Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (2): 499-507.doi: 10.12307/2025.898
Previous Articles Next Articles
Application of laser microporous decellularized scaffolds in tissue regeneration
Gu Jianmei1, Yuan Kunshan1, Zhou Qiang1, Zhang Haijun1, 2, 3
- 1National & Local Joint Engineering Laboratory for Biomedical Materials Modification Technology, Dezhou 253000, Shandong Province, China; 2Tongji University School of Medicine, Shanghai 200000, China; 3Shanghai Tenth People’s Hospital, Tongji University, Shanghai 200000, China
-
Received:2024-10-15Accepted:2024-11-22Online:2026-01-18Published:2025-07-03 -
Contact:Zhang Haijun, PhD, Professor, Doctoral supervisor, National & Local Joint Engineering Laboratory for Biomedical Materials Modification Technology, Dezhou 253000, Shandong Province, China; Tongji University School of Medicine, Shanghai 200000, China; Shanghai Tenth People’s Hospital, Tongji University, Shanghai 200000, China -
About author:Gu Jianmei, Master candidate, National & Local Joint Engineering Laboratory for Biomedical Materials Modification Technology, Dezhou 253000, Shandong Province, China
CLC Number:
Cite this article
Gu Jianmei, Yuan Kunshan, Zhou Qiang, Zhang Haijun, , . Application of laser microporous decellularized scaffolds in tissue regeneration[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 499-507.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
2.1 激光在生物医学领域的应用发展历程 自1960年世界上第一台红宝石激光器诞生以来,激光医学领域经历了从初步探索到成熟应用的发展历程[18]。1961年,医学界迎来了一个重要的里程碑,美国推出了世界第一台医用激光机,Campbell首次通过该设备对剥离的视网膜进行凝固治疗,这标志着激光技术在生物医学领域的首次应用[19]。随后在1963年,红宝石激光被首次用于治疗良性皮肤损害和纹身[20],进一步拓宽了激光在生物医学领域的应用范围。进入20世纪70年代,激光技术在生物医学中的应用取得了显著进展。1972年,二氧化碳激光与显微镜结合用于喉部组织的止血手术[21],不仅提高了手术操作的精确性,也减少了术中创伤。紧接着1973年,光纤传输激光首次被用于内窥镜下的激光治疗[22],这一创新为激光介入治疗的发展奠定了基础。1981年,联合国世界卫生组织正式宣布激光医学作为医学的一个新分支[23],这一认定标志着激光医学的正式确立。1983年,激光技术的应用领域进一步扩展到了冠状动脉和周围血管成形术[24],为心血管疾病的治疗提供了新的选择。在这一时期,激光医学的临床应用模式初步形成,主要包括强激光治疗、弱激光治疗和光动力治疗,这些模式至今仍被广泛使用。到了2010年,中国专家编写了激光医学临床诊疗指南和临床技术操作规范,为激光医学的临床应用提供了标准化的指导[25],图4体现了激光在生物医学领域应用的重要时间节点。激光在生物医学领域的应用已经涵盖了癌症治疗和肿瘤消融、脑外科手术、癫痫治疗、体内碎石、心脏病学研究、皮肤病学研究、皮肤再生、脂肪分解、组织工程等多个重要领域[26],见图5。这些治疗手段的发展,不仅提高了治疗效果,也极大地改善了患者的生活质量。除了临床治疗技术,激光医学在诊断领域也取得了同步发展。激光多普勒技术、光学相干成像术、激光荧光光谱技术等高灵敏度和高分辨率的激光诊断技术相继问世,为疾病的早期诊断和治疗提供了强有力的工具。"

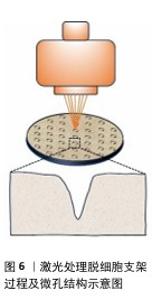
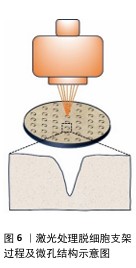
