胞因子和趋化因子(包括肿瘤坏死因子α和白细胞介素6)以及病原体相关分子模式,诱发炎症和组织损伤[73]。在炎症后期,M1型巨噬细胞可转变为具有抗炎和促进组织修复作用的M2型,它可分为4种不同的亚型:M2a、M2b、M2c、M2d。其中,M2a型分泌白细胞介素10、转化生长因子β、胰岛素样生长因子、纤连蛋白、CC家族趋化因子配体22、CC家族趋化因子配体17等,促进创面愈合和纤维化;M2b型分泌白细胞介素1、白细胞介素6、CC家族趋化因子配体1、CC家族趋化因子配体20等物质,具有有效的抗炎和免疫调节特性;M2c型可分泌转化生长因子β、白细胞介素10、基质金属蛋白酶、CC家族趋化因子配体18等,同时可发挥吞噬、免疫抑制、血管生成、组织纤维化发展等作用;M2d是一种被Toll样受体激活的巨噬细胞,特异性表达血管内皮生长因子和白细胞介素10,也被称为肿瘤相关巨噬细胞,参与血管生成和肿瘤进展[74]。总之,M2型巨噬细胞参与辅"

助性T细胞2型免疫反应、寄生虫清除和炎症抑制等过程,它们还具有很强的吞噬能力,清除碎片和凋亡细胞,促进组织修复和伤口愈合、血管生成和纤维化[75-77]。在纤维化器官中,比如在肝脏和肾脏中巨噬细胞表现出双重作用[78-79]:一方面,巨噬细胞通过促进炎症细胞募集、产生促纤维化介质推动纤维化的进程;另一方面,巨噬细胞分泌的基质金属蛋白酶可通过降解细胞外基质,减轻纤维化[61]。 中性粒细胞是先天免疫反应的第一反应者和重要组成部分[66],来源于骨髓中的造血干细胞[80]。中性粒细胞在组织中的作用具有双重性,它们可通过吞噬作用和形成细胞外陷阱来防止感染并清除病原体,从而发挥保护作用;另一方面,它们通过产生有毒活性氧,分泌促炎因子、促血管生成因子及促纤维化介质等,激活成纤维细胞,促进器官损伤及推动纤维化进程。此外,这些因子还可引发持续的炎症反应甚至促进癌症的发生[66]。 淋巴细胞包括T细胞和B细胞,在炎症反应和免疫调节过程中发挥重要作用[81]。调节性T细胞产生双调蛋白促进急性组织损伤后的修复,而在慢性疾病——非酒精性脂肪性肝炎中,双调蛋白通过表皮生长因子受体信号转导激活肝星状细胞,使它活化为肝肌成纤维细胞,促进肝纤维化发展[65]。 其他免疫细胞也参与纤维化进程,如自然杀伤细胞在纤维化中有双重作用。在肾纤维化中, CD56bright自然杀伤细胞分泌的促炎细胞因子干扰素γ可通过激活M1巨噬细胞、调节效应T细胞反应、诱导主要组织相容性复合体分子表达以促进抗原呈递,还能上调趋化因子增强免疫细胞浸润,从而加剧炎症反应,推动肾纤维化进程[62]。研究表明,在肝纤维化形成早期,自然杀伤细胞发挥抑制纤维化的作用,该作用可能与自然杀伤细胞分泌细胞因子如干扰素γ、穿孔素、颗粒酶及抗体依赖性细胞毒作用杀伤早期活化的肝星状细胞以及活化的自然杀细胞诱导肝星状细胞的凋亡有关[64]。 2.3.2 成纤维细胞 成纤维细胞为具有产生结缔组织能力的间充质细胞,由形态相似但具有特定功能的高度异质性间充质细胞群体组成。除了在维持组织细胞外基质稳态方面发挥重要作用外[82],成纤维细胞对调节炎症、癌症进展和血管生成等生物过程也至关重要[57]。在组织损伤时,成纤维细胞受到多种因素刺激(包括生化信号、机械因素以及表观遗传修饰作用等)而被激活为肌成纤维细胞,参与组织修复。其中,生化信号包括转化生长因子β1、白细胞介素4、白细胞介素6、白细胞介素11、白细胞介素13、血管紧张素Ⅱ、内皮素1、血小板源性生长因子、溶血磷脂酸受体1和Toll样受体4等[83]。在机械因素(比如皮肤的拉伸、压缩、扭转)中,机械应力刺激成纤维细胞激活转化为肌成纤维细胞,从而合成新的胶原蛋白并将其重塑为具有机械稳定性的瘢痕组织的关键因素[84]。尽管成纤维细胞的可塑性可能给检测和分类带来干扰,但这正是其关键特征。成纤维细胞激活可能引发一系列功能表现,包括增殖和迁移能力增强、细胞外基质分泌与交联、胶原蛋白及其他细胞外基质成分的降解、吞噬作用,甚至基础免疫功能。同时,只有具备收缩的功能和特征的成纤维细胞才被视为肌成纤维细胞[16]。 作为一种表型,而非独特的细胞类型[82],肌成纤维细胞在修复过程中发挥关键作用,主要通过以下方式:高表达α-平滑肌肌动蛋白;合成和分泌细胞外基质成分,尤其是胶原蛋白;分泌整合素及多种细胞因子,如血管内皮生长因子、血小板源性生长因子和转化生长因子β[85]。随后,肌成纤维细胞面临3种结局:失活变成低活性成纤维细胞;变成瘢痕或老化细胞;通过凋亡的方式被大量淘汰[86]。虽然肌成纤维细胞的功能在急性损伤修复中至关重要,但它们在慢性损伤或持续炎症时的异常激活可导致细胞外基质过度沉积、局部瘢痕形成及弥漫性纤维化,甚至有助于肿瘤发生[63]。与成纤维细胞一样,肌成纤维细胞也具有异质性,在不同的器官其来源有所区别,心脏肌成纤维细胞主要由心肌成纤维细胞激活活化而来[52],而在肝脏主要由活化的肝星状细胞和门静脉成纤维细胞活化而来[87]。也有一些研究表明,在急性肝损伤中,造血干细胞被认为是肝肌成纤维细胞的主要来源[88]。无论损伤发生在身体的哪个部位,绝大多数的肌成纤维细胞前体是间充质来源,最常见的间充质肌成纤维细胞前体包括真正的成纤维细胞[89]、脂肪细胞[90]、血管平滑肌细胞[91]、周细胞、多能间充质基质或干细胞[92]。此外,在特定条件下,以组织特异性的方式对肌成纤维细胞池作出贡献的细胞有上皮细胞[93](通过上皮-间充质转化)、血管内皮细胞[94](通过内皮-间充质转化)以及巨噬细胞[95],在这些细胞类型中,成纤维细胞向肌成纤维细胞的活化被认为是肌成纤维细胞最重要的来源[85]。 2.3.3 上皮细胞 上皮-间充质转化是指上皮细胞通过特定程序转化为具有间质表型细胞的生物学过程,被认为是肌成纤维细胞的重要来源。病理条件下的上皮-间充质转化可导致正常上皮细胞减少、组织正常结构遭到破坏,同时胶原纤维的产生增多[96],在这一过程中,上皮细胞会丧失原有的极性、细胞间连接力和细胞骨架结构[97]。上皮-间充质转化分为3种亚型,其中2型上皮-间充质转化与组织修复反应(如纤维化)有关。在持续的慢性炎症中,2型上皮-间充质转化促进肌成纤维细胞异常形成并引发进行性纤维化,过度的细胞外基质沉积导致器官实质破坏[98]。除上皮-间充质转化外,上皮屏障功能受损后紧密连接蛋白和黏附连接蛋白表达下调,细胞极性丧失,上皮屏障被破坏可直接导致上皮通透性改变,刺激物或细胞因子作用于间质,直接与间质反应导致纤维化[67]。此外,上皮细胞死亡(包括细胞凋亡、铁死亡、铜死亡和自噬等多种形式)中上皮细胞凋亡是纤维化中的关键步骤[67]。在肺纤维化过程中,转化生长因子β可通过诱导2型肺泡上皮细胞的凋亡介导其促纤维化作用[99]。衰老细胞通过分泌多种可溶性因子(包括细胞因子、生长因子和基质金属蛋白酶)来影响附近的细胞,这称为衰老相关分泌表型[100]。获得衰老相关分泌表型的2型肺泡上皮细胞释放了许多炎症和促纤维化因子,这些因子在纤维化微环境中通过自分泌和旁分泌作用促进肺纤维化[99]。综上所述,上皮细胞可通过上皮-间充质转化、上皮屏障破坏、细胞死亡(包括细胞凋亡、铁死亡、铜死亡和自噬)、细胞衰老等变化参与纤维化的发生发展[67]。 2.3.4 内皮细胞 在生理条件下,血管内皮细胞支持成纤维细胞的发育和再生,而在外源性刺激下,内皮细胞被激活,血管完整性被破坏,直接引发或促进已经存在的炎症反应[101]。内皮细胞通过分泌结缔组织生长因子、纤溶酶原激活物抑制剂1、骨桥蛋白、纤连蛋白和成纤维细胞特异性蛋白1来促进炎症细胞的募集,引起炎症反应[102]。在转化生长因子β等促纤维化介质驱动下,内皮细胞通过内皮-间充质转化成为肌成纤维细胞来源之一[40] ,同时衰老的内皮细胞也可以通过内皮-间充质转化促进器官纤维化[103]。研究表明,内皮细胞中过量表达转录因子Sox9除了可通过诱导细胞外基质、生长因子和炎症基因表达促进基质沉积,还可通过激活邻近成纤维细胞使这些成纤维细胞迁移并沉积基质,这一过程部分由分泌的生长因子CCN2(Sox9的直接靶标)介导;而在内皮细胞中敲除Sox9则能减轻心脏、肺和肝脏的纤维化,改善器官功能[104]。此外,缺氧诱导的内皮细胞来源外泌体中含有赖氨酰氧化酶样蛋白2,它通过激活成纤维细胞增加胶原交联和组织硬度,导致纤维化组织特有的僵硬度增加[102]。因此,内皮细胞可通过内皮-间充质转化直接增加肌成纤维细胞池,也可通过衰老和/或分泌多种促纤维化和促炎递质,间接促进成纤维细胞的活化和炎症/免疫细胞的募集,进一步促进纤维化[68]。 2.4 纤维化过程中发生异常的主要信号通路 见表4。 在纤维化进程中,各信号通路可通过促进或抑制靶基因的表达进一步促进纤维化的发展,详细机制见图4。 2.4.1 转化生长因子β信号通路 转化生长因子β家族是纤维化相关疾病的关键细胞因子,包括转化生长因子β1、转化生长因子β2和转化生长因子β3三种亚型。其中,转化生长因子β1是已知最早被描述且最有效诱导肌成纤维细胞激活的促纤维化因子之一,它与受体结合后可通过激活经典信号通路——转化生长因子β1/Smads信号通路诱导Smad2和Smad3的磷酸化,随后与Smad4形成Smad2-Smad3-Smad4复合物,并进入细胞核与促纤维化基因启动子中的Smad结合元件结合增强其转录[123]。转化生长因子β通路在器官纤维化(如肝纤维化[87]、心肌纤维化)的发展中起关键作用[124]。此外,Smad2-Smad3-Smad4复合物还与其他因子共同作用,例如与Yes相关蛋白(yes associated protein,YAP)和具有PDZ结合结构域的转录共激活因子(transcriptional co-activator with PDZ - binding motif,TAZ)、心肌蛋白相关的转录因子A、T细胞因子和Wnt信号相关淋巴增强因子等共同作用,形成共转录因子,促进与肌成纤维细胞相关的基因转录,例如编码α-平滑肌肌动蛋白和Ⅰ型胶原蛋白的基因,这些基因的表达上调促使纤维化蛋白的合成[82]。除经典的Smad依赖性信号通路外,转化生长因子β受体还可通过非经典——非Smad依赖信号通路,如丝裂原活化蛋白激酶信号通路、p38/c-Jun氨基末端激酶信号通路、磷脂酰肌醇3-激酶/蛋白激酶B/雷帕霉素靶蛋白信号通路、Ras同源基因家族蛋白A鸟苷三磷酸酶信号通路等,促进与细胞外基质相关基因的表达,进一步调控纤维化过程[125]。例"
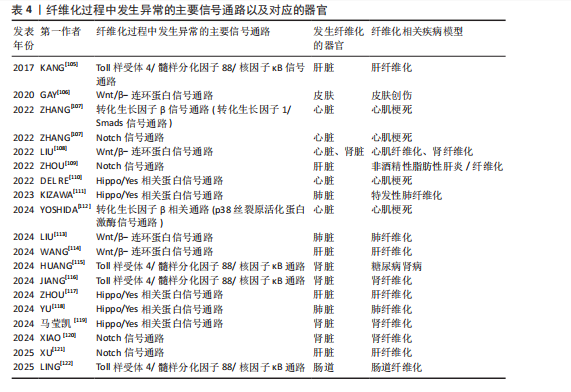
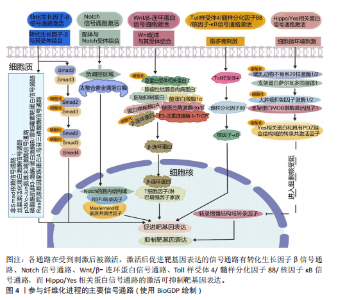
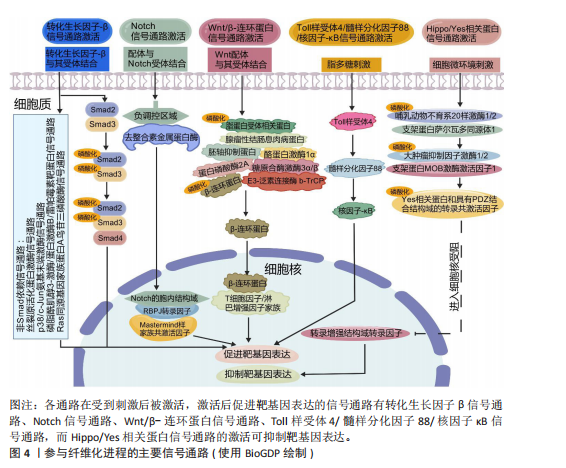
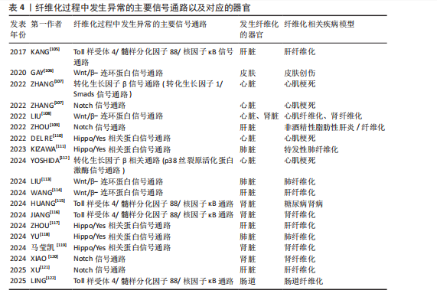
如,心脏成纤维细胞中的蛋白激酶N通过激活p38丝裂原活化蛋白激酶信号通路促进成纤维细胞向肌成纤维细胞的活化,增强心肌梗死后Ⅰ型及Ⅲ型胶原蛋白的分泌和细胞外基质的合成[112]。在系统性硬化症、硬化性皮肤、慢性移植物抗宿主病和特发性肺纤维化等纤维化疾病中,Wnt5a/c-Jun氨基末端激酶/Rho相关卷曲螺旋蛋白激酶信号通过快速激活潜伏的转化生长因子β刺激成纤维细胞活化为肌成纤维细胞,促进组织纤维化[126]。 2.4.2 Wnt/β-连环蛋白信号通路 在发育进化和癌症治疗领域,Wnt信号通路被认为是一种基本的生长调节通路[107]。Wnt信号通路在胚胎发育过程中通过时空特异性激活调控成纤维细胞的分化命运,并在成年组织中通过动态平衡成纤维细胞活性参与组织修复与病理性纤维化的双向调控。Wnt信号通路分为两类:β-连环蛋白依赖性信号传导(经典通路)、β-连环蛋白非依赖性信号传导(非经典通路)。经典Wnt/β-连环蛋白通路通常高度保守,通过自分泌/旁分泌方法将细胞外Wnt配体与膜受体结合而被激活[108]。Wnt与其受体的结合诱导胚轴抑制蛋白与磷酸化脂蛋白受体相关蛋白的结合,阻止胚轴蛋白与腺瘤性结肠息肉病蛋白、酪蛋白激酶1α和糖原合酶激酶3α/β、蛋白磷酸酶2A以及E3-泛素连接酶 b-TrCP组成的复合体对β-连环蛋白的磷酸化与泛素化,降低了β-连环蛋白在蛋白酶体中的降解,胞质中聚集增多的β-连环蛋白转入细胞核,随后在细胞核中结合T细胞因子/淋巴增强因子家族的转录因子以上调靶基因的表达[127]。 近年研究表明,Wnt信号通路在组织损伤中被特异性激活以驱动局部修复;然而,该信号通路持续活化可通过异常调控细胞增殖与纤维化进程最终造成器官功能障碍或病理性重塑。比如在皮肤创伤修复过程中,肌成纤维细胞中短暂激活的Wnt活性可促进新生毛囊的再生愈合[63],而Wnt持续性激活导致纤维化反应和毛发再生失败[106]。短暂的Wnt/β-连环蛋白激活刺激急性肾损伤后的组织再生,而持续(不受控制的)Wnt/β-连环蛋白信号传导促进慢性肾病、足细胞损伤和蛋白尿、急性肾损伤和囊性肾病期间持续组织损伤的肾纤维化[108]。典型Wnt信号通路通过乳酸脱氢酶A/缺氧诱导因子1α转录复合物促进星状细胞糖酵解和肝纤维化。肝星状细胞特异性敲低乳酸脱氢A可减轻小鼠肝星状细胞活化和肝纤维化[114]。m6A修饰导致的DKK3上调激活Wnt/β-连环蛋白信号通路,增加线粒体分裂因子转录表达,引发线粒体功能障碍和氧化应激,从而促进肾纤维化进展[128]。Wnt信号通路除了独立作用,还可与转化生长因子β信号通路相互作用,在肌成纤维细胞的活化过程中发挥关键作用[107]。比如在肺纤维化中,Wnt/β-连环蛋白信号通路可与转化生长因子β1协同作用增强β-连环蛋白驱动的纤维化。Wnt/β-连环蛋白信号通路诱导上皮-间充质转化,从而调节组织修复和细胞外基质成分(如纤连蛋白和基质金属蛋白酶)的沉积[113]。在心肌纤维化中,Wnt/β-连环蛋白信号通路为转化生长因子β介导的关键下游分子通路。受到转化生长因子β刺激后,Wnt分泌并通过转化生长因子激酶1信号通路激活Wnt/β-连环蛋白信号通路,促进成纤维细胞活化为肌成纤维细胞,从而导致心肌纤维化[108]。 2.4.3 Notch信号通路 Notch信号通路在胚胎发育早期活跃[109],但在发育成熟的机体组织中,如正常成年肾脏和肝细胞中Notch活性维持在较低水平[109,129]。然而在组织损伤或应激条件下,Notch信号通路可被迅速激活,对干细胞和祖细胞的自我更新、分化命运选择、细胞间相互作用以及组织器官的形态发生和稳态维持至关重要[109]。配体的结合使Notch受体的近膜负调控域发生构象变化,随后经去整合素金属蛋白酶和γ-分泌酶复合体切割,释放Notch胞内结构域进入细胞核,与转录因子RBPJ及Mastermind样(MAML)家族的共激活因子形成Notch转录激活复合体,激活靶基因表达[130]。在发生心肌梗死时,Notch信号通路通过多种机制参与调控心肌损伤的修复过程,通过诱导干细胞分化为心肌修复提供新的细胞来源,通过促进血管生成以改善心肌的血液供应;此外,Notch信号通路还可通过减轻心肌纤维化、减少心肌细胞凋亡和降低氧化应激等机制进一步改善心脏再生能力,保护心脏功能[107]。