Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (16): 3388-3399.doi: 10.12307/2025.426
Previous Articles Next Articles
REG-augmented decellularized porcine cornea/hydroxyethyl methacrylate in situ integrated composite artificial cornea
Xin Yuan, Wu Xixi, Quan Liang, Zhang Hengtong, Ao Qiang
- College of Biomedical Engineering, Sichuan University, Chengdu 610064, Sichuan Province, China
-
Received:2024-03-29Accepted:2024-05-10Online:2025-06-08Published:2024-09-03 -
Contact:Ao Qiang, Professor, College of Biomedical Engineering, Sichuan University, Chengdu 610064, Sichuan Province, China -
About author:Xin Yuan, College of Biomedical Engineering, Sichuan University, Chengdu 610064, Sichuan Province, China -
Supported by:National Key R&D Program of China, No. 2023YFC2410403 (to AQ)
CLC Number:
Cite this article
Xin Yuan, Wu Xixi, Quan Liang, Zhang Hengtong, Ao Qiang. REG-augmented decellularized porcine cornea/hydroxyethyl methacrylate in situ integrated composite artificial cornea[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3388-3399.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
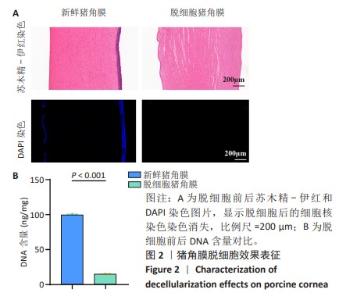
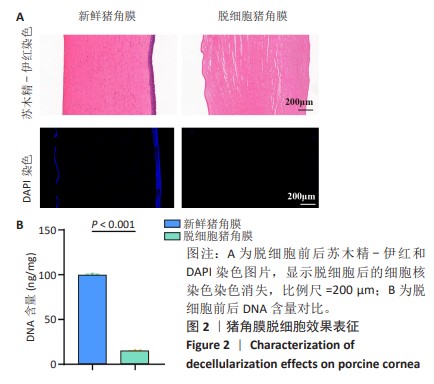
2.1 猪角膜脱细胞效果表征 如图2A所示,通过苏木精-伊红染色观察到脱细胞后细胞核染色几乎完全消失,表明脱细胞过程去除了大部分细胞中的DNA成分,而细胞外基质的结构得以保留;为了更直观地确认细胞核的去除情况,使用DAPI染料染色细胞核,观察到新鲜猪角膜样本中大量细胞核呈现出蓝色荧光,而经过脱细胞处理后蓝色荧光几乎完全消失。结合苏木精-伊红染色结果,脱细胞过程有效去除了角膜中的DNA成分。 通过DNA剩余含量定量分析来进一步评估脱细胞处理对组织样本的影响,在脱细胞前的样本中存在大量的细胞核,测得的DNA含量较高,为(99.83±0.57) ng/mg;经过脱细胞处理后,样本中的DNA含量显著降低,为(15.00±0.37) ng/mg,见图2B。DNA含量定量检测结果进一步证实了细胞成分的去除。 "