2.2 激光微图技术对脱细胞支架性能的影响 随着激光技术在生物医学领域的不断深入,其在生物材料加工中的优势也逐渐显现,特别是在组织工程领域,激光微图技术对脱细胞支架性能的影响尤为显著。当激光作用于生物组织时,这些组织会吸收激光能量并转化为热能,根据加热的具体程度,激光能够实现逐步且选择性的热损伤效果[27]。当组织温度逐渐攀升至某一临界点时,将引发蛋白质的一系列复杂变化,包括变性、凝固以及脱水等过程。而若激光能量足够强大,则会导致组织消融,这一过程中伴随着液体成分的迅速汽化以及大分子的热解现象[28]。激光处理使得加工点处的生物材料被去除,适当的参数设置可以在脱细胞支架上留下孔洞,达到表面改性的目的。 值得一提的是,激光微图技术对脱细胞支架的加工处理,对支架的孔隙结构、机械性能、细胞渗透性均会产生影响,并且这种影响在一定程度上可控。借助激光微图技术,可以有目的地调节界面的通透性,产生的微孔将为后续的再细胞化过程提供充足的空间,以满足生物组织修复与重建的迫切需求。 2.2.1 孔隙结构 激光微图技术以其卓越的精确性和稳定性,在调控孔隙的几何形态方面展现出了巨大的潜力。通过精细调整各项指标,如孔道密度、直径以及连通性,这项技术能够实现高度定制化的微孔结构,激光处理脱细胞支架过程及微孔结构见图6。有研究表明,利用二氧化碳激光技术,可以在支架表面引入点阵排列的锥形微孔[29]。这一过程中,激光通过光学系统聚焦,于焦点处达到最小光斑直径与最高能量密度,随着激光深入材料内部,光斑逐渐扩大,能量密度降低,从而形成了独特的锥形孔结构。 进一步地,另一项研究探究了激光参数设置对脱细胞支架结构的影响。实验发现,较低的输出功率、较短的脉冲宽度与较少的重复次数仅能在材料浅层产生微孔;脉冲宽度越小,对目标孔隙外的组织热损伤越小;当重复次数增加时,孔深提升至接近壁厚的一半。然而,过高的激光参数会导致整体结构受损。特别地,通过精细调节至特定的参数组合(例如,实验中采用的10 W功率、17 ms脉冲宽度及4次重复),可以实现微孔深度达到壁厚的9/10,同时保持细胞外基质轻微受损,其形态与功能与天然气管软骨相似[30]。 同时,为了控制微孔结构的均匀性,在激光处理前对脱细胞支架进行冷冻干燥,可以消除与水合组织相关的激光传播(折射、反射、导热性),从而进一步优化激光微图的效果[31]。研究者对冷冻干燥工艺的改进,将恒定冷却速率技术用于生产具有均匀孔隙结构的支架,生产出平均孔径范围为85-325 μm的胶原-糖胺聚糖支架[32]。 "

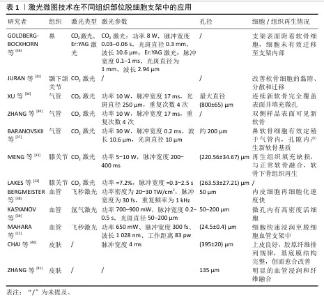
2.2.2 力学性能 机械稳定性是支架设计中的关键考量因素,它直接关系到支架在生物体内的行为和长期功能。为了深入探究激光处理对支架机械性能的影响,有研究对激光作用前后的脱细胞半月板进行了全面的机械完整性评估,结果显示,虽然激光处理导致了非约束应力松弛期间的弹性模量、瞬时应力以及拉伸试验中的周向极限强度有所降低,但这些变化并未显著增加基本生理条件下支架失效的风险,其性能仍然维持在接近生理值的范围内[33]。 而在关于激光微图脱细胞气管的研究中,发现了力学性能的不同表现:与原生气管相比,经激光处理的脱细胞气管最大断裂力和压缩弹性模量均出现较为严重的降低,不过这一现象在播种软骨细胞并体内培养12周后发生逆转,由于软骨再生,气管有足够的强度和弹性来维持管状形状;然而,对于未进行细胞播种的激光微图脱细胞气管来说,其力学性能表现依旧不理想[34],这一发现提示细胞接种对于脱细胞支架的再生也是必要的。随后,对上述2篇研究的力学性能进行分析,推测这种差异可能是由于脱细胞与激光处理的不同顺序引起的,先进行激光处理再进行脱细胞处理往往更加损害脱细胞支架的力学性能,这种猜想也在另一项研究中得到证实。MATUSKA等[35]对比了不同处理顺序脱细胞支架,结果显示相较于在脱细胞处理之前进行激光处理,采取先脱细胞后激光处理的支架,在压缩模量上实现了1.3-2.2倍的提升,同时峰值应力也相应地提高了1.4-2.2倍,这一显著差异强调了处理顺序在将激光微图技术应用于脱细胞支架制备过程中的重要性。 2.2.3 细胞渗透 孔径作为影响细胞黏附和增殖的关键因素,一直是生物医学材料设计中的重要考量。当平均孔径设定为120 μm时,细胞在48 h时内的初始附着和增殖能力得到了显著提升[32]。这一发现为研究者提供了一个重要的孔径范围参考,即在该范围内,细胞能够更有效地与材料进行相互作用。进一步地,考虑到CO2激光的常见波长为10.6 μm,这一特性决定了光束能够聚焦到的最小光斑尺寸,进而影响了实际的烧蚀直径,通常落在几百微米的范围内,而这个尺寸范围恰好与细胞黏附与增殖的最佳孔径窗口相吻合,为激光微图技术在生物医学材料制备中的应用提供了有力的支持[31,35]。MAHARA等[12]通过激光消融在脱细胞血管移植物中以不同间距进行微孔处理,研究发现巨噬细胞和成纤维细胞的再细胞化速度均快于无孔对照组织;当微孔以小于250 μm的距离排列时,2周后几乎整个区域都被细胞占据。换言之,激光微图技术制造的孔隙密度对细胞迁移速率也有影响,进而影响宿主细胞的浸润效率,小于250 μm可能会加速细胞迁移到脱细胞组织中。 综上所述,激光微图技术在脱细胞支架的制备过程中发挥着举足轻重的作用。为了获得最优的细胞整合与重塑效果,需要综合考虑整个工艺流程中的多个因素,包括支架的否冻干处理、激光微图技术与脱细胞技术工艺顺序、激光微图技术参数(输出功率、脉冲宽度和重复次数等)设定、脱细胞工艺(如试剂种类、试剂浓度、试剂用量、处理时间、机械作用、环境温度等)以及孔隙直径及密度,以选择最优路径,从而进一步推动激光微图技术在脱细胞支架领域的应用与发展。 2.3 激光微图技术在不同组织部位脱细胞支架中的应用 正是基于这些精细的工艺控制,激光微图技术的应用范围得以扩展到不同的组织部位,目前该技术在软骨组织(如气管、膝关节、颞下颌关节、鼻等)和非软骨组织(如血管、皮肤等)中都有应用。在这些不同的组织中,激光微图技术通过微孔结构设计,促进了细胞的渗透和营养物质的交换,从而加速了组织的修复与再生。见表1。 "
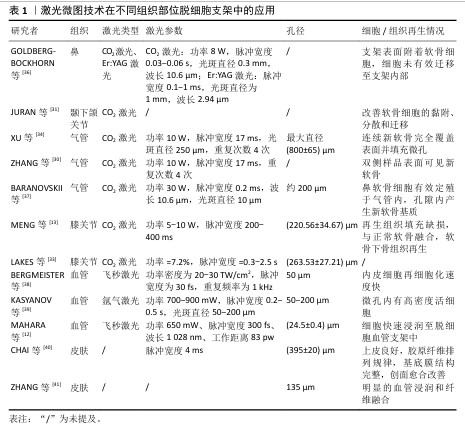