而Notch信号的异常激活会导致纤维化的进一步发展,比如在非酒精性脂肪性肝炎/纤维化患者中,由细胞间Toll样受体4触发JAG1/Notch信号通路诱导Notch活性显著升高,而Notch激活的肝细胞通过调控相关基因的表达最终激活肝星状细胞,诱导肝纤维化[109]。研究发现,源自中性粒细胞的Notch信号通路可促进中性粒细胞胞外陷阱的形成,从而加剧非酒精性脂肪性肝炎期间的细胞衰老和脂毒性,进一步导致肝脂肪变性和纤维化[121]。HOXA5通过直接结合其基因启动子抑制JAG1的转录,抑制肾纤维化过程中的JAG1/Notch信号。然而高甲基化可导致HOXA5缺失,同时高甲基化激活JAG1表达及Notch信号传导,进而促进肾纤维化[120]。 2.4.4 Toll样受体4/髓样分化因子88/核因子κB信号通路 Toll样受体4是免疫系统和炎症反应的关键调节因子[96]。Toll样受体包含一个高度保守的受体家族,可识别病原体相关的分子模式,并允许宿主检测微生物感染[131]。当机体受到微生物如革兰阴性菌感染时,细菌细胞壁的成分脂多糖可诱导Toll样受体4的激活,并招募其下游的关键分子——髓样分化因子88,随即触发一系列复杂的信号级联反应,最终导致下游核因子κB的激活[132],产生促炎细胞因子,包括白细胞介素1β、白细胞介素6和肿瘤坏死因子α[133]。在肝纤维化中,Toll样受体4通过调节转化生长因子β信号通路以及增强由转化生长因子β或库普弗细胞诱导的肝星状细胞活化,促进肝脏炎症和纤维化[131]。研究表明,在饮食诱导的肥胖小鼠中,慢性间歇性缺氧诱导该小鼠肝纤维化的发生,这种现象与Toll样受体4介导的丝裂原活化蛋白激酶和核因子κB信号传导有关[105]。Toll样受体4/髓样分化因子88/核因子κB信号通路的激活是导致肾功能受损的重要机制之一。研究表明,链脲佐菌素诱导的Toll样受体4?/?糖尿病小鼠肾损伤减轻,包括白蛋白尿减少、系膜基质扩张和肾小球纤维化减轻、基质蛋白和炎症因子丰度降低。因此,下调Toll样受体4/髓样分化因子88/核因子κB信号通路可以延缓糖尿病肾病的纤维化和炎症反应[115]。在肠道纤维化中,Toll样受体4被确定为肠道慢性炎症和纤维化的重要媒介,可影响肌成纤维细胞活性[134]。此外,许多细胞外基质蛋白可以作为Toll样受体4配体,从而引发免疫应答。研究表明,Tenascin-C与Toll样受体4的结合可诱导胶原合成和白细胞介素6和转化生长因子β的分泌[135]。研究表明,雌激素受体β可通过抑制Ⅰ型胶原蛋白、纤维连接蛋白、α-平滑肌肌动蛋白和N-钙黏蛋白的表达减轻转化生长因子β诱导的成纤维细胞活化和迁移,可能通过调控Toll样受体4/髓样分化因子88/核因子κB信号通路和转化生长因子β/Smad信号通路调控肠道纤维化[122]。溴氰菊酯是一种Ⅱ型拟除虫菊酯杀虫剂,研究发现溴氰菊酯暴露增加了内质网应激介导的TXNDC5的表达,通过激活Toll样受体4/髓样分化因子88/核因子κB信号通路和转化生长因子β/Smad信号通路,导致小鼠肾脏炎症和纤维化[116]。 2.4.5 Hippo/YAP信号通路 Hippo信号通路在调节器官大小和维持组织稳态方面发挥关键作用[107]。经典Hippo/YAP信号通路是一种进化上保守的蛋白质激酶级联反应,主要由细胞质中的调节激酶模块和细胞核中的转录模块构成[136]。在微环境信号刺激下,Hippo/YAP信号通路被激活,哺乳动物不育系20样激酶1/2与支架蛋白萨尔瓦多同源体1形成异源二聚体,随后磷酸化大肿瘤抑制因子激酶1/2及其共激活因子支架蛋白MOB激酶激活因子1,进而激活磷酸化YAP和TAZ,磷酸化的YAP和TAZ随后被滞留在细胞质中,并通过泛素化途径降解。当Hippo/YAP信号通路被激活时会触发一系列磷酸化级联反应,最终抑制YAP和TAZ,阻止YAP和TAZ转位到细胞核与转录增强结构域转录因子家族结合,继而阻断转录增强结构域转录因子介导的基因表达[137]。Hippo/YAP信号调节成纤维细胞活化和分化,并能影响多种器官组织损伤后纤维化的程度[110]。Hippo信号通路可以与转化生长因子β、Wnt信号通路协同促进纤维化[111],YAP和TAZ还可受到机械信号的刺激而被激活,独立于转化生长因子β信号传导调节肺成纤维细胞基因的表达促进纤维化的发展,机械激活的YAP和TAZ和纤溶酶原激活物抑制物1加强基质刚度并形成一个正反馈回路,导致持续性纤维化发生[111]。此外,在心肌梗死后3-5 d YAP在心脏成纤维细胞中上调,心肌成纤维细胞YAP可诱导并增强心脏中与损伤相关的纤维化和炎症性重塑。对YAP和TAZ两个基因的靶向抑制可防止心脏功能障碍,并减少损伤后的纤维化[110]。病理性血管生成可通过促进胶原积累而加剧肝纤维化。血管紧张素Ⅱ是肝纤维化进展过程中内皮-间充质转化的关键调节因子,内皮细胞中YAP活性的变化可以影响血管紧张素Ⅱ对血管生成的调节,抑制YAP活性可显著减弱血管紧张素Ⅱ对内皮细胞的调节作用,表明血管紧张素Ⅱ依赖于Hippo/YAP信号转导来重组血管生成并促进肝纤维化[117]。研究表明,百草枯致肺纤维化与肺上皮细胞衰老、YAP/TAZ有关,YAP和TAZ的激活促进衰老的肺上皮细胞逃避凋亡,衰老的肺上皮细胞持续分泌衰老相关分泌表型因子促进纤维化。靶向YAP和TAZ可以通过减少促纤维化下游基因表达以及诱导衰老的肺上皮细胞凋亡,减轻肺纤维化[118]。 2.5 表观遗传学在纤维化中的作用 表观遗传学是指不改变DNA序列的情况下通过化学修饰或染色质结构重塑等方式调控基因表达和活性的生物学机制。研究表明,虽然一些表观遗传标记非常稳定,但在实际研究中确实发现一些表观遗传标记并不稳定。例如,环境因素对表观遗传修饰的影响至关重要,在胚胎发育和个体生长过程中,环境暴露会导致表观遗传标记发生改变。产前和围产期环境对整个生命中的表观遗传标记和基因表达有显著影响,在生命后期,环境因素持续作用于表观基因组。同卵双胞胎随着年龄增长,DNA甲基化组会出现多样化,这说明环境干扰使得部分表观遗传修饰难以稳定遗传[138]。因此,表观遗传调控可以是可遗传的,即在细胞分裂过程中传递给子代细胞,也可以是不可遗传的,仅在一定环境或发育条件下短暂影响基因表达[138],这些调控方式主要包括DNA甲基化、组蛋白修饰和非编码RNA调控,它们在细胞命运决定、组织分化、环境适应性以及疾病的发生发展中发挥关键作用。 2.5.1 DNA甲基化 DNA甲基化由DNA甲基化转移酶催化,将甲基基团添加到DNA的CpG岛中的胞嘧啶第5位碳原子上,形成5-甲基胞嘧啶。启动子区域的高甲基化通常抑制基因转录,而基因的编码区域的低甲基化一般促进基因表达[139]。研究表明,在肾纤维化过程中,转化生长因子β受体3、Smad3和Smad6等关键基因的甲基化状态变化可导致其表达水平异常,进而参与纤维化信号通路的失调。此外,基因RASAL1(RAS protein activator-like 1)的转录抑制与其启动子区域的高甲基化密切相关,这促进了肌成纤维细胞的持续活化,从而加剧了肾纤维化的病理进程[129]。 2.5.2 组蛋白修饰 染色质的基本结构是核小体,由DNA和组蛋白组成。组蛋白修饰是调节纤维化基因表达的重要表观遗传机制,包括甲基化、乙酰化、磷酸化、泛素化等。其中,甲基化和乙酰化在纤维化的发病机制中研究最为深入,组蛋白甲基化由组蛋白甲基转移酶催化,组蛋白乙酰化由组蛋白乙酰转移酶和组蛋白去乙酰化酶共同调节。此外,组蛋白甲基化促进/抑制基因转录取决于修饰的氨基酸位点、甲基化状态及其基因组定位。组蛋白乙酰化与基因转录的激活相关。溴结构域和末端外结构域蛋白家族与组蛋白N端尾部的乙酰化赖氨酸结合,招募其他调控因子,形成转录起始复合物,从而促进基因转录激活。研究表明,转化生长因子β/Smads信号通路通过激活组蛋白乙酰转移酶,调控促纤维化基因Ⅰ型胶原α1和Ⅲ型胶原α1的表达,进而驱动细胞外基质的过度沉积和纤维化进程[140]。 2.5.3 非编码RNA 微小核糖核酸(miRNA)主要通过抑制mRNA的翻译和促进其降解来负向调控基因表达[139],它在纤维化器官中具有促纤维化和抗纤维化双重作用。研究表明,miRNA 21在多种器官纤维化中发挥促纤维化作用,而miRNA 29家族在心脏、肝脏、肺等纤维化中起抗纤维化作用[141]。其他非编码RNA如长链非编码RNA和环状RNA对miRNA有内源性竞争作用,它们能作为miRNA海绵结合miRNA,保留miRNA结合位点。因为被结合后的miRNA无法再结合到它们的靶基因上,以达到竞争性地抑制miRNA的转录调控来调节miRNA活性的目的[142]。 2.6 细胞外基质交联调节 在纤维化过程中,活化的肌成纤维细胞会过度分泌Ⅱ型和Ⅲ型胶原蛋白以及纤连蛋白和弹性蛋白,这些蛋白质的异常积累可通过调控细胞外基质重塑酶,如基质金属蛋白酶、去整合素金属蛋白酶和astacin家族金属蛋白酶来实现动态平衡。基质金属蛋白酶和去整合素金属蛋白酶的活性受组织金属蛋白酶抑制剂家族蛋白的调控,从而防止细胞外基质的过度降解[143]。细胞外基质积累和重塑之间的失衡被认为是纤维化反复出现的特征。除了降解酶的调控外,细胞外基质蛋白还会受到赖氨酰氧化酶、赖氨酸羟化酶和转谷氨酰胺酶影响。其中,赖氨酰氧化酶家族包括赖氨酰氧化酶和4个赖氨酰氧化酶样蛋白。赖氨酰氧化酶活性在纤维化进程中发挥关键作用[22],该家族需依赖分泌的铜离子对细胞外基质中的胶原蛋白和弹性蛋白进行翻译后修饰,进而促使纤维发生共价交联,这是维持胶原稳定性的必要条件[1]。赖氨酸羟化酶虽然不直接参与交联反应,但它的活性对赖氨酰氧化酶介导的交联反应至关重要。这些酶通过将胶原上的赖氨酸转化为羟赖氨酸形成许多交联位点促进交联。此外,转谷氨酰胺酶家族中转谷氨酰胺酶2表达最广泛,转谷氨酰胺酶2不仅能介导交联反应,还能通过其蛋白质结合能力促进纤维化[22]。 2.7 抗纤维化治疗方式以及对应的靶点 见表5。"


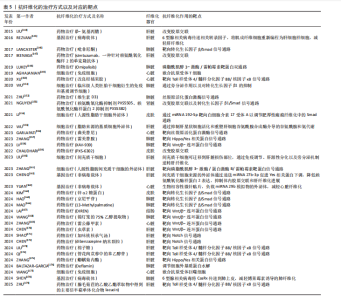
2.7.1 药物治疗 靶向转化生长因子β信号通路:药物吡非尼酮是已获得批准用于治疗特发性肺纤维化的药物之一[146],吡非尼酮通过靶向经典的转化生长因子β信号通路抑制Smad3磷酸化,进一步抑制促纤维化介质的合成、转化生长因子β1的表达以及成纤维细胞的增殖和活化,发挥抗纤维化作用[125]。此外,靶向非经典转化生长因子β信号通路中磷脂酰肌醇3-激酶的药物Omipalisib,是一种磷脂酰肌醇3-激酶/雷帕霉素靶蛋白双通路抑制剂,它降低了转化生长因子β1诱导的胶原积累,抑制肺成纤维细胞增殖和胶原蛋白积累,减弱原代人肺成纤维细胞和体外特发性肺纤维化肺切片中成纤维细胞增殖和转化生长因子β诱导的胶原合成[148]。靶向丝裂原活化蛋白激酶信号通路的药物曲美替尼,通过抑制细胞外调节蛋白激酶的磷酸化显著减少转化生长因子β1的激活,进而下调Ⅰ型胶原α1和Ⅲ型胶原α1基因以及POSTN和ACTA2纤维化基因的表达,这样可改善非再灌注心肌梗死小鼠的心肌功能,防止纤维化重构[156]。研究表明,在博来霉素诱导的肺纤维化小鼠模型中,维生素D3通过下调转化生长因子β激活的成纤维细胞中PSAT1的表达,显著抑制p38和细胞外调节蛋白激酶1/2的磷酸化水平,从而靶向阻断丝裂原活化蛋白激酶信号通路的激活,降低胶原特异性标志物羟脯氨酸的含量,抑制Ⅰ型胶原蛋白的合成及过度沉积,调控细胞外基质重塑过程,具有改善肺纤维化病理进程的潜在治疗价值[152]。需要注意的是,转化生长因子β1是强大的促纤维化细胞因子,长期抑制转化生长因子β1产生可能加速细胞增殖和肿瘤发生[81],因此,通过局部或位点特异性调节转化生长因子β相关分子或信号通路来抑制纤维化的方法更可取[180]。锌α2糖蛋白可有效抑制转化生长因子β/Smad通路,抑制瘢痕形成过程中细胞过度增殖,提示它对瘢痕疙瘩和增生性瘢痕具有潜在的治疗意义[164]。研究表明,京尼平苷与转化生长因子β1、Smad2、Smad3、p38具有良好的结合活性,它可以通过抑制转化生长因子β/Smad和p38丝裂原活化蛋白激酶信号通路显著下调肺纤维化组织中转化生长因子β1、Smad2/3、p38、结缔组织生长因子的表达,改善博莱霉素诱导的小鼠肺纤维化[165]。13-Methylpalmatine通过抑制成纤维细胞分化显示出良好的抗纤维化潜力,与现有的抗纤维化药物不同,13-Methylpalmatine特异性靶向ITGA5/转化生长因子β/Smad信号通路,提供了一种新颖且可能更有效的治疗方法[166]。 靶向Wnt/β-连环蛋白信号通路:研究发现,小分子化合物XAV939能选择性抑制β-连环蛋白介导的转录,作用机制是通过稳定降解复合物的限速组分——胚轴抑制蛋白促进β-连环蛋白的降解;采用定量化学蛋白质组学方法进一步揭示,XAV939通过抑制多聚ADP-核糖基化酶tankyrase1和tankyrase2来维持胚轴抑制蛋白稳定性,这两种tankyrase亚型均能与胚轴抑制蛋白高度保守结构域相互作用,通过泛素-蛋白酶体途径促进其降解[181]。研究表明,XAV-939通过抑制Wnt信号通路逆转二氧化硅诱导的硅肺中上皮-间充质转化,减轻肺纤维化[158]。DIREN通过调节Wnt/β-连环蛋白信号通路抑制成纤维细胞迁移,减少胶原分泌,从而改善葡聚糖硫酸钠诱导的结肠纤维化[167]。研究发现,中药锦灯笼的体积分数75%乙醇提取物可通过抑制炎症反应和细胞外基质沉积改善博来霉素诱导的肺纤维化,同时通过减少β-连环蛋白核积聚并促进其磷酸化来抑制Wnt/β-连环蛋白通路[168]。雷公藤甲素抑制Wnt/β-连环蛋白信号通路,降低胶原蛋白的表达,抑制小鼠体外心脏成纤维细胞的增殖和分化,从而减轻心功能损伤和心肌组织病理损伤[169]。虫草素通过抑制Sox9介导的Wnt/β-连环蛋白信号通路中β-连环蛋白的核易位来减弱Wnt/β-连环蛋白通路的激活,进而抑制肝星状细胞的活化并减轻糖尿病相关肝纤维化[170]。 靶向Notch信号通路:加味桃核承气汤是中药桃核承气汤的改良制剂,可通过抑制Notch信号通路抑制肝星状细胞活化和调节巨噬细胞向M2表型极化,同时降低M1表型,最终发挥抗肝纤维化的作用[171]。研究表明,负载Notch抑制剂dibenzazepine 的胆红素与聚乙二醇链共价结合形成的活性氧响应型纳米颗粒,能在H2O2刺激下快速释放药物并有效清除肝细胞内的活性氧。dibenzazepine纳米颗粒可显著改善葡萄糖耐受不良,减轻肝脂质积累和炎症,改善非酒精性脂肪性肝炎诱导的肝纤维化;此外,dibenzazepine纳米颗粒没有明显的肝外不良反应[172]。 靶向Toll样受体4/髓样分化因子88/核因子κB信号通路:半乳糖凝集素3是一种心力衰竭患者纤维化和炎症相关的生物标志物。半乳糖凝集素3抑制剂改良柑橘果胶通过下调半乳糖凝集素3、Toll样受体4和髓样分化因子88表达抑制核因子κB-p65的激活,减轻心肌纤维化。