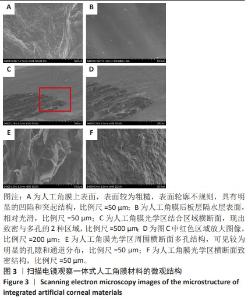
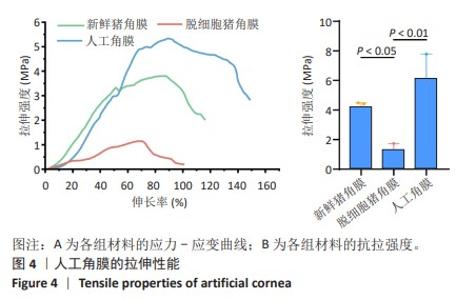
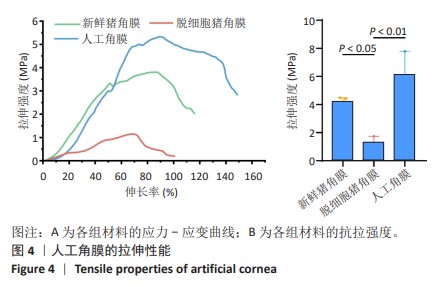
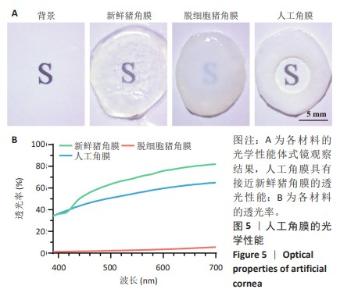
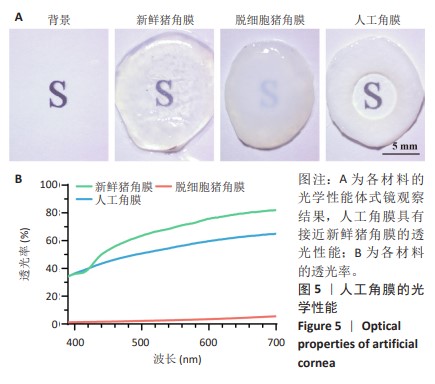
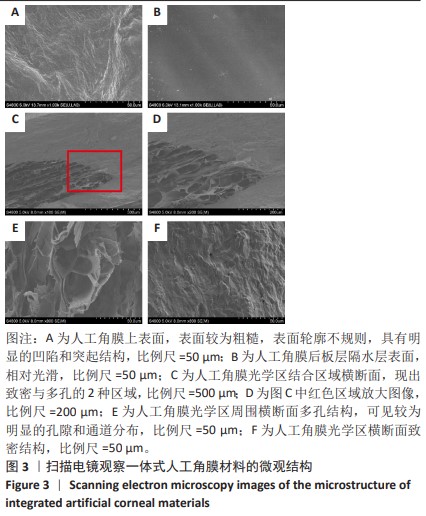
2.2 人工角膜结构形貌及物理性能表征 通过扫描电镜观察R-GSAC的上下表面和截面,上表面和下表面具有不同的形貌。如图3A所示,R-GSAC的上表面较为粗糙,表面轮廓不规则,具有明显的凹陷和突起结构,呈现出一种不均匀的外观,存在着不规则的起伏纹理分布,这些纹理呈现出不同的大小和形状,形成一种相对粗糙的表面结构。图3B所示为R-GSAC的下表面,由高分子材料聚甲基丙烯酸羟乙酯构建隔水层,表面相对光滑。 图3C,D所示截面中呈现出致密与多孔的2种区域,在2个区域的结合部位呈现明显梯度结构,可为基质细胞长入人工角膜后结合提供相比垂直结构更大的接触面积与结合强度。图3E所示多孔结构为未固化的脱细胞基质区域,表现为较为明显的孔隙和通道分布,这些孔隙具有不同的大小和形状,呈现出一种不规则的分布结构,环绕中心致密区域形成了紧密连接的多孔结构。图3F所示致密区域为原位固化后的光学区域,具有较高的密度。APCS的裙边中大部分为层状的胶原,在R-GSAC制作的反复冻融过程中通过隔热材料限制裙边中的水由周围向中央结晶在人工角膜裙边中形成微通道,冷冻干燥后在扫描电镜下呈现出疏松多孔的结构特征,如图3E。R-GSAC中央的光学区为聚甲基丙烯酸羟乙酯的固化区,水分含量极少,在扫描电镜下光学区具有较高的密度,呈现出相对均匀且紧密的表面结构,与周围的多孔裙边形成鲜明的对比。 "
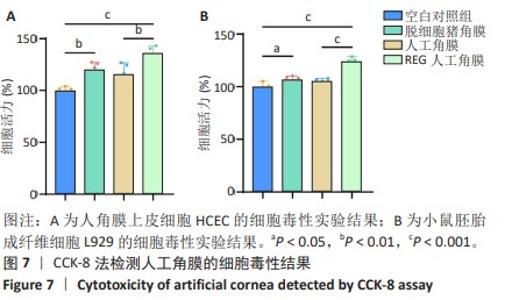
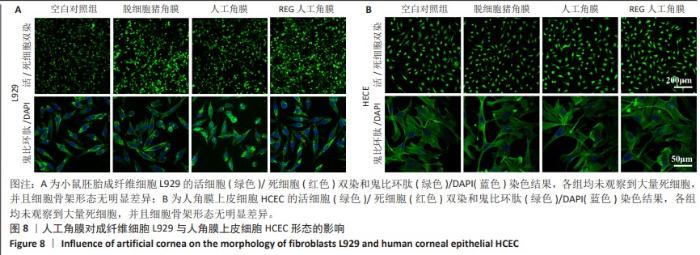
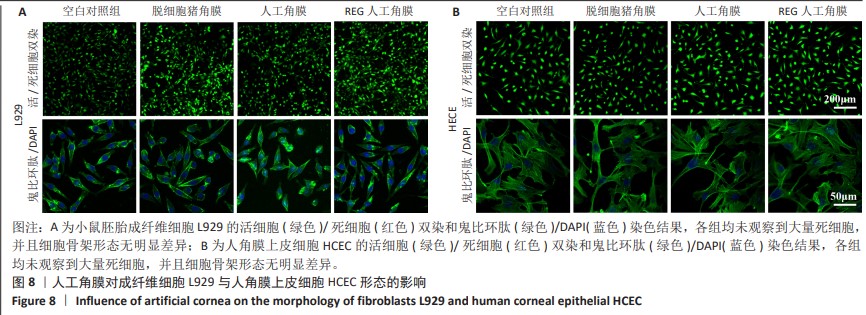
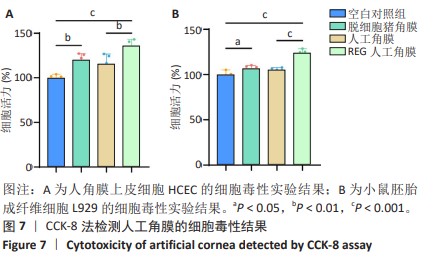
2.6 人工角膜细胞毒性实验 CCK-8检测结果显示,所有实验组对比空白对照组的增殖率大于1,表明APCS、GSAC及REG多肽均未表现出对细胞正常生长的抑制作用,并且细胞外基质和REG可以促进细胞的增殖生长,见图7。说明经过固化处理构建梯度结构后的GSAC并未在人工角膜中引入具有细胞毒性的成分。由于光学区和后板层隔水区的影响,固化后GSAC的浸提体系进行物质交换的接触面积相较于APCS更小,使细胞外基质对细胞的增殖生长的效果相较APCS组略低,但并无明显统计学差异。由于R-GSAC组加入了促进细胞迁移的活性多肽,使得其增殖率高于APCS组。活/死细胞双染和细胞骨架的染色结果如图8所示,各组均未观察到大量死细胞,并且细胞骨架形态无明显差异,说明人工角膜中各组分不会引起细胞形态的畸变。"
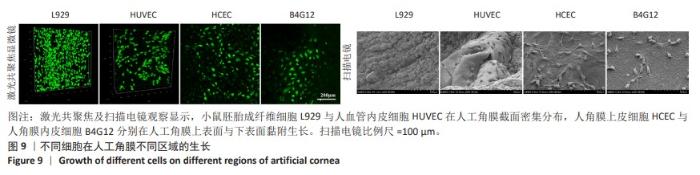