2.3.1 鼻 由于创伤、肿瘤或先天性病变导致的鼻结构功能缺损和病变大多需要复杂的重建手术[42-43],由于软骨分化水平高、组织代谢缓慢,缺乏固有的再生能力及明显的血管化,这类缺损的重建在耳鼻喉科备受关注。组织工程化软骨移植为鼻缺损带来希望[44],脱细胞猪软骨是一种理想的鼻缺损修复材料。然而,软骨细胞在脱细胞猪软骨支架上的迁移与覆盖需要数周时间,为了加速这一过程,GOLDBERG-BOCKHORN等[36]比较了二氧化碳激光和Er:YAG激光对支架表面修饰及软骨细胞迁移的效果,他们发现激光形成的腔内细胞数量明显多于未经处理的表面,并且随时间增长而显著增加。尽管如此,由于激光作用导致的胶原纤维变性,热变性区阻碍了软骨细胞向支架内迁移,这种趋势在经CO2激光处理的支架中更加明显。 为了减少胶原蛋白结构的热损伤,飞秒激光可作为改变支架表面的一种选择,由于其脉冲持续时间很短,在电子有时间热化并将吸收的激光脉冲能量传递到晶格之前,激光脉冲已经结束,因此对材料的热效应可以忽略不计[45]。飞秒激光已在角膜屈光手术中得到应用[46-47]。一项研究表明,飞秒激光在猪角膜中的附带损伤为15 μm,在巩膜和关节软骨中的损伤更是分别小于4 μm和1 μm,而切口深度则达到几毫米[48]。 2.3.2 颞下颌关节 颞下颌关节作为机械活动关节,其骨关节炎的发病机制高度复杂,包括炎症、机械负荷、软骨退化等,可引起剧烈疼痛和功能障碍,严重的退变或颞下颌关节损伤需要手术切除[49-50]。天然衍生支架植入是治疗手段之一,同样存在细胞长入困难问题,细胞向内生长受到致密细胞外基质纤维微环境(胶原蛋白、弹性蛋白、糖胺聚糖)的限制,这阻碍了细胞浸润与再生[35]。JURAN等[31]证明激光微孔化支架改善了细胞重塑,随着培养时间的增长,通过激光微图技术增加的人工浸润路径最终改善了软骨细胞的黏附、分散和迁移,有助于再现天然颞下颌关节的特征。"


2.3.3 气管 气管具有独特的解剖结构和生理环境,目前长段气管缺损的功能重建仍是一个未解决的难题[51-52]。近年来,脱细胞气管支架给气管重建带来新的希望[53]。然而,气管中软骨组织的致密结构和易受病原体影响的特点,使得快速细胞化成为必要。XU等[54]利用激光微孔技术从脱细胞气管基质中再生组织工程气管,并植入于裸鼠皮下,证明了开发最佳气管替代物的可能性,该替代物既保留了气管原有的管状形状,又具有足够的气管软骨再生能力。上述研究局限于异位植入,BARANOVSKII等[37]研究了鼻软骨细胞在脱细胞激光微孔化气管软骨(LPTC)组织的孔洞中定植并产生软骨基质的能力,脱细胞激光微孔化气管软骨成功地移植到家兔气管壁上,无脱位或气管狭窄迹象,鼻软骨细胞在激光微孔化气管软骨细胞间隙周围有大量的糖胺聚糖,产生了新的软骨基质。上述例子均采用软骨细胞进行播种,而气管组织由不同类型的细胞(软骨细胞、上皮细胞、内皮细胞和平滑肌细胞)组成[55],原生气管细胞系及结构的复杂性要求研究者们需要在大动物模型及临床上进一步验证。 2.3.4 膝关节 随着人口老龄化的加剧和运动人数的增加,世界范围内关节软骨损伤患者的数量逐年增加,关节软骨的重建和功能修复同样是一大挑战[56-57]。组织工程策略,如将自体软骨细胞或干细胞植入三维支架,已被证明是软骨组织再生的有效方法[58-59]。MENG等[13]评估了激光微孔化骨软骨植入物(LMP-OI)在山羊负重区关节骨软骨缺损模型中的再生能力,植入6个月组织学结果显示,LMP-OI+骨髓间充质干细胞组中新生软骨的厚度与周围原始组织的厚度相等,修复组织中细胞组织均匀,Ⅱ型胶原蛋白的表达较LMP-OI组明显增加,各项指标均优于对照组和LMP-OI组,12个月时该组依旧修复效果最好;LMP-OI+骨髓间充质干细胞组在6,12个月时的总组织学评分明显低于其他两组,而且大量修复组织已被确定为“透明软骨”,这些结果表明,LMP-OI +骨髓间充质干细胞治疗能够成功修复山羊骨软骨缺损。另外,对照组和 LMP-OI 组均存在空洞、炎症及水肿信号,相比之下,LMP-OI+骨髓间充质干细胞组有轻微水肿,无炎症现象;术后12个月,对照组和LMP-OI组的水肿信号均显著减少,LMP-OI+骨髓间充质干细胞组水肿信号几乎完全消失[13]。以上证据表明,激光微孔化骨软骨植入物促进了细胞长入,体内安全性评估良好,是修复大型动物骨软骨缺损的理想支架材料,可能在骨软骨损伤治疗的临床治疗中发挥积极的作用。 对于含水量丰富的软骨组织,以往研究者大多选择二氧化碳激光器进行改性,这源于二氧化碳对水有较大的吸收,尽管如此,该激光处理带来的组织热损伤不可忽视。由于水在2.0 μm附近有一个尖锐的吸收峰,在3.0 μm处有一个最强的吸收峰,这两个波段的激光与生物组织作用时,作用精确,并且对邻近组织的热损伤较小。Er:YAG激光波长为2.94 μm,接近水在3.0 μm的最强吸收峰,此时热损伤较小,加工效果较为理想。 2.3.5 血管 血管功能障碍是众多疾病的根源,其引发的损害往往严重且不可逆,对人类健康构成重大威胁[60-61],血管替代疗法(VRT)应运而生,旨在帮助患者克服危及生命的疾病或创伤性损伤。然而,传统自体血管移植存在供体部位损伤及血管来源有限的问题,血管组织工程技术因此被视为减少或消除这些难题的潜力途径[62-63]。KASYANOV等[39]采用氩激光技术,在脱细胞小肠黏膜下层平面片和管状脱细胞小肠黏膜下层支架上加工直径为50,100和200 μm的微孔,将绿色荧光基因(GFP)标记的细胞悬浮在原位可交联的透明质酸基水凝胶中,并进行离心浇铸,成功实现了支架的再细胞化,激光加工的微孔内呈现出高密度的活细胞。BERGMEISTER 等[38]利用飞秒激光开发了脱细胞血管移植物,并将其植入羊的颈动脉,6周时实现快速再细胞化,激光处理促进脱细胞基质向新血管组织转化;并与非穿孔移植物的对侧植入进行比较,6周时检查穿孔植入物,与未穿孔的标本相比,显示出明显更厚的新内膜和清晰的新生血管迹象,这些结果表明,激光穿孔引起的基质孔隙度增加促进了移植物与宿主细胞的重塑和重建。但是,假体的长期功能仍有待确定,基质降解和组织重组的时间进程问题、假体的免疫原性以及在异种动物实验中的可行性等问题仍需确定。"

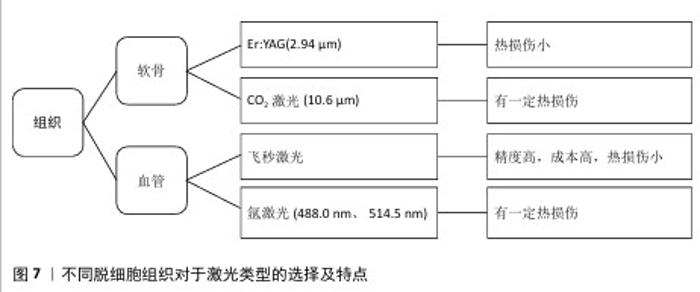
2.3.6 皮肤 由于糖尿病性皮肤溃疡等慢性疾病或严重烧伤等创伤导致的皮肤再生是一个重大挑战。目前,皮肤移植主要依赖自体皮肤移植和脱细胞皮肤替代品(例如INTEGRA?)[64]。为提高烧伤创面的愈合质量,CHAI等[40]在激光的辅助下,制备了一种由猪脱细胞真皮基质组成的复合皮肤移植物,激光微孔化猪脱细胞真皮基质复合皮肤移植物可促进创面愈合。在烧伤修复领域,存在供体皮肤短缺、同种异体或异种皮肤费用高、临时替代和伤口愈合后肢体功能不理想等问题。有研究表明,烧伤变性皮肤可以恢复正常的真皮形成和功能[65]。ZHANG等[41]的研究表明,利用激光微图技术可以将非感染创面的深度Ⅱ型烧伤皮肤转化为微孔脱细胞真皮基质,皮下植入实验后有明显的血管浸润和纤维融合,未见炎症细胞浸润。该微孔脱细胞真皮基质具有生物性能好、免疫原性低、真皮相容性好、细胞因子活性强等优点,有望成为真皮基质的合适替代品。 对于非软骨组织来说,尤其是血管这类较为精细的组织,往往会选择飞秒激光等精度较高的激光加工方式,这源于移植血管孔径精读高,也避免了额外的热损伤。另外,氩气激光对血红蛋白有较高吸收,因此氩激光器也是一种选择。不同脱细胞组织对于激光类型的选择及特点见图7。"