改良柑橘果胶还降低促炎因子(白细胞介素1β、白细胞介素18和肿瘤坏死因子α)的表达来抑制炎症,改善心脏功能[150]。棉子糖通过抑制Toll样受体4/髓样分化因子88/核因子κB信号通路以及靶向核因子E2相关因子2改善非酒精性脂肪性肝病中脂质代谢紊乱诱导的细胞焦亡、炎症和纤维化[173]。腺毛菊苣是一种中药,lacucin是从腺毛菊苣的乙酸乙酯萃取物中得到的主要倍半萜单体化合物,lacucin可通过肠道菌群调节Toll样受体4/髓样分化因子88/丝裂原活化蛋白激酶/核因子κB信号通路减轻肝纤维化[179]。管花肉苁蓉中的苯乙醇苷,通过调节肠道菌群-肝脏轴有效降低血清脂多糖和肝脏脂多糖结合蛋白水平,抑制与肝脏蛋白表达相关的脂多糖/Toll样受体4/髓样分化因子88/核因子κB信号通路,减轻肝纤维化[174]。 靶向Hippo/YAP信号通路:抑制Hippo/YAP的下游YAP和TAZ信号可以减少器官纤维化。YAP和TAZ既能够单独发挥作用,也能够协同合作发挥作用,单敲除其中一个因子可以减少成纤维细胞的激活,双敲除可显著增强抗纤维化作用[182-183]。但有研究报道,YAP和TAZ似乎有不同的作用,在肺纤维化中,上皮细胞中过表达YAP可促进纤维化发展,而过表达TAZ则驱动肺泡上皮再生和纤维化消退,这表明靶向YAP而非TAZ介导的转录可能有助于促进1型肺泡上皮细胞再生和治疗肺纤维化[184]。蟛蜞菊内酯具有显著的抗肝纤维化作用,蟛蜞菊内酯通过抑制YAP和TAZ的表达显著减轻肝纤维化和肝损伤[175]。雷美替胺是一种美国食品药品监督管理局批准的褪黑激素受体激动剂,雷美替胺通过抑制Hippo通路核心组分YAP1从细胞质到细胞核的易位,减轻胶原沉积和肺纤维化[157]。 改变胶原交联:不可逆赖氨酰氧化酶抑制剂β-氨基丙腈通过抑制胶原的交联以减轻纤维化,加速纤维化消退[144,185]。使用抗赖氨酰氧化酶样蛋白2抗体simtuzumab抑制赖氨酰氧化酶样蛋白2可减少肝、肺纤维化及转化生长因子介导的肌成纤维细胞激活[147,186]。研究表明,赖氨酰氧化酶抑制剂(PXS-5505)或赖氨酰氧化酶样蛋白2抑制剂(PXS-5382)可通过抑制细胞外基质蛋白(α-平滑肌肌动蛋白、纤连蛋白和Ⅰ型胶原蛋白)、基质金属蛋白酶2、基质金属蛋白酶9、炎症标志物(肿瘤坏死因子α和单核细胞趋化蛋白1)以及转化生长因子β1/Smad信号通路的表达,减轻环孢素A导致的进行性肾病[153]。研究人员开发了不可逆的赖氨酰氧化酶抑制剂PXS-4787和PXS-6302,其中PXS-4787是有效的泛赖氨酰氧化酶抑制剂,PXS-6302稳定性更好且适合局部应用。在小鼠和猪的模型实验中,局部应用PXS-6302能减少皮肤纤维化、改善瘢痕外观且不降低组织强度,具有治疗瘢痕和其他纤维化疾病的潜力[159]。 调节细胞外基质蛋白水解:基质金属蛋白酶可降解细胞外基质蛋白,而组织金属蛋白酶抑制剂可抑制基质金属蛋白酶的活性。使用抗组织金属蛋白酶抑制剂抗体可下调组织金属蛋白酶抑制剂水平,促进细胞外基质蛋白降解,部分逆转肝纤维化;纤溶酶原激活剂也参与细胞外基质蛋白的降解,抑制纤溶酶原激活物抑制剂1可减少细胞外基质沉积[81]。基质金属蛋白酶相关机制在心肌梗死后的临床试验中具有一定的疗效,但基质金属蛋白酶长期受抑制会干扰胶原网络的生理功能[52]。最近的研究表明,从白羽扇豆中提取的蛋白质成分Deflamin在结肠腺癌和溃疡性结肠炎的动物模型中显著抑制基质金属蛋白酶9的催化活性,可减轻纤维化进程;同时,Deflamin可减轻小鼠慢性阻塞性肺疾病模型的肺气肿和支气管周围纤维化[176]。 2.7.2 细胞治疗 干细胞治疗:干细胞组织工程与再生医学备受关注[187-190]。研究表明,临床级人类胚胎干细胞衍生的免疫和基质调节细胞可通过静脉注射抑制肺损伤小鼠模型的肺部炎症和纤维化,并以剂量依赖的方式显著提高受体小鼠存活率,改善肺损伤的机制可能是免疫和基质调节细胞的旁分泌作用以及该细胞对转化生长因子β1的抑制,这种免疫和基质调节细胞优于原代的脐带间充质干细胞和吡非尼酮,在小鼠和猴子中具有出色的疗效和安全性[151]。研究表明,间充质干细胞可迁移到肝脏损伤部位,通过免疫调节、肝源性分化以及旁分泌机制逆转肝纤维化[160]。研究表明,人源性脂肪干细胞外泌体通过miRNA 192-5p靶向白细胞介素17受体A以调节肥厚性瘢痕纤维化中的Smad信号通路,减少胶原蛋白沉积、成纤维细胞向肌成纤维细胞的转分化以及增生性瘢痕的形成[154]。研究表明,脂肪来源的基质细胞外泌体可以通过抑制肝星状细胞活化和重塑肝细胞谷氨酰胺合成酶介导的谷氨酰胺和氨代谢,改善小鼠肝功能和减少细胞外基质沉积,有效改善肝纤维化[155]。研究发现,人源性脂肪间充质干细胞外泌体抗肝纤维化的关键机制是抑制磷脂酰肌醇3-激酶/蛋白激酶B/雷帕霉素靶蛋白信号通路,通过加速凋亡和阻滞G1期抑制活化的肝星状细胞增殖,有效抑制促纤维化蛋白的表达和上皮-间充质转化,减弱肝星状细胞的激活并抑制肝纤维化的进展[161]。同时,干细胞治疗的临床应用受到免疫排斥、潜在的癌变、治疗效果不稳定和过高的成本等挑战的阻碍[191]。 免疫细胞治疗:另一种细胞治疗近些年来被广泛研究——嵌合抗原受体-T细胞治疗。靶向成纤维细胞激活蛋白的嵌合抗原受体-T细胞被证明可以到达心脏,消融被激活的成纤维细胞,减少高血压心脏病小鼠模型中的胶原沉积[149]。研究表明,通过基因工程改造巨噬细胞使其表达靶向成纤维细胞激活蛋白的嵌合抗原受体——嵌合抗原受体-巨噬细胞,不仅在体外能有效吞噬表达成纤维细胞激活蛋白的心脏成纤维细胞,也能够迁徙至心肌损伤处并靶向吞噬成纤维细胞激活蛋白阳性肌成纤维细胞,进而显著减少心肌缺血再灌注损伤小鼠模型中心肌纤维化程度,改善心脏功能[177]。 2.7.3 基因治疗 病毒载体具有高效的基因传递能力,可以将目的基因导入靶细胞并实现长期稳定表达。腺相关病毒载体已被广泛应用于基因治疗研究,它具有安全性相对较高、免疫原性低等优点。在纤维化疾病的基因治疗中,腺相关病毒载体可用于将抗纤维化相关基因递送至纤维化组织的细胞中,以增强其抗纤维化作用。比如,气管内使用6型腺相关病毒作为载体将CasRx传递到肺上皮,通过同时降解编码胚轴抑制蛋白1和胚轴抑制蛋白2的mRNA可逆地激活Wnt信号,不仅能促进肺泡Ⅱ型细胞增殖和肺泡再生,还能减轻博莱霉素诱导的肺纤维化[178]。还有研究通过6型腺相关病毒载体传递相关转录因子将肌成纤维细胞重新编程为肝细胞样细胞,减轻肝纤维化[145]。但腺相关病毒载体的应用仍存在一定的局限性,比如潜在的免疫原性、较高的细胞毒性以及在血液循环和血清环境中的不稳定性,这些因素可能影响它的临床应用效果[192-193]。 为弥补病毒载体腺相关病毒的不足,非病毒载体的研究正悄然兴起。比如一种具有脂质双层膜的纳米级小泡——外泌体,作为天然载体,外泌体的优点是免疫原性低、细胞毒性低、循环稳定性高[194-195]。研究表明,间充质干细胞来源外泌体通过递送miRNA 27b-3p促进YAP下调,降低赖氨酰氧化酶样蛋白2表达,抑制体内胶原交联和肝纤维化进展[162]。值得注意的是,外泌体在体内存在半衰期短、组织滞留率低的特点,直接注射外泌体在心肌梗死治疗中的应用受到限制,导致疗效难以达到预期。研究表明,注射的外泌体很容易被冲洗掉,在心肌注射后3 h就无法检测到,这严重影响疗效[196]。已知miRNA 29b过表达可通过抑制转化生长因子β1/Smads信号通路达到抗纤维化的目 的[197]。为了克服外泌体缺陷的同时增加其疗效,研究采用基于明胶的生物相容性微针贴片作为递送平台,负载miRNA 29b模拟物的外泌体,以有效调控心肌梗死后心脏纤维化,从而减轻炎症、缩小梗死面积、改善心脏功能并抑制病理性纤维化的进展[163]。"

| [1] LEASK A, NAIK A,STRATTON RJ. Back to the future: targeting the extracellular matrix to treat systemic sclerosis. Nat Rev Rheumatol. 2023;19(11):713-723. [2] KHANAM A, SALEEB PG, KOTTILIL S. Pathophysiology and treatment options for hepatic fibrosis: can it be completely cured?. Cells. 2021;10(5):1097. [3] HUMPHREYS BD. Mechanisms of renal fibrosis. Annu Rev Physiol. 2018;80:309-326. [4] MOSS BJ, RYTER SW, ROSAS IO. Pathogenic mechanisms underlying idiopathic pulmonary fibrosis. Annu Rev Pathol. 2022; 17:515-546. [5] LIU M, LÓPEZ DE JUAN ABAD B,CHENG K. Cardiac fibrosis: myofibroblast-mediated pathological regulation and drug delivery strategies. Adv Drug Deliv Rev. 2021;173: 504-519. [6] GRIFFIN MF, DESJARDINS-PARK HE, MASCHARAK S, et al. Understanding the impact of fibroblast heterogeneity on skin fibrosis. Dis Model Mech. 2020;13(6):dmm044164. [7] PAROLA M,PINZANI M. Liver fibrosis in NAFLD/NASH: from pathophysiology towards diagnostic and therapeutic strategies. Mol Aspects Med. 2024;95: 101231. [8] TULETA I,FRANGOGIANNIS NG. Diabetic fibrosis. Biochim Biophys Acta Mol Basis Dis. 2021;1867(4):166044. [9] 陈柳燕,梁潘,覃武金,等.四方木皮提取物对皮肤急性创伤模型大鼠的治疗作用研究[J].药物评价研究,2025,48(4):844-855. [10] HORN P,TACKE F. Metabolic reprogramming in liver fibrosis. Cell Metab. 2024;36(7): 1439-1455. [11] CHEN C, ZHANG J, YU T, et al. LRG1 contributes to the pathogenesis of multiple kidney diseases: a comprehensive review. Kidney Dis (Basel). 2024;10(3):237-248. [12] GEORGE PM, SPAGNOLO P, KREUTER M, et al. Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir Med. 2020;8(9):925-934. [13] PEÑA OA,MARTIN P. Cellular and molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol. 2024;25(8):599-616. [14] HENDERSON NC, RIEDER F,WYNN TA. Fibrosis: from mechanisms to medicines. Nature. 2020;587(7835):555-566. [15] SOLIMAN AM,BARREDA DR. Acute inflammation in tissue healing. Int J Mol Sci. 2022;24(1):641. [16] SCHUSTER R, YOUNESI F, EZZO M, et al. The role of myofibroblasts in physiological and pathological tissue repair. Cold Spring Harb Perspect Biol. 2023;15(1):a041231. [17] PENG D, FU M, WANG M, et al. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21(1):104. [18] ENZO M, RASTRELLI M, ROSSI C, et al. The Wnt/β-catenin pathway in human fibrotic-like diseases and its eligibility as a therapeutic target. Mol Cell Ther. 2015; 3(1):1. [19] BAKALENKO N, KUZNETSOVA E,MALASHICHEVA A. The complex interplay of TGF-β and Notch signaling in the pathogenesis of fibrosis. Int J Mol Sci. 2024; 25(19):10803. [20] ABDEL-AZIZ AM, FATHY EM, HAFEZ HM, et al. TLR4/ MyD88/NF-κB signaling pathway involved in the protective effect of diacerein against lung fibrosis in rats. Hum Exp Toxicol. 2023;42:9603271231200213. [21] KIM CL, CHOI SH, MO JS. Role of the Hippo pathway in fibrosis and cancer. Cells. 2019; 8(5):468. [22] LLOYD SM,HE Y. Exploring extracellular matrix crosslinking as a therapeutic approach to fibrosis. Cells. 2024;13(5):438. [23] GINÈS P, KRAG A, ABRALDES JG, et al. Liver cirrhosis. Lancet. 2021;398(10308):1359-1376. [24] ZHANG R, JIANG J, YU Y, et al. Analysis of structural components of decellularized scaffolds in renal fibrosis. Bioact Mater. 