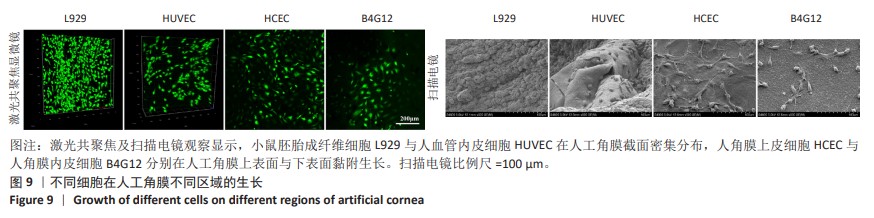
2.7 人工角膜不同区域特征细胞的生长 在角膜伤口愈合重建过程中,角膜基质细胞会先转变为成纤维细胞迁移到伤口处进行伤口修复,因此对于角膜基质细胞的表征使用小鼠胚胎成纤维细胞L929来代替,在人工角膜的横截面上观察生长情况。角膜上皮和内皮细胞使用人角膜上皮细胞HCEC和人角膜内皮细胞B4G12,分别观察在人工角膜的上表面和下表面的生长情况。此外,使用人血管内皮细胞来表征血管细胞在人工角膜横截面的黏附生长情况。 如图9所示,通过激光共聚焦及扫描电镜分别观察到大量小鼠胚胎成纤维细胞L929和人血管内皮细胞在人工角膜的横截面上生长,在材料的上表面及下表面也分别有大量人角膜上皮细胞HCEC和人角膜内皮细胞B4G12黏附生长,表明实验制备的人工角膜具有良好的生物相容性,能够为细胞提供适宜的黏附生长环境。由于上表面固化区域表面是聚甲基丙烯酸羟乙酯与生物活性物质脱细胞基质的分子互穿网络,而下表面隔水区则完全由高分子材料构成,因此在扫描电微镜下可以观察到相比材料下表面,上表面能够黏附更密集的细胞,表明实验构建的人工角膜表面能够有效促进角膜上皮细胞的黏附,有利于促进角膜的上皮化过程。"
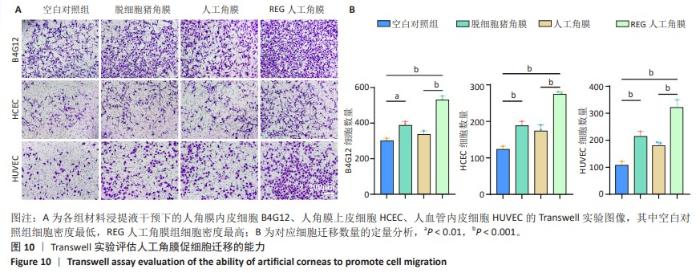
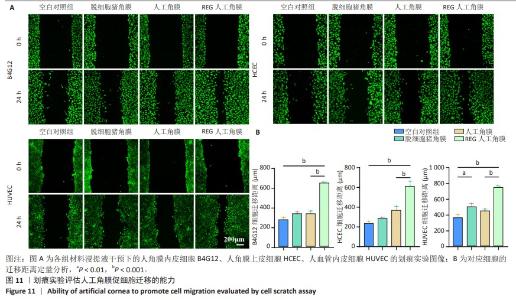
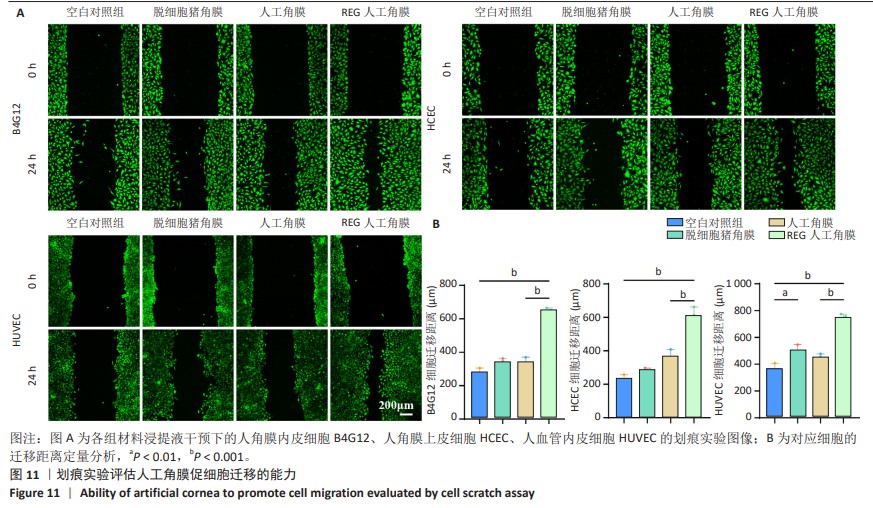
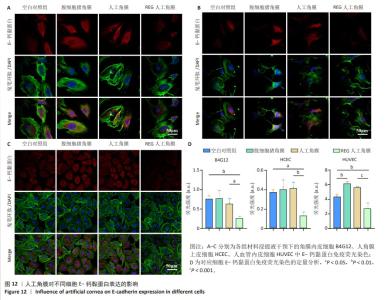
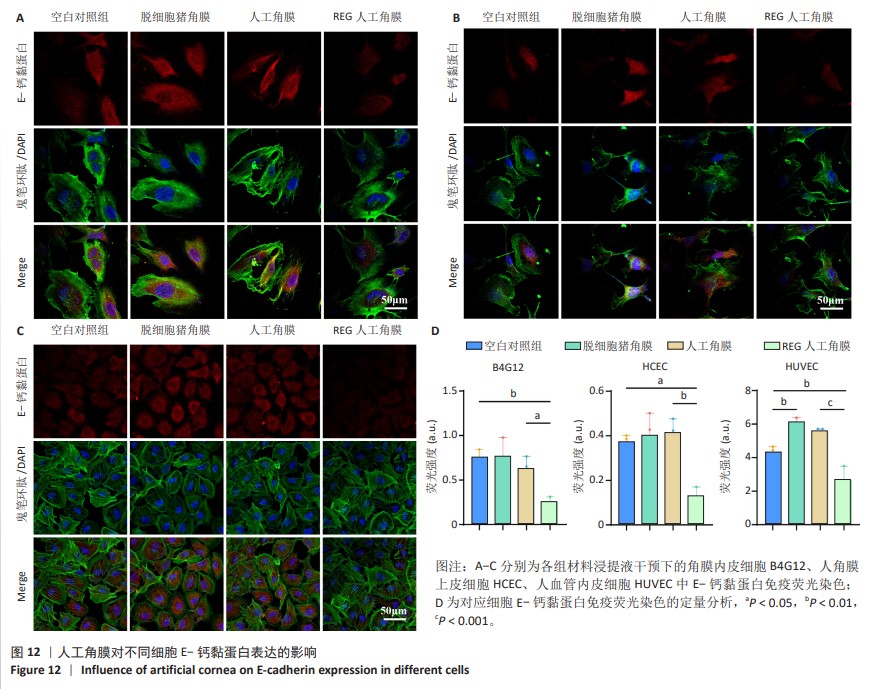
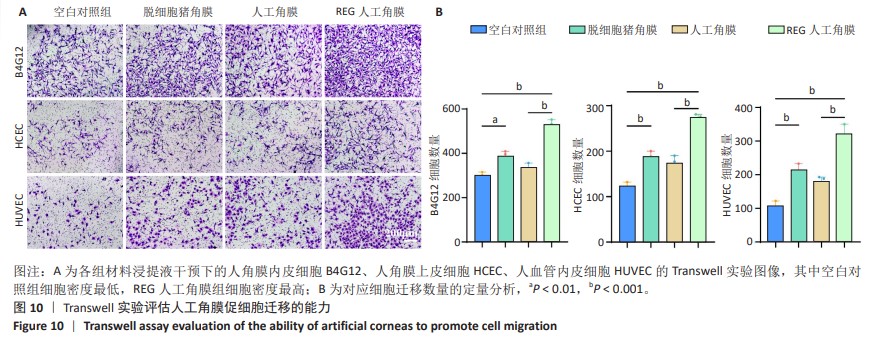
2.8 REG在人工角膜中对细胞迁移的促进作用 采用Transwell实验评估了APCS、GSAC及R-GSAC对人角膜上皮细胞HCEC、人角膜内皮细胞B4G12及人血管内皮细胞迁移能力的影响[31],结果见图10A所示。实验组和空白对照组均有细胞通过Transwell孔膜迁移到孔膜的下表面,其中R-GSAC组呈现出密集的细胞聚集,而空白对照组中的细胞密度最低。通过计数细胞迁移到下表面的细胞数量,见图10B,R-GSAC组孔膜下侧的细胞数量明显高于其他组,表明负载REG的材料对细胞迁移能力的促进作用较强;APCS和GSAC组细胞数量相比空白对照组也有明显差异,说明细胞外基质和REG均可以促进细胞的迁移过程,而REG对细胞的迁移过程影响更为显著。 细胞划痕实验结果如图11所示,在划痕形成后的初始时间点,观察到划痕区域呈现出界限分明的线性缺口,随着时间的推移,24 h后观察到划痕边缘的细胞向划痕中心迁移使缺口宽度减小,其中R-GSAC组划痕边缘的细胞迁移距离显著大于其他组。通过测量划痕宽度计算细胞迁移距离,APCS组和GSAC组细胞迁移距离大于空白对照组,R-GSAC组细胞迁移距离大于其他3组。 通过查阅相关通路,REG活性五肽通过促进细胞合成多种基质金属蛋白酶分解细胞外基质和多种黏附蛋白,进而促进细胞的迁移[29]。E-钙黏蛋白是一种细胞间黏附蛋白,在细胞间连接中起关键作用[32]。通过抗体标记细胞上的E-钙黏蛋白,在荧光显微镜下观察标记后的细胞,如图12所示。R-GSAC组中E-钙黏蛋白的荧光强度明显降低,这种现象可能是由于该组中表达的基质金属蛋白酶对E-钙黏蛋白的降解作用,基质金属蛋白酶作为蛋白酶已被证实具有降解和改变细胞外基质的能力,其中包括E-钙黏蛋白,R-GSAC中基质金属蛋白酶的存在可能导致E-钙黏蛋白的降解,进而降低了其荧光信号强度。 "
