Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (4): 522-527.doi: 10.12307/2024.994
Previous Articles Next Articles
Changes in pulmonary pericytes and tube formation of pulmonary vascular endothelial cells in mouse models of broncho-pulmonary dysplasia
Hu Guangzhi, Lu Hongyan
- Department of Pediatrics, Affiliated Hospital of Jiangsu University, Zhenjiang 212000, Jiangsu Province, China
-
Received:2022-12-10Accepted:2023-01-29Online:2024-02-08Published:2023-07-13 -
Contact:Lu Hongyan, Chief physician, Professor, Department of Pediatrics, Affiliated Hospital of Jiangsu University, Zhenjiang 212000, Jiangsu Province, China -
About author:Hu Guangzhi, Master, Department of Pediatrics, Affiliated Hospital of Jiangsu University, Zhenjiang 212000, Jiangsu Province, China -
Supported by:the National Natural Science Foundation of China, No. 82171702 (to LHY); Nature Science Foundation of Jiangsu Province, No. BK20201226 (to LHY)
CLC Number:
Cite this article
Hu Guangzhi, Lu Hongyan. Changes in pulmonary pericytes and tube formation of pulmonary vascular endothelial cells in mouse models of broncho-pulmonary dysplasia[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 522-527.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
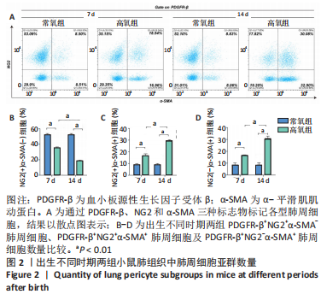
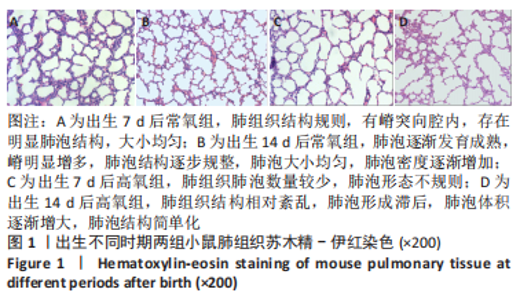
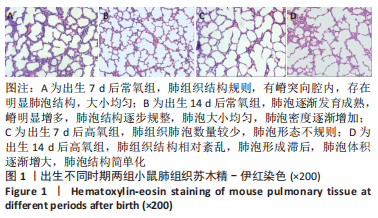
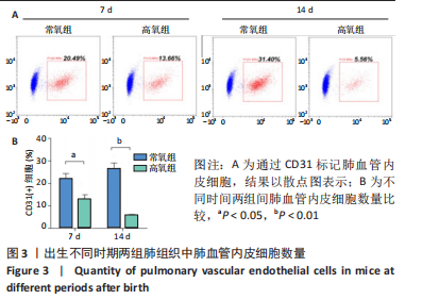
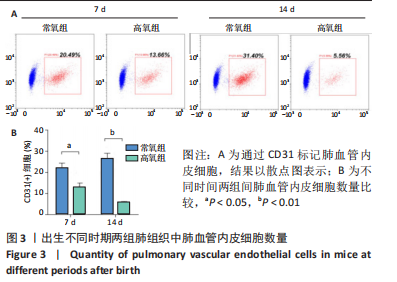
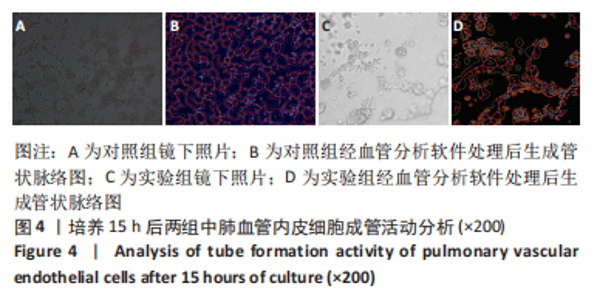
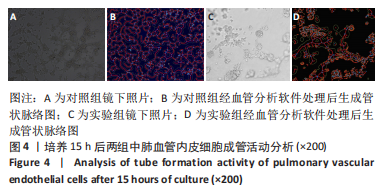
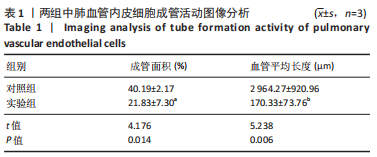
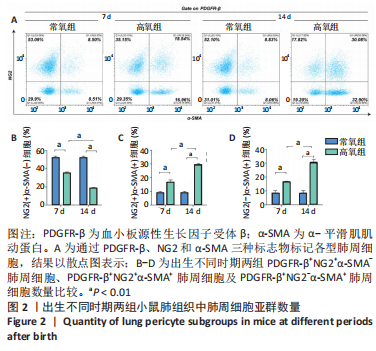
流式细胞术检测结果:与常氧组相比,高氧组出生7,14 d后肺组织中PDGFR-β+NG2-α-SMA+和PDGFR-β+NG2+α-SMA+肺周细胞数量均显著上升(P < 0.01),PDGFR-β+NG2+α-SMA-肺周细胞数量显著下降(P < 0.01)。与出生7 d后相比,高氧组出生14 d后的PDGFR-β+NG2+α-SMA-肺周细胞数量显著下降(P < 0.01),PDGFR-β+NG2+α-SMA+和PDGFR-β+NG2-α-SMA+肺周细胞数量均显著上升(P < 0.01),见图2。与常氧组相比,高氧组出生7,14 d后的肺血管内皮细胞数显著下降(P < 0.05),见图3。"
| [1] JOSHI S, KOTECHA S. Lung growth and development. Early Hum Dev. 2007;(12):83. [2] MORRISEY EE, HOGAN B. Preparing for the First Breath: Genetic and Cellular Mechanisms in Lung Development. Dev Cell. 2010;18(1):8-23. [3] WHITE ES. Commentary: A Breath of Fresh Air on the Mesenchyme: Impact of Impaired Mesenchymal Development on the Pathogenesis of Bronchopulmonary Dysplasia. Front Med (Lausanne). 2016;3:13. [4] LU X, GONG J, DENNERY PA, et al. Endothelial-to-mesenchymal transition: Pathogenesis and therapeutic targets for chronic pulmonary and vascular diseases. Biocheml Pharmacol. 2019;168:100-107. [5] 翟丽丽,杨迷玲,李争艳,等.周细胞的研究进展[J].现代肿瘤医学,2011,19(8):4. [6] Dessalles CA, Babataheri A, Barakat AI. Pericyte mechanics and mechanobiology. J Cell Sci. 2021;134(6):jcs240226. [7] HUNG CF, WILSON CL, SCHNAPP LM. Pericytes in the Lung. Adv Exp Med Biol. 2019;1122:41-58. [8] YAMAGUCHI M, HIRAI S, TANAKA Y, et al. Pericyte-myofibroblast transition in the human lung. Biochem Biophys Res Commun. 2020; 528(2):269-275. [9] RICARD N, TU L, LE HIRESS M, et al. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation. 2014;129(15):1586-1597. [10] WILSON CL, STEPHENSON SE, HIGUERO JP, et al. Characterization of human PDGFR-β-positive pericytes from IPF and non-IPF lungs. Am J Physiol Lung Cell Mol Physiol. 2018;315(6):L991-L1002. [11] BORDENAVE J, TU L, BERREBEH N, et al. Lineage Tracing Reveals the Dynamic Contribution of Pericytes to the Blood Vessel Remodeling in Pulmonary Hypertension. Arterioscler Thromb Vasc Biol. 2020;40(3): 766-782. [12] HUNG CF, MITTELSTEADT KL, BRAUER R, et al. Lung pericyte-like cells are functional immune sentinel cells. Am J Physiol Lung Cell Mol Physiol. 2017;312(4):L556-L567. [13] WU Y, LI P, GOODWIN AJ, et al. miR-145a Regulates Pericyte Dysfunction in a Murine Model of Sepsis. J Infect Dis. 2020;222(6):1037-1045. [14] DUMPA V, BHANDARI V. Surfactant, steroids and non-invasive ventilation in the prevention of BPD. Semin Perinatol. 2018;42(7):444-452. [15] 朱玥,卢红艳,郝晓波,等.SUMO 化修饰 C/EBPα在高氧暴露所致支气管肺发育不良早产大鼠中的动态表达及作用[J].中国当代儿科杂志,2018,20(5):68-74. [16] EILKEN HM, DIÉGUEZ-HURTADO R, SCHMIDT I, et al. Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1. Nat Commun. 2017;8(1):1574. [17] EHRENKRANZ RA, WALSH MC, VOHR BR, et al.Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353-1360. [18] SCHMIDT AR, RAMAMOORTHY C. Bronchopulmonary dysplasia. Pediatr Anesth. 2022;32(2):174-180. [19] CUMMINGS KW, BHALLA S. Pulmonary vascular diseases. Clin Chest Med. 2015;36(2):235-248, viii. [20] DENG X, BAO Z, YANG X, et al. Molecular mechanisms of cell death in bronchopulmonary dysplasia. Apoptosis. 2022. doi: 10.1007/s10495-022-01791-4. [21] KATO K, DIÉGUEZ-HURTADO R, PARK DY, et al. Pulmonary pericytes regulate lung morphogenesis. Nat Commun. 2018;9(1):2448. [22] ARMULIK A, GENOVÉ G, BETSHOLTZ C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193-215. [23] CRISAN M, YAP S, CASTEILLA L, et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell. 2008;3(3):301-313. [24] RICARD N, TU L, LE HIRESS M, et al. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation. 2014;129(15):1586. [25] PAYNE LB, ZHAO H, JAMES CC, et al. The Pericyte Microenvironment during Vascular Development. Microcirculation. 2019;26(2):e12554. [26] 蒋福林,艾冬青,官秋玥.周细胞概念及在血管形成信号转导通路研究中的进展[J].中国组织工程研究,2015,19(46):7504-7508. [27] ALARCON-MARTINEZ L, YEMISCI M, DALKARA T. Pericyte morphology and function. Histol Histopathol. 2021;36(6):18314. [28] CHI FH, WILSON CL, CHOW YH, et al. Effect of lung pericyte-like cell ablation on the bleomycin model of injury and repair. Am J Physiol. 2022;322(4):L607-L616. [29] LIU X, ROWAN SC, LIANG J, et al. Categorization of Lung Mesenchymal Cells in Development and Fibrosis. iScience. 2021;24(6):102551. [30] MATHEW R. Signaling Pathways Involved in the Development of Bronchopulmonary Dysplasia and Pulmonary Hypertension. Children (Basel). 2020;7(8):100. [31] HUNG C, LINN G, CHOW YH, et al. Role of Lung Pericytes and Resident Fibroblasts