2021;6(7):2187-2197. [25] KOUDSTAAL T, FUNKE-CHAMBOUR M, KREUTER M, et al. Pulmonary fibrosis: from pathogenesis to clinical decision-making. Trends Mol Med. 2023;29(12):1076-1087. [26] HAN M, LIU Z, LIU L, et al. Dual genetic tracing reveals a unique fibroblast subpopulation modulating cardiac fibrosis. Nat Genet. 2023;55(4):665-678. [27] ZHONG Y, ZHANG Y, LU B, et al. Hydrogel loaded with components for therapeutic applications in hypertrophic Scars and Keloids. Int J Nanomedicine. 2024;19:883-899. [28] GULL W. Clinical lecture on chronic bright’s disease with contracted kidney (arterio-capillary fibrosis). Br Med J. 1872; 2(626):707-709. [29] DUCKWORTH D. Clinical lecture on hypertrophic fibrosis of the liver. Br Med J. 1892;1(1618):1-2. [30] MACCALLUM JB. A contribution to the knowledge of the pathology of fragmentation and segmentation, and fibrosis of the myocardium. J Exp Med. 1899;4(3-4):409-424. [31] EMANUEL JG. A case of congenital obliteration of the bile ducts in which there was fibrosis of the pancreas and of the spleen. Br Med J. 1907;2(2433):385-387. [32] JEX-BLAKE AJ. Fibrosis of the left lung. Proc R Soc Med. 1909;2(Sect Study Dis Child): 194-196. [33] PARSONS JI. Fibrosis of the uterus causing persistent hæmorrhagia. Proc R Soc Med. 1910;3(Obstet Gynaecol Sect):240-241. [34] STOCKMAN R. The clinical symptoms and treatment of chronic subcutaneous fibrosis. Br Med J. 1911;1(2616):352-355. [35] JOHNSON RL, ZIFF M. Lymphokine stimulation of collagen accumulation. J Clin Invest. 1976;58(1):240-252. [36] KOVACS EJ, DIPIETRO LA. Fibrogenic cytokines and connective tissue production. FASEB J. 1994;8(11):854-861. [37] WYNN T. Cellular and molecular mechanisms of fibrosis. J Pathol. 2007;214(2):199-210. [38] ZEISBERG M,KALLURI R. Cellular mechanisms of tissue fibrosis. 1. common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol. 2013;304(3):C216-C225. [39] KIM KK, SHEPPARD D,CHAPMAN HA. TGF-β1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol. 2018;10(4):a022293. [40] LURJE I, GAISA NT, WEISKIRCHEN R, et al. Mechanisms of organ fibrosis: emerging concepts and implications for novel treatment strategies. Mol Aspects Med. 2023;92:101191. [41] GREENMAN R,WESTON CJ. CCL24 and fibrosis: a narrative review of existing evidence and mechanisms. Cells. 2025; 14(2):105. [42] TARU V, SZABO G, MEHAL W, et al. Inflammasomes in chronic liver disease: Hepatic injury, fibrosis progression and systemic inflammation. J Hepatol. 2024; 81(5):895-910. [43] SHARMA S, LE GUILLOU D, CHEN JY. Cellular stress in the pathogenesis of nonalcoholic steatohepatitis and liver fibrosis. Nat Rev Gastroenterol Hepatol. 2023;20(10):662-678. [44] HUANG DQ, TERRAULT NA, TACKE F, et al. Global epidemiology of cirrhosis — aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023;20(6):388-398. [45] ABEDINI A, LEVINSOHN J, KLÖTZER KA, et al. Single-cell multi-omic and spatial profiling of human kidneys implicates the fibrotic microenvironment in kidney disease progression. Nat Genet. 2024;56(8):1712-1724. [46] LIANG Z, TANG Z, ZHU C, et al. Intestinal CXCR6+ ILC3s migrate to the kidney and exacerbate renal fibrosis via IL-23 receptor signaling enhanced by PD-1 expression. Immunity. 2024;57(6):1306-1323. [47] NICULAE A, GHERGHINA ME, PERIDE I, et al. Pathway from acute kidney injury to chronic kidney disease: molecules involved in renal fibrosis. Int J Mol Sci. 2023;24(18):14019. [48] HETTIARACHCHI SU, LI YH, ROY J, et al. Targeted inhibition of PI3 kinase/mTOR specifically in fibrotic lung fibroblasts suppresses pulmonary fibrosis in experimental models. Sci Transl Med. 2020; 12(567):eaay3724. [49] HUANG Y, HONG W, WEI X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J Hematol Oncol. 2022;15(1):129. [50] YANG B, QIAO Y, YAN D, et al. Targeting Interactions between fibroblasts and macrophages to treat cardiac fibrosis. Cells. 2024;13(9):764. [51] WANG W, HU M, LIU H, et al. Global burden of disease study 2019 suggests that metabolic risk factors are the leading drivers of the burden of ischemic heart disease. Cell Metab. 2021;33(10):1943-1956. [52] LóPEZ B, RAVASSA S, MORENO MU, et al. Diffuse myocardial fibrosis: mechanisms, diagnosis and therapeutic approaches. Nat Rev Cardiol. 2021;18(7):479-498. [53] MA ZG, YUAN YP, WU HM, et al. Cardiac fibrosis: new insights into the pathogenesis. Int J Biol Sci. 2018;14(12):1645-1657. [54] SADEK H,OLSON EN. Toward the goal of human heart regeneration. Cell Stem Cell. 2020;26(1):7-16. [55] FRANGOGIANNIS NG. Cardiac fibrosis: cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med. 2019;65:70-99. [56] WANG Y, LI Q, TAO B, et al. Fibroblasts in heart scar tissue directly regulate cardiac excitability and arrhythmogenesis. Science. 2023;381(6665):1480-1487. [57] TALBOTT HE, MASCHARAK S, GRIFFIN M, et al. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell. 2022;29(8):1161-1180. [58] ZHANG N, XUE L, YOUNAS A, et al. Co-delivery of triamcinolone acetonide and verapamil for synergistic treatment of hypertrophic scars via carboxymethyl chitosan and Bletilla striata polysaccharide-based microneedles. Carbohydr Polym. 2022;284:119219. [59] JESCHKE MG, WOOD FM, MIDDELKOOP E, et al. Scars. Nat Rev Dis Primers. 2023;9(1):64. [60] MURAKAMI T,SHIGEKI S. Pharmacotherapy for Keloids and Hypertrophic Scars. Int J Mol Sci. 2024;25(9):4674. [61] WYNN TA, VANNELLA KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450-462. [62] LAW BMP, WILKINSON R, WANG X, et al. Interferon-γ production by tubulointerstitial human CD56bright natural killer cells contributes to renal fibrosis and chronic kidney disease progression. Kidney Int. 2017;92(1):79-88. [63] PLIKUS MV, WANG X, SINHA S, et al. Fibroblasts: origins, definitions, and functions in health and disease. Cell. 2021; 184(15):3852-3872. [64] 高元,章孝成,胡媛,等.自然杀伤细胞抑制血吸虫病肝纤维化作用的研究[J].中国寄生虫学与寄生虫病杂志,2022, 40(2):168-174. [65] SAVAGE TM, FORTSON KT, DE LOS SANTOS-ALEXIS K, et al. Amphiregulin from regulatory T cells promotes liver fibrosis and insulin resistance in non-alcoholic steatohepatitis. Immunity. 2024;57(2):303-318. [66] HERRO R,GRIMES HL. The diverse roles of neutrophils from protection to pathogenesis. Nat Immunol. 