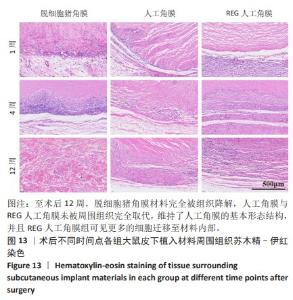
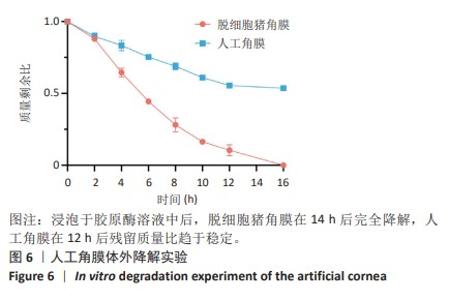
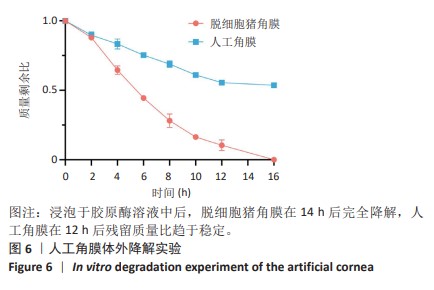
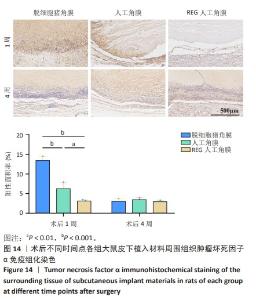
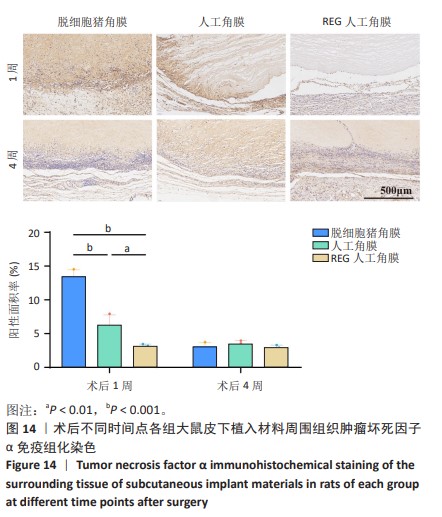
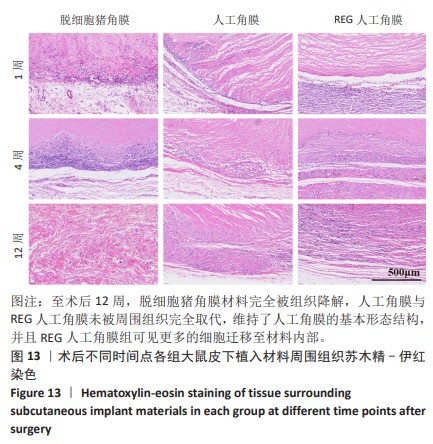
2.9 SD大鼠皮下植入生物相容性 2.9.1 实验动物数量分析 27只大鼠全部进入结果分析。 2.9.2 苏木精-伊红染色结果 术后不同时间点各组大鼠植入材料周围组织的苏木精-伊红染色结果,见图13。APCS中存在大量亲水性的胶原分子,植入皮下组织后吸水溶胀,由于溶胀作用使胶原分子间隙扩大,细胞更容易侵入导致APCS降解速率过快。术后1周,APCS组材料内胶原纤维由于吸水作用层状分布略微分散,有少量细胞进入角膜材料内,GSAC组与R-GSAC组材料内胶原纤维可见明显的层状结构,有较少的细胞进入材料内。术后4周,APCS组材料内的胶原纤维基本不可见层状结构,并且有大量细胞进入材料内部,GSAC组和R-GSAC组仍可见规则取向的胶原纤维,R-GSAC组材料内具有更密集的细胞长入。术后12周,APCS组材料完全被组织降解,GSAC组和R-GSAC组材料清晰可见,R-GSAC组材料中相比GSAC组可见更密集的细胞。结果表明,GSAC和R-GSAC中具有不可降解的聚合物组分聚甲基丙烯酸羟乙酯,未被周围组织完全取代,维持了人工角膜的基本形态结构,并且REG促进更多的细胞迁移至R-GSAC内部。"
| [1] CABRERA-AGUAS M, KHOO P, WATSON SL. Infectious keratitis: A review. Clin Exp Ophthalmol. 2022;50(5):543-562. [2] GAYTON JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405-412. [3] MOSHIRFAR M, MOODY JJ, BARKE MR, et al. The historical development and an overview of contemporary keratoprostheses. Surv Ophthalmol. 2022;67(4):1175-1199. [4] TEY KY, CHEONG EZK, ANG M. Potential applications of artificial intelligence in image analysis in cornea diseases: a review. Eye Vis (Lond). 2024;11(1):10. [5] RIAU AK, LWIN NC, GELFAND L, et al. Surface modification of corneal prosthesis with nano-hydroxyapatite to enhance in vivo biointegration. Acta Biomater. 