| [1] RAHMATI M, MILLS DK, URBANSKA AM, et al. Electrospinning for tissue engineering applications. Progress Mater Sci. 2021;117: 100721. [2] GUO L, LIANG Z, YANG L, et al. The role of natural polymers in bone tissue engineering. J Control Release. 2021;338:571-582. [3] LI Q, CHANG B, DONG H, et al. Functional microspheres for tissue regeneration. Bioact Mater. 2023;25:485-499. [4] GONG M, SUN J, LIU G, et al. Graphene oxide‑modified 3D acellular cartilage extracellular matrix scaffold for cartilage regeneration. Mater Sci Eng C Mater Biol Appl. 2021:119:111603. [5] ZHANG X, CHEN X, HONG H, et al. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact Mater. 2022;10: 15-31. [6] MOUSAVI MS, AMOABEDINY G, MAHFOUZI SH, et al. Enhanced articular cartilage decellularization using a novel perfusion-based bioreactor method. J Mech Behav Biomed Mater. 2021:119:104511. [7] COLLINS MN, REN G, YOUNG K, et al. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv Funct Mater. 2021; 31(21):2010609. [8] BROWN M, LI J, MORAES C, et al. Decellularized extracellular matrix: New promising and challenging biomaterials for regenerative medicine. Biomaterials. 2022; 289:121786. [9] BILODEAU C, SHOJAIE S, GOLTSIS O, et al. TP63 basal cells are indispensable during endoderm differentiation into proximal airway cells on acellular lung scaffolds. NPJ Regen Med. 2021;6(1):12. [10] TALLAPANENI V, KALAIVANI C, PAMU D, et al. Acellular Scaffolds as Innovative Biomaterial Platforms for the Management of Diabetic Wounds. Tissue Eng Regen Med. 2021;18(5):713-734. [11] GOLEBIOWSKA AA, INTRAVAIA JT, SATHE VM, et al. Decellularized extracellular matrix biomaterials for regenerative therapies: Advances, challenges and clinical prospects. Bioact Mater. 2024;32:98-123. [12] MAHARA A, KOJIMA K, YAMAMOTO M, et al. Accelerated tissue regeneration in decellularized vascular grafts with a patterned pore structure. J Mater Chem B. 2022;10(14):2544-2550. [13] MENG H, LIU X, LIU R, et al. Decellularized laser micro-patterned osteochondral implants exhibit zonal recellularization and self-fixing for osteochondral regeneration in a goat model. J Orthop Translat. 2024:46: 18-32. [14] ZHANG Y, FENG G, XU G, et al. Microporous acellular extracellular matrix combined with adipose-derived stem cell sheets as a promising tissue patch promoting articular cartilage regeneration and interface integration. Cytotherapy. 