2024;25(12): 2209-2219. [67] LUO L, ZHANG W, YOU S, et al. The role of epithelial cells in fibrosis: mechanisms and treatment. Pharmacol Res. 2024;202: 107144. [68] ROMANO E, ROSA I, FIORETTO BS, et al. The contribution of endothelial cells to tissue fibrosis. Curr Opin Rheumatol. 2024; 36(1):52-60. [69] 单云,朱晓琳,唐蕾,等.巨噬细胞相关组织纤维化中细胞外囊泡的作用机制研究进展[J].细胞与分子免疫学杂志,2022, 38(5):466-471. [70] ALMEIDA L, DHILLON-LABROOY A, SPARWASSER T. The evolutionary tug-of-war of macrophage metabolism during bacterial infection. Trends Endocrinol Metab. 2024; 35(3):235-248. [71] LI CX, GONG ZC, YU JW. Deliberation concerning the role of M1-type macrophage subset in oral carcinogenesis. J Exp Clin Cancer Res. 2024;43(1):220. [72] YANG Q, GUO N, ZHOU Y, et al. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm Sin B. 2020;10(11):2156-2170. [73] CHEN Y, WANG T, LIANG F, et al. Nicotinamide phosphoribosyltransferase prompts bleomycin-induced pulmonary fibrosis by driving macrophage M2 polarization in mice. Theranostics. 2024; 14(7):2794-2815. [74] LUO M, ZHAO F, CHENG H, et al. Macrophage polarization: an important role in inflammatory diseases. Front Immunol. 2024;15:1352946. [75] WYNN TA, CHAWLA A, POLLARD JW. Macrophage biology in development, homeostasis and disease. Nature. 2013; 496(7446):445-455. [76] SHAPOURI‐MOGHADDAM A, MOHAMMADIAN S, VAZINI H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425-6440. [77] HORWOOD NJ. Macrophage polarization and bone formation: a review. Clin Rev Allergy Immunol. 2016;51(1):79-86. [78] 白小洋,张旭,海龙,等.巨噬细胞极化在肝纤维化中的调控作用机制[J].临床肝胆病杂志,2024,40(3):611-615. [79] CHEN H, LIU N, ZHUANG S. Macrophages in renal injury, repair, fibrosis following acute kidneyinjury and targeted therapy. Front Immunol. 2022;13:934299. [80] HUANG X, NEPOVIMOVA E, ADAM V, et al. Neutrophils in cancer immunotherapy: friends or foes? Mol Cancer. 2024;23(1):107. [81] KISSELEVA T, BRENNER D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18(3):151-166. [82] YOUNESI FS, MILLER AE, BARKER TH, et al. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat Rev Mol Cell Biol. 2024;25(8):617-638. [83] SCHUSTER R, ROCKEL JS, KAPOOR M, et al. The inflammatory speech of fibroblasts. Immunol Rev. 2021;302(1):126-146. [84] SAWANT M, HINZ B, SCHÖNBORN K, et al. A story of fibers and stress: Matrix‐embedded signals for fibroblast activation in the skin. Wound Repair Regen. 2021; 29(4):515-530. [85] ZHANG H, ZHOU Y, WEN D, et al. Noncoding RNAs: master regulator of fibroblast to myofibroblast transition in fibrosis. Int J Mol Sci. 2023;24(2):1801. [86] HINZ B, LAGARES D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol. 2020;16(1): 11-31. [87] YANG X, LI Q, LIU W, et al. Mesenchymal stromal cells in hepatic fibrosis/cirrhosis: from pathogenesis to treatment. Cell Mol Immunol. 2023;20(6):583-599. [88] RUSSELL JO, CAMARGO FD. Hippo signalling in the liver: role in development, regeneration and disease. Nat Rev Gastroenterol Hepatol. 2022;19(5):297-312. [89] JIANG DS, GUO RJ, MACHENS HG, et al. Diversity of fibroblasts and their roles in wound healing. Cold Spring Harb Perspect Biol. 2023;15(3):a041222. [90] SHOOK B, RIVERA GONZALEZ G, EBMEIER S, et al. The role of adipocytes in tissue regeneration and stem cell niches. Annu Rev Cell Dev Biol. 2016;32(1):609-631. [91] ZHAI M, LEI Z, SHI Y, et al. TEAD1-mediated trans-differentiation of vascular smooth muscle cells into fibroblast-like cells contributes to the stabilization and repair of disrupted atherosclerotic plaques. Adv Sci (Weinh). 2025;12(5):e2407408. [92] SOLIMAN H, THERET M, SCOTT W, et al. Multipotent stromal cells: one name, multiple identities. Cell Stem Cell. 2021; 28(10):1690-1707. [93] ROCK JR, BARKAUSKAS CE, CRONCE MJ, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011; 108(52):E1475-E1483. [94] ZHAO J, PATEL J, KAUR S, et al. Sox9 and Rbpj differentially regulate endothelial to mesenchymal transition and wound scarring in murine endovascular progenitors. Nat Commun. 2021;12(1):2564. [95] SINHA M, SEN CK, SINGH K, et al. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nat Commun. 2018;9(1):936. [96] ZHAO M, WANG L, WANG M, et al. Targeting fibrosis: mechanisms and clinical trials. Signal Transduct Target Ther. 2022;7(1):206. [97] 李贺生,李春婵,郭华慧,等.基于“肌成纤维细胞激活”及其信号通路探讨中药防治肾间质纤维化的研究进展[J].中草药,2025,56(8):2995-3004. [98] MARCONI GD, FONTICOLI L, RAJAN TS, et al. Epithelial-mesenchymal transition (EMT): the type-2 EMT in wound healing, tissue regeneration and organ fibrosis. Cells. 2021;10(7):1587. [99] PARIMON T, YAO C, STRIPP BR, et al. Alveolar epithelial type ii cells as drivers of lung fibrosis in idiopathic pulmonary fibrosis. Int J Mol Sci. 2020;21(7):2269. [100] RAMADHIANI R, IKEDA K, HIRATA KI, et al. Endothelial cell senescence exacerbates pulmonary fibrosis potentially through accelerated endothelial to mesenchymal transition. Kobe J Med Sci. 2021;67(3):E84-E91. [101] ZHOU X, ZHANG C, YANG S, et al. Macrophage-derived MMP12 promotes fibrosis through sustained damage to endothelial cells. J Hazard Mater. 2024;461: 132733. [102] SUN X, NKENNOR B, MASTIKHINA O, et al. Endothelium-mediated contributions to fibrosis. Semin Cell Dev Biol. 2020;101:78-86. [103] TSOU PS, SHI B, VARGA J. Role of cellular senescence in the pathogenesis of systemic sclerosis. Curr Opin Rheumatol. 2022;34(6):343-350. [104] TROGISCH FA, ABOUISSA A, KELES M, et al. Endothelial cells drive organ fibrosis in mice by inducing expression of the transcription factor SOX9. Sci Transl Med. 2024;16(736):eabq4581. [105] KANG HH, KIM IK, LEE HI, et al. Chronic intermittent hypoxia induces liver fibrosis in mice with diet-induced obesity via TLR4/MyD88/MAPK/NF-kB signaling pathways. Biochem Biophys Res Commun. 2017;490(2):349-355. [106] GAY D, GHINATTI G, GUERRERO-JUAREZ CF, et al. Phagocytosis of Wnt inhibitor SFRP4 by late wound macrophages drives chronic Wnt activity for fibrotic skin healing. Sci Adv. 2020;6(12):eaay3704. [107] ZHANG Q, WANG L, WANG S, et al. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct Target Ther. 2022;7(1):78. [108] LIU J, XIAO Q, XIAO J, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7(1):3. [109] ZHOU B, LIN W, LONG Y, et al. Notch signaling pathway: architecture, disease, and therapeutics. Signal Transduct Target Ther. 2022;7(1):95. [110] DEL RE DP. Hippo-Yap signaling in cardiac and fibrotic remodeling. Curr Opin Physiol. 2022;26:100492. [111] KIZAWA R, ARAYA J, FUJITA Y. Divergent roles of the Hippo pathway in the pathogenesis of idiopathic pulmonary fibrosis: tissue homeostasis and fibrosis. Inflamm Regen. 