2020;107:299-312. [6] KINOSHITA S, KOIZUMI N, UENO M, et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N Engl J Med. 2018; 378(11):995-1003. [7] ZHANG C, DU L, SUN P, et al. Construction of tissue-engineered full-thickness cornea substitute using limbal epithelial cell-like and corneal endothelial cell-like cells derived from human embryonic stem cells. Biomaterials. 2017;124:180-194. [8] WANG X, ELBAHRAWI RT, ABDUKADIR AM, et al. A proposed model of xeno-keratoplasty using 3D printing and decellularization. Front Pharmacol. 2023:14:1193606. doi: 10.3389/fphar.2023.1193606. [9] HE B, WANG J, XIE M, et al. 3D printed biomimetic epithelium/stroma bilayer hydrogel implant for corneal regeneration. Bioact Mater. 2022;17:234-247. [10] CATALÀ P, THURET G, SKOTTMAN H, et al. Approaches for corneal endothelium regenerative medicine. Prog Retin Eye Res. 2022;87: 100987. [11] ZHAO J, TIAN M, LI Y, et al. Construction of tissue-engineered human corneal endothelium for corneal endothelial regeneration using a crosslinked amniotic membrane scaffold. Acta Biomater. 2022;147: 185-197. [12] GHOSH A, SINGH VK, SINGH V, et al. Recent Advancements in Molecular Therapeutics for Corneal Scar Treatment. Cells. 2022;11(20):3310. [13] DOHLMAN C. The Boston Keratoprosthesis-The First 50 Years: Some Reminiscences. Annu Rev Vis Sci. 2022;8(1):1-32. [14] LI Z, WANG Q, ZHANG SF, et al. Timing of glaucoma treatment in patients with MICOF: A retrospective clinical study. Front Med (Lausanne). 