2019;21(8):856-869. [15] DASKALOVA A, ANGELOVA LJI. Ultra‑short laser patterning of silk fibroin thin films–a potential scaffold platform in tissue engineering applications. Industry 4.0. 2022;7(1):14-17. [16] NüRNBERGER S, SCHNEIDER C, KEIBL C, et al. Repopulation of decellularised articular cartilage by laser-based matrix engraving. EBioMedicine. 2021;64:103196. [17] MOON HI, KIM S, BYUN JE, et al. Facile and rapid fabrication of wearable biosensors via femtosecond laser-directed micro-patterning with large-sized reduced graphene oxide for physiological monitoring. Chem Eng J. 2024;479:147819. [18] NASIM H, JAMIL Y. Diode lasers: From laboratory to industry. Optics Laser Technol. 2014;56:211-222. [19] 吕磊,张正厚,隋丽云,等.激光在医学基础和临床研究中的应用[J].中国临床康复,2006,10(17):152-154. [20] GOLDMAN L, BLANEY DJ, KINDEL DJ, et al. Pathology of the effect of the laser beam on the skin. Nature. 1963:197:912-914. [21] JAKO GJ. Laser surgery of the vocal cords an experimental study with carbon dioxide lasers on dogs. Laryngoscope. 1972;82(12): 2204-2216. [22] NATH G, GORISCH W, KIEFHABER PJE. First laser endoscopy via a fiberoptic transmission system. Endoscopy. 1973;5(4):208-213. [23] 邱海霞,顾瑛.激光医学研究生培养方法初探[J].中国激光医学杂志,2017,26(4): 221-224. [24] 盖晓波,周形海.激光血管成形术研究近况[J].解放军医学杂志,1987(6):463-465. [25] 顾瑛.激光医学[J].物理,2010,39(8): 515-521. [26] KHALKHAL E, REZAEI-TAVIRANI M, ZALI MR, et al. The Evaluation of Laser Application in Surgery: A Review Article. J Lasers Med Sci. 2019;10(Suppl 1):S104-S111. [27] STEINER R. Laser-Tissue Interactions//RAULIN C, KARSAI S. Laser and IPL Technology in Dermatology and Aesthetic Medicine. Berlin, Heidelberg; Springer Berlin Heidelberg. 2011:23-36. [28] LECARPENTIER GL, MOTAMEDI M, MCMATH LP, et al. Continuous wave laser ablation of tissue: analysis of thermal and mechanical events. IEEE Trans Biomed Eng. 1993;40(2):188-200. [29] LI Y, XU Y, LIU Y, et al. Decellularized cartilage matrix scaffolds with laser-machined micropores for cartilage regeneration and articular cartilage repair. Mater Sci Eng C Mater Biol Appl. 2019;105:110139. [30] ZHANG Y, XU Y, LIU Y, et al. Porous decellularized trachea scaffold prepared by a laser micropore technique. J Mech Behav Biomed Mater. 2019;90:96-103. [31] JURAN CM, DOLWICK MF, MCFETRIDGE PS. Engineered Microporosity: Enhancing the Early Regenerative Potential of Decellularized Temporomandibular Joint Discs. Tissue Eng Part A. 2015;21(3-4): 829-839. [32] MURPHY CM, HAUGHG, O’BRIEN FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31(3): 461-466. [33] LAKES EH, MATUSKA AM, MCFETRIDGE PS, et al. Mechanical Integrity of a Decellularized and Laser Drilled Medial Meniscus. J Biomech Eng. 2016;138(3): 4032381. [34] XU Y, LI D, YIN Z, et al. Tissue-engineered trachea regeneration using decellularized trachea matrix treated with laser micropore technique. Acta Biomater. 