2023;43(1):45. [112] YOSHIDA S, YOSHIDA T, INUKAI K, et al. Protein kinase N promotes cardiac fibrosis in heart failure by fibroblast-to-myofibroblast conversion. Nat Commun. 2024;15(1):7638. [113] LIU D, GUAN Y. Mechanism of action of miR-15a-5p and miR-152-3p in paraquat-induced pulmonary fibrosis through Wnt/β-catenin signaling mediation. PeerJ. 2024; 12:e17662. [114] WANG F, CHEN L, KONG D, et al. Canonical wnt signaling promotes HSC glycolysis and liver fibrosis through an LDH-A/HIF-1α transcriptional complex. Hepatology. 2024;79(3):606-623. [115] HUANG L, LIN T, SHI M, et al. Liraglutide ameliorates inflammation and fibrosis by downregulating the TLR4/MyD88/NF-κB pathway in diabetic kidney disease. Am J Physiol Regul Integr Comp Physiol. 2024;327(4):R410-R422. [116] JIANG Z, YANG F, CAO H, et al. Deltamethrin exposure caused renal inflammation and renal fibrosis via upregulating endoplasmic reticulum stress-mediated TXNDC5 level in mice. Pestic Biochem Physiol. 2024;206: 106180. [117] ZHOU Y, LIANG P, BI T, et al. Angiotensin II depends on Hippo/YAP signaling to reprogram angiogenesis and promote liver fibrosis. Cell Signal. 2024;123:111355. [118] YU Y, CHU C, WANG K, et al. YAP/TAZ activation mediates PQ-induced lung fibrosis by sustaining senescent pulmonary epithelial cells. Respir Res. 2024;25(1):212. [119] 马莹凯,王永安,骆媛.Hippo/YAP信号通路在组织纤维化中的作用研究进展[J].中国药理学与毒理学杂志,2024, 38(11):859-871. [120] XIAO X, WANG W, GUO C, et al. Hypermethylation leads to the loss of HOXA5, resulting in JAG1 expression and NOTCH signaling contributing to kidney fibrosis. Kidney Int. 2024;106(1):98-114. [121] XU M, XU H, LING YW, et al. Neutrophil extracellular traps-triggered hepatocellular senescence exacerbates lipotoxicity in non-alcoholic steatohepatitis. J Adv Res. 2025:S2090-1232(25)00175-4. doi: 10.1016/j.jare.2025.03.015. [122] LING F, CHEN Y, LI J, et al. Estrogen receptor β activation mitigates colitis-associated intestinal fibrosis via Iinhibition of TGF-β/Smad and TLR4/MyD88/NF-κB signaling pathways. Inflamm Bowel Dis. 2025;31(1):11-27. [123] TU M, LU C, JIA H, et al. SULF1 expression is increased and promotes fibrosis through the TGF-β1/SMAD pathway in idiopathic pulmonary fibrosis. J Transl Med. 2024;22(1):885. [124] HANNA A, HUMERES C, FRANGOGIANNIS NG. The role of Smad signaling cascades in cardiac fibrosis. Cell Signal. 2021;77: 109826. [125] PARK CH, YOO TH. TGF-β inhibitors for therapeutic management of kidney fibrosis. Pharmaceuticals (Basel). 2022;15(12):1485. [126] TRINH-MINH T, CHEN CW, TRAN MANH C, et al. Noncanonical WNT5A controls the activation of latent TGF-β to drive fibroblast activation and tissue fibrosis. J Clin Invest. 2024;134(10):e159884. [127] MAURICE MM, ANGERS S. Mechanistic insights into Wnt–β-catenin pathway activation and signal transduction. Nat Rev Mol Cell Biol. 2025;26(5):371-388. [128] SONG J, CHEN Y, CHEN Y, et al. DKK3 promotes renal fibrosis by increasing MFF-mediated mitochondrial dysfunction in Wnt/β-catenin pathway-dependent manner. Ren Fail. 2024;46(1):2343817. [129] HUANG R, FU P, MA L. Kidney fibrosis: from mechanisms to therapeutic medicines. Signal Transduct Target Ther. 2023;8(1):129. [130] WANG H, ZANG C, LIU XS, et al. The Role of Notch Receptors in Transcriptional Regulation. J Cell Physiol. 2015;230(5): 982-988. [131] SEKI E, DE MINICIS S, ÖSTERREICHER CH, et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat Med. 2007;13(11): 1324-1332. [132] ZHENG XY, SUN CC, LIU Q, et al. Compound LM9, a novel MyD88 inhibitor, efficiently mitigates inflammatory responses and fibrosis in obesity-induced cardiomyopathy. Acta Pharmacol Sin. 2020;41(8):1093-1101. [133] ZHANG S, CHEN Q, JIN M, et al. Notoginsenoside R1 alleviates cerebral ischemia/reperfusion injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway through microbiota-gut-brain axis. Phytomedicine. 2024;128:155530. [134] D’ALESSIO S, UNGARO F, NOVIELLO D, et al. Revisiting fibrosis in inflammatory bowel disease: the gut thickens. Nat Rev Gastroenterol Hepatol. 2022;19(3):169-184. [135] LI L, FU H, LIU Y. The fibrogenic niche in kidney fibrosis: components and mechanisms. Nat Rev Nephrol. 2022;18(9): 545-557. [136] SUN M, SUN Y, FENG Z, et al. New insights into the Hippo/YAP pathway in idiopathic pulmonary fibrosis. Pharmacol Res. 2021; 169:105635. [137] ZHENG XH, WANG LL, ZHENG MZ, et al. RGFP966 inactivation of the YAP pathway attenuates cardiac dysfunction induced by prolonged hypothermic preservation. J Zhejiang Univ Sci B. 2020;21(9):703-715. [138] OSPELT C. A brief history of epigenetics. Immunol Lett. 2022;249:1-4. [139] ZHANG YS, TU B, SONG K, et al. Epigenetic hallmarks in pulmonary fibrosis: new advances and perspectives. Cell Signal. 2023;110:110842. [140] AGUADO-ALVARO LP, GARITANO N, PELACHO B. Fibroblast diversity and epigenetic regulation in cardiac fibrosis. Int J Mol Sci. 2024;25(11):6004. [141] O’REILLY S. Epigenetics in fibrosis. Mol Aspects Med. 2017;54:89-102. [142] LING H, WANG XC, LIU ZY, et al. Noncoding RNA network crosstalk in organ fibrosis. Cell Signal. 2024;124:111430. [143] BONNANS C, CHOU J,WERB Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014; 15(12):786-801. [144] LIU SB, IKENAGA N, PENG ZW, et al. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J. 2016;30(4):1599-1609. [145] REZVANI M, ESPAÑOL-SUÑER R, MALATO Y, et al. In vivo Hepatic reprogramming of myofibroblasts with AAV vectors as a therapeutic strategy for liver fibrosis. Cell Stem Cell. 2016;18(6):809-816. [146] LANCASTER LH, DE ANDRADE JA, ZIBRAK JD, et al. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur Respir Rev. 2017; 26(146):170057. [147] IKENAGA N, PENG ZW, VAID KA, et al. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut. 2017;66(9):1697-1708. [148] LUKEY PT, HARRISON SA, YANG S, et al. A randomised, placebo-controlled study of omipalisib (PI3K/mTOR) in idiopathic pulmonary fibrosis. Eur Respir J. 2019;53(3): 1801992. [149] AGHAJANIAN H, KIMURA T, RURIK JG, et al. Targeting cardiac fibrosis with engineered T cells. Nature. 2019;573(7774):430-433. [150] XU GR, ZHANG C, YANG HX, et al. Modified citrus pectin ameliorates myocardial fibrosis and inflammation via suppressing galectin-3 and TLR4/MyD88/NF-κB signaling pathway. Biomed Pharmacother. 