2022;9:986176. [15] DONG Y, YANG J, WANG L, et al. An improved biofunction of Titanium for keratoprosthesis by hydroxyapatite-coating. J Biomater Appl. 2014; 28(7):990-997. [16] GABRIEL BS, ROBBINS CB, WISELY CE, et al. Incidence, risk factors, and treatment of retroprosthetic membranes following Boston keratoprosthesis eyes and the impact of glaucoma surgery. Graefes Arch Clin Exp Ophthalmol. 2024. doi: 10.1007/s00417-024-06445-6 [17] CHAROENROOK V, MICHAEL R, DE LA PAZ MF, et al. Comparison of long-term results between osteo-odonto-keratoprosthesis and tibial bone keratoprosthesis. Ocul Surf. 2018;16(2):259-264. [18] ARORA A, PANDEY SK, ROYCHOUDHURY A, et al. Piezoelectric harvest of osteo-odonto-lamina in modified osteo-odonto keratoprosthesis: A maxillofacial perspective. Natl J Maxillofac Surg. 2018;9(2):167. [19] HISHAM M, SALIH AE, BUTT H. 3D Printing of Multimaterial Contact Lenses. ACS Biomater Sci Eng. 2023;9(7):4381-4391. [20] RAESIAN P, RAD MS, KHODAVERDI E, et al. Preparation and characterization of fluorometholone molecular imprinted soft contact lenses as ocular controlled drug delivery systems. J Drug Deliv Sci Technol. 2021;64:102591. [21] JIRÁSKOVÁ N, ROZSIVAL P, BUROVA M, et al. AlphaCor artificial cornea: clinical outcome. Eye. 2011;25(9):1138-1146. [22] COASSIN M, ZHANG C, GREEN WR, et al. Histopathologic and Immunologic Aspects of AlphaCor Artificial Corneal Failure. Am J Ophthalmol. 