2017;58:113-121. [35] MATUSKA AM, MCFETRIDGE PS. Laser micro-ablation of fibrocartilage tissue: Effects of tissue processing on porosity modification and mechanics. J Biomed Mater Res B Appl Biomater. 2018;106(5): 1858-1868. [36] GOLDBERG-BOCKHORN E, SCHWARZ S, SUBEDI R, et al. Laser surface modification of decellularized extracellular cartilage matrix for cartilage tissue engineering. Lasers Med Sci. 2018;33(2):375-384. [37] BARANOVSKII D, DEMNER J, NÜRNBERGER S, et al. Engineering of Tracheal Grafts Based on Recellularization of Laser-Engraved Human Airway Cartilage Substrates. Cartilage. 2022;13(1):19476035221075951. [38] BERGMEISTER H, BOECK P, KASIMIR MT, et al. Effect of laser perforation on the remodeling of acellular matrix grafts . J Biomed Mater Res B Appl Biomater. 2005; 74B(1):495-503. [39] KASYANOV VA, HODDE J, HILES MC, et al. Rapid biofabrication of tubular tissue constructs by centrifugal casting in a decellularized natural scaffold with laser-machined micropores. J Mater Sci Mater Med. 2009;20(1):329-337. [40] CHAI JK, LIANG LM, YANG HM, et al. Preparation of laser micropore porcine acellular dermal matrix for skin graft: An experimental study. Burns. 2007;33(6): 719-725. [41] ZHANG Y, ZENG Y, XIN G, et al. Biological function evaluation and effects of laser micro-pore burn-denatured acellular dermal matrix. Burns. 2018;44(2):350-358. [42] LAVERNIA L, BROWN WE, WONG BJF, et al. Toward tissue-engineering of nasal cartilages. Acta Biomater. 2019;88:42-56. [43] BROWN WE, LAVERNIA L, BIELAJEW BJ, et al. Human nasal cartilage: Functional properties and structure-function relationships for the development of tissue engineering design criteria. Acta Biomater. 2023;168:113-124. [44] FARAHANI PK. Application of Tissue Engineering and Biomaterials in Nose Surgery. JPRAS Open. 2024;40:262-272. [45] GEMINI L, AL-BOURGOL S, MACHINET G, et al. Ablation of Bone Tissue by Femtosecond Laser: A Path to High-Resolution Bone Surgery. Materials. 2021;14(9):2429. [46] MOSHIRFAR M, MEGERDICHIAN A, WEST WB, et al. Comparison of Visual Outcome After Hyperopic LASIK Using a Wavefront-Optimized Platform Versus Other Excimer Lasers in the Past Two Decades. Ophthalmol Ther. 2021;10(3):547-563. [47] JABBOUR S, BOWER KS. Refractive Surgery in the US in 2021. JAMA. 2021;326(1):77-78. [48] TIAN K, XIANG M, WEN X, et al. Tissue Ablation with Multi-Millimeter Depth and Cellular-Scale Collateral Damage by a Femtosecond Mid-Infrared Laser Tuned to the Amide-I Vibration. Laser Photonics Rev. 2024;18(2):2300421. [49] LU K, MA F, YI D, et al. Molecular signaling in temporomandibular joint osteoarthritis. J Orthop Translat. 