2020;126:110071. [151] WU J, SONG D, LI Z, et al. Immunity-and-matrix-regulatory cells derived from human embryonic stem cells safely and effectively treat mouse lung injury and fibrosis. Cell Res. 2020;30(9):794-809. [152] ZHU W, DING Q, WANG L, et al. Vitamin D3 alleviates pulmonary fibrosis by regulating the MAPK pathway via targeting PSAT1 expression in vivo and in vitro. Int Immunopharmacol. 2021;101(Pt B):108212. [153] NGUYEN LT, SAAD S, SHI Y, et al. Lysyl oxidase inhibitors attenuate cyclosporin A-induced nephropathy in mouse. Sci Rep. 2021;11(1):12437. [154] LI Y, ZHANG J, SHI J, et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res Ther. 2021;12(1):221. [155] WU B, FENG J, GUO J, et al. ADSCs-derived exosomes ameliorate hepatic fibrosis by suppressing stellate cell activation and remodeling hepatocellular glutamine synthetase-mediated glutamine and ammonia homeostasis. Stem Cell Res Ther. 2022;13(1):494. [156] GARLAPATI V, MOLITOR M, MICHNA T, et al. Targeting myeloid cell coagulation signaling blocks MAP kinase/TGF-β1–driven fibrotic remodeling in ischemic heart failure. J Clin Invest. 2023;133(4):e156436. [157] ZHANG L, CHENG T, CHEN W, et al. Preventive effects of Ramelteon on bleomycin-induced pulmonary fibrosis in mice. Naunyn Schmiedebergs Arch Pharmacol. 2023;397(6):4153-4163. [158] LV Z, XU H, SI X, et al. XAV‐939 inhibits epithelial‐mesenchymal transformation in pulmonary fibrosis induced by crystalline silica via the Wnt signaling pathway. Environ Toxicol. 2023;38(2):460-471. [159] CHAUDHARI N, FINDLAY AD, STEVENSON AW, et al. Topical application of an irreversible small molecule inhibitor of lysyl oxidases ameliorates skin scarring and fibrosis. Nat Commun. 2022;13(1):5555. [160] LIU P, QIAN Y, LIU X, et al. Immunomodulatory role of mesenchymal stem cell therapy in liver fibrosis. Front Immunol. 2023;13:1096402. [161] ZHANG Z, SHANG J, YANG Q, et al. Exosomes derived from human adipose mesenchymal stem cells ameliorate hepatic fibrosis by inhibiting PI3K/Akt/mTOR pathway and remodeling choline metabolism. J Nanobiotechnology. 2023;21(1):29. [162] CHENG F, YANG F, WANG Y, et al. Mesenchymal stem cell-derived exosomal miR-27b-3p alleviates liver fibrosis via downregulating YAP/LOXL2 pathway. J Nanobiotechnology. 2023; 21(1):195. [163] YUAN J, YANG H, LIU C, et al. Microneedle patch loaded with exosomes containing microRNA‐29b prevents cardiac fibrosis after myocardial infarction. Adv Healthc Mater. 2023;12(13):e2202959. [164] KIM SH, OH JM, ROH H, et al. Zinc-alpha-2-glycoprotein peptide downregulates type I and III collagen expression via suppression of TGF-β and p-Smad 2/3 pathway in Keloid fibroblasts and rat incisional model. Tissue Eng Regen Med. 2024;21(7):1079-1092. [165] HAO J, YIN JB, WANG YX, et al. Geniposide ameliorates bleomycin-induced pulmonary fibrosis in mice by inhibiting TGF-β/Smad and p38MAPK signaling pathways. PLoS One. 2024;19(9):e0309833. [166] MAO Q, LIU J, YAN Y, et al. 13-Methylpalmatine alleviates bleomycin-induced pulmonary fibrosis by suppressing the ITGA5/TGF-β/Smad signaling pathway. Phytomedicine. 2025;140:156545. [167] LAI W, WANG Y, HUANG C, et al. DIREN mitigates dss-induced colitis in mice and attenuates collagen deposition via inhibiting the Wnt/β-catenin and focal adhesion pathways. Biomed Pharmacother. 2024;175:116671. [168] WANG T, XU LT, LI PP, et al. Physalis calyx seu fructus inhibited pulmonary fibrosis through regulating Wnt/β-catenin signaling pathway. Phytomedicine. 2024;131:155797. [169] ZHANG Y, LU F. Molecular mechanism of triptolide in myocardial fibrosis through the Wnt/β-catenin signaling pathway. Scand Cardiovasc J. 2024;58(1):2295785. [170] CHEN S, SUO J, WANG Y, et al. Cordycepin alleviates diabetes mellitus-associated hepatic fibrosis by inhibiting SOX9-mediated Wnt/β-catenin signal axis. Bioorg Chem. 2024;153:107812. [171] SHAO C, XU H, SUN X, et al. Jiawei Taohe Chengqi decoction inhibition of the notch signal pathway affects macrophage reprogramming to inhibit HSCs activation for the treatment of hepatic fibrosis. J Ethnopharmacol. 2024;321:117486. [172] CHEN J, ZHANG J, XIA Y, et al. Reactive oxygen species-responsive delivery of a notch inhibitor to alleviate nonalcoholic steatohepatitis by inhibiting Hepatic de novo lipogenesis and inflammation. Mol Pharm. 2024;21(6):2922-2936. [173] LIU J, ZHENG Y, YANG S, et al. Targeting antioxidant factor Nrf2 by raffinose ameliorates lipid dysmetabolism-induced pyroptosis, inflammation and fibrosis in NAFLD. Phytomedicine. 2024;130:155756. [174] QI X, SUN H, LIU J, et al. Phenylethanol glycoside from cistanche tubulosa attenuates BSA-induced liver fibrosis in rats by modulating the Gut Microbiota–Liver axis. Pharmaceuticals. 2024;17(9):1149. [175] ZHANG W, GAO K, BAI Y, et al. Wedelolactone attenuates liver fibrosis and hepatic stellate sell activation by suppressing the Hippo pathway. Rejuvenation Res. 2024;27(6):207-219. [176] BALTAZAR-GARCÍA EA, VARGAS-GUERRERO B, LIMA A, et al. Deflamin attenuated lung tissue damage in an ozone-induced COPD Murine model by regulating MMP-9 catalytic activity. Int J Mol Sci. 2024;25(10):5063. [177] WANG J, DU H, XIE W, et al. CAR-Macrophage therapy alleviates myocardial ischemia-reperfusion injury. Circ Res. 2024; 135(12):1161-1174. [178] SHEN S, WANG P, WU P, et al. CasRx-based wnt activation promotes alveolar regeneration while ameliorating pulmonary fibrosis in a mouse model of lung injury. Mol Ther. 2024;32(11):3974-3989. [179] ZHU L, LV B, GAO Y, et al. Lactucin alleviates liver fibrosis by regulating the TLR4-MyD88-MAPK/NF-κB signaling pathway through intestinal flora. Arch Biochem Biophys. 2025;766:110341. [180] HAMMERICH L, TACKE F. Hepatic inflammatory responses in liver fibrosis. Nat Rev Gastroenterol Hepatol. 2023;20(10):633-646. [181] HUANG SM, MISHINA YM, LIU S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009; 461(7264):614-620. [182] LINK PA, CHOI KM, DIAZ ESPINOSA AM, et al. Combined control of the fibroblast contractile program by YAP and TAZ. Am J Physiol Lung Cell Mol Physiol. 2022;322(1):L23-L32. [183] REGGIANI F, GOBBI G, CIARROCCHI A, et al. YAP and TAZ are not identical twins. Trends Biochem Sci. 2021;46(2):154-168. [184] LYU H, WARREN R, KLINKHAMMER K, et al. Hippo signaling impairs alveolar epithelial regeneration in pulmonary fibrosis. Elife. 