2007;144(5):699-704.e4. [23] LIU T, SHEN M, HUANG L, et al. Characterization of hyperelastic mechanical properties for youth corneal anterior central stroma based on collagen fibril crimping constitutive model. J Mech Behav Biomed Mater. 2020;103:103575. [24] LAGALI N. Corneal Stromal Regeneration: Current Status and Future Therapeutic Potential. Curr Eye Res. 2020;45(3):278-290. [25] HAO Y, ZHOU J, TAN J, et al. Preclinical evaluation of the safety and effectiveness of a new bioartificial cornea. Bioact Mater. 2023;29: 265-278. [26] FERNÁNDEZ-PÉREZ J, MADDEN PW, BRADY RT, et al. The effect of prior long-term recellularization with keratocytes of decellularized porcine corneas implanted in a rabbit anterior lamellar keratoplasty model PLoS One. 2021;16(6):e0245406. [27] WANG X, SHAKEEL A, SALIH AE, et al. A scalable corneal xenograft platform: simultaneous opportunities for tissue engineering and circular economic sustainability by repurposing slaughterhouse waste. Front Bioeng Biotechnol. 2023;11:1133122. [28] LJUBIMOV AV, SAGHIZADEH M. Progress in corneal wound healing. Prog Retin Eye Res. 2015;49:17-45. [29] WANG SY, KIM H, KWAK G, et al. Development of Biocompatible HA Hydrogels Embedded with a New Synthetic Peptide Promoting Cellular Migration for Advanced Wound Care Management. Adv Sci (Weinh). 2018;5(11):1800852. [30] ZHANG L, CHEN L, XIANG Y, et al. Multifunctional integrally-medicalized hydrogel system with internal synergy for efficient tissue regeneration. Chem Eng J. 2021;406:126839. [31] HSU HC, KE YL, LAI YH, et al. Chondroitin Sulfate Enhances Proliferation and Migration via Inducing β-Catenin and Intracellular ROS as Well as Suppressing Metalloproteinases through Akt/NF-ϰB Pathway Inhibition in Human Chondrocytes. J Nutr. 2022;26(3): 307-313. [32] NZIGOU MOMBO B, BIJONOWSKI BM, RAAB CA, et al. Reversible photoregulation of cell-cell adhesions with opto-E-cadherin. Nat Commun. 2023;14(1):6292. [33] LEE S, KIM MS, JUNG SJ, et al. ERK activating peptide, AES16-2M promotes wound healing through accelerating migration of keratinocytes. Sci Rep. 2018;8(1):14398. [34] MAURIS J, WOODWARD AM, CAO Z, et al. Molecular basis for MMP9 induction and disruption of epithelial cell-cell contacts by galectin-3. J Cell Sci. 2014;127(Pt 14):3141-3148. |
| [1] | Dang Xiaowen, Huang Hailiang, Huang Lei, Wang Yajie . Research frontiers and hotspots of carbon nanomaterials in biomedical field over the past 10 years [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 752-760. |
| [2] | Wang Renzhi, Chen Yuanfen, Li Jinwei. 3D printed hollow pipe double-crosslinked hydrogel tissue engineering scaffold [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3432-3439. |
| [3] | Israrguli · Maimeti, Jia Sen, Liu Jia. Bone morphogenetic protein 2-loaded hydrogel induces osteogenic differentiation of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3301-3310. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||