2022;32:21-27. [50] WHYTE A, BOEDDINGHAUS R, BARTLEY A, et al. Imaging of the temporomandibular joint. Clin Radiol. 2021;76(1):76.e21-76.e35. [51] RAVINDRA A, D’ANGELO W, ZHANG L, et al. Human Bronchial Epithelial Cell Growth on Homologous Versus Heterologous Tissue Extracellular Matrix. J Surg Res. 2021;263: 215-223. [52] HUO Y, XU Y, WU X, et al. Functional Trachea Reconstruction Using 3D-Bioprinted Native-Like Tissue Architecture Based on Designable Tissue-Specific Bioinks. Adv Sci. 2022;9(29):2202181. [53] ZHANG B, SUN F, LU Y, et al. A novel decellularized trachea preparation method for the rapid construction of a functional tissue engineered trachea to repair tracheal defects. J Mater Chem B. 2022;10(25): 4810-4822. [54] XU Y, LI Y, LIU Y, et al. Surface modification of decellularized trachea matrix with collagen and laser micropore technique to promote cartilage regeneration. Am J Transl Res. 2019; 11(9):5390-5403. [55] DHASMANA A, SINGH A, RAWAL S. Biomedical grafts for tracheal tissue repairing and regeneration “Tracheal tissue engineering: an overview”. Tissue Eng Regen Med. 2020;14(5):653-672. [56] JIANG S, TIAN G, YANG Z, et al. Enhancement of acellular cartilage matrix scaffold by Wharton’s jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact Mater. 2021;6(9):2711-2728. [57] ZHANG JY, XIANG XN, YU X, et al. Mechanisms and applications of the regenerative capacity of platelets-based therapy in knee osteoarthritis. Biomed Pharmacother. 2024:178:117226. [58] JIA L, ZHANG P, CI Z, et al. Acellular cartilage matrix biomimetic scaffold with immediate enrichment of autologous bone marrow mononuclear cells to repair articular cartilage defects. Mater Today Bio. 2022:15:100310. [59] 刘雪剑,刘士臣,孙百川,等.激光微孔化脱细胞骨软骨支架的制备及表征[J].中国组织工程研究,2018,22(18):2836-2842. [60] FORD TJ, ONG P, SECHTEM U, et al. Assessment of Vascular Dysfunction in Patients Without Obstructive Coronary Artery Disease. JACC Cardiovasc Interv. 2020;13(16):1847-1864. [61] ILANLOU S, KHAKBIZ M, AMOABEDINY G, et al. Preclinical studies of acellular extracellular matrices as small-caliber vascular grafts. Tissue Cell. 2019;60:25-32. [62] WANG X, CHAN V, CORRIDON PR. Decellularized blood vessel development: Current state-of-the-art and future directions. Front Bioeng Biotechnol. 2022; 10:951644. [63] WANG X, CHAN V, CORRIDON PR. Acellular Tissue-Engineered Vascular Grafts from Polymers: Methods, Achievements, Characterization, and Challenges. Polymers. 2022;14(22):4825. [64] BOCK N, PHAM LB, NGUYEN TB, et al. Polydopamine coating of uncrosslinked chitosan as an acellular scaffold for full thickness skin grafts. Carbohydr Polym. 2020;245:116524. [65] YU G, YE L, TAN W, et al. A novel dermal matrix generated from burned skin as a promising substitute for deep‑degree burns therapy. Mol Med Rep. 2016;13(3):2570-2582. |