2023;12:e85092. [185] POPOV Y, SVERDLOV DY, SHARMA AK, et al. Tissue transglutaminase does not affect fibrotic matrix stability or regression of liver fibrosis in mice. Gastroenterology. 2011;140(5):1642-1652. [186] BARRY-HAMILTON V, SPANGLER R, MARSHALL D, et al. Allosteric inhibition of lysyl oxidase–like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16(9):1009-1017. [187] WANG Y, ZHANG Y, LI T, et al. Adipose mesenchymal stem cell derived exosomes promote keratinocytes and fibroblasts embedded in collagen/platelet-rich plasma scaffold and accelerate wound healing. Adv Mater. 2023;35(40):e2303642. [188] ZHANG Y, SHENG R, CHEN J, et al. Silk fibroin and sericin differentially potentiate the paracrine and regenerative functions of stem cells through multiomics analysis. Adv Mater. 2023;35(20):e2210517. [189] ZHANG J, CHAN HF, WANG H, et al. Stem cell therapy and tissue engineering strategies using cell aggregates and decellularized scaffolds for the rescue of liver failure. J Tissue Eng. 2021;12:2041731420986711. [190] WEI H, LI F, XUE T, et al. MicroRNA-122-functionalized DNA tetrahedron stimulate hepatic differentiation of human mesenchymal stem cells for acute liver failure therapy. Bioact Mater. 2023;28:50-60. [191] XU Y, ZHANG Y, TIAN H, et al. Smart microneedle arrays integrating cell-free therapy and nanocatalysis to treat liver fibrosis. Adv Sci (Weinh). 2024;11(31): e2309940. [192] CORTI M, LIBERATI C, SMITH BK, et al. Safety of intradiaphragmatic delivery of adeno-associated virus-mediated alpha-glucosidase (rAAV1-CMV-hGAA) gene therapy in children affected by pompe disease. Hum Gene Ther Clin Dev. 2017; 28(4):208-218. [193] LESIZZA P, PROSDOCIMO G, MARTINELLI V, et al. Single-dose intracardiac injection of pro-regenerative micrornas improves cardiac function after myocardial infarction. Circ Res. 2017;120(8):1298-1304. [194] LI SP, LIN ZX, JIANG XY, et al. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol Sin. 2018;39(4):542-551. [195] PENG H, JI W, ZHAO R, et al. Exosome: a significant nano-scale drug delivery carrier. J Mater Chem B. 2020;8(34):7591-7608. [196] GALLET R, DAWKINS J, VALLE J, et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017; 38(3):201-211. [197] HORITA M, FARQUHARSON C,STEPHEN LA. The role of miR‐29 family in disease. J Cell Biochem. 2021;122(7):696-715. [198] REESE-PETERSEN AL, HOLM NIELSEN S, BüLOW SAND JM, et al. The sclerotic component of metabolic syndrome: fibroblast activities may be the central common denominator driving organ function loss and death. Diabetes Obes Metab. 2024;26(7):2554-2566. [199] GUO JL, GRIFFIN M, YOON JK, et al. Histological signatures map anti-fibrotic factors in mouse and human lungs. Nature. 2025;641(8064):993-1004. [200] CHO S, RHEE S, MADL CM, et al. Selective inhibition of stromal mechanosensing suppresses cardiac fibrosis. Nature. 2025; 642(8068):766-775. |
| [1] | Hou Chaowen, Li Zhaojin, Kong Jianda, Zhang Shuli. Main physiological changes in skeletal muscle aging and the multimechanism regulatory role of exercise [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1464-1475. |
| [2] | Xu Yixuan, Yao Jun, Liu Xulu, Li Xinlian, Liu Zhixiong, Zhang Zhihong. Vancomycin-containing porcine skin acellular extracellular matrix hydrogel promotes wound healing in skin infections [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5214-5228. |
| [3] | Gu Jianmei, Yuan Kunshan, Zhou Qiang, Zhang Haijun, , . Application of laser microporous decellularized scaffolds in tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 499-507. |
| [4] | Gao Feng, Wang Jiliang, Wang Hongbo, Yang Yongsheng, Liu Yuan, Fu Su. Extracellular matrix stiffness affects the proliferation activity of bone marrow stromal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(13): 3226-3232. |
| [5] | Wu Xianyuan, Zhang Nini, Huang Guilin. Gene transfection technology and tissue fibrosis repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(13): 3424-3434. |
| [6] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [7] | Shui Jing, He Yu, Jiang Nan, Xu Kun, Song Lijuan, Ding Zhibin, Ma Cungen, Li Xinyi. Astrocytes regulate remyelination in central nervous system [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7889-7897. |
| [8] | Zhang Xiaoyu, Wei Shanwen, Fang Jiawei, Ni Li. Prussian blue nanoparticles restore mitochondrial function in nucleus pulposus cells through antioxidation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7318-7325. |
| [9] | Wang Wanchun, , Yi Jun, Yan Zhangren, Yang Yue, Dong Degang, Li Yumei. 717 Jiedu Decoction remodels homeostasis of extracellular matrix and promotes repair of local injured tissues in rats after Agkistrodon halys bite [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6457-6465. |
| [10] | Song Yuting, Wen Chunlei, Li Yi, Bai Xue, Gao Hong, Hu Tingju, Wang Zijun, Yan Xu. Effects of myocardial extracellular matrix remodeling on connexin 43 and its Ser368 phosphorylation and electrical conduction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6212-6218. |
| [11] | Sun Yahui, Wang Yufeng, Guo Chao, Yao Junjie, Ji Yuanyuan, Li Zhongxu, Lou Huijuan, Jiang Jinglei, Sun Yiping, Xu Jing, Cong Deyu. Effect of massage on extracellular matrix collagen deposition in skeletal muscle of type 2 diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5549-5555. |
| [12] | Ji Yaqiong, Ning Zhongping. Protective effect of paeoniflorin on angiotensin II-induced fibrosis in cardiac fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5382-5389. |
| [13] | Zhou Lijun, Zhang Keyuan, Xu Feihu, Wang Xi, Yu Li, Dong Shiming, Xu Junyu, Guo Yufeng, Ma Hairong, Ding Hong. Effect and mechanism of circular RNA SEC24A on proliferation and apoptosis of synovial fibroblasts in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5086-5092. |
| [14] | Wang Dan, Zhu Xiaojun, Li Zhicheng, Li Na. Construction of tissue engineered urethra by combining acellular matrix with exosomes in small intestinal submucosa [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 4907-4914. |
| [15] | Yue Jinru, Zhang Yumin, Liu Jingshu, Bu Yanan, Wu Jingruo, Chen Jia, Wang Jianru. Effects of extracellular vesicles treated with Duhuo Jisheng Decoction on rheumatoid arthritis fibroblast-like synovial cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 4915-4926. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||