| [1] | Jiang Kan, Alimujiang·Abudourousuli, Shalayiding·Aierxiding, Aikebaierjiang·Aisaiti, Kutiluke·Shoukeer, Aikeremujiang·Muheremu. Biomaterials and bone regeneration: research hotspots and analysis of 500 influential papers [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 528-536. |
| [2] | Dang Xiaowen, Huang Hailiang, Huang Lei, Wang Yajie . Research frontiers and hotspots of carbon nanomaterials in biomedical field over the past 10 years [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 752-760. |
| [3] | Wang Sifan, He Huiyu, Yang Quan, Han Xiangzhen. miRNA-378a overexpression of macrophage cell line composite collagen sponge: anti-inflammation and tissue repair promotion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 789-799. |
| [4] | Israrguli · Maimeti, Jia Sen, Liu Jia. Bone morphogenetic protein 2-loaded hydrogel induces osteogenic differentiation of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3301-3310. |
| [5] | Xin Yuan, Wu Xixi, Quan Liang, Zhang Hengtong, Ao Qiang. REG-augmented decellularized porcine cornea/hydroxyethyl methacrylate in situ integrated composite artificial cornea [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3388-3399. |
| [6] | Wang Renzhi, Chen Yuanfen, Li Jinwei. 3D printed hollow pipe double-crosslinked hydrogel tissue engineering scaffold [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3432-3439. |
| [7] | Yu Xingge, Lin Kaili. Application of nanocomposite hydrogels in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5441-5446. |
| [8] | Zhou Pengfei, Lin Jing, Chen Yuying, Lin Minkui. Canine dental pulp stem cells-polyglycolic acid scaffold complex for canine periodontal tissue defect [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5526-5531. |
| [9] |
Feng Yuan, Han Zhiqi, Zhou Nuo.
Endothelial progenitor cells promote vasculogenesis and osteogenesis in the repair of bone defects [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(25): 4046-4053. |
| [10] |
Ji Hangyu, Gu Jun, Xie Linghan, Wu Xiaotao.
Application of stem cells, tissue engineering scaffolds and neurotrophic factors in the treatment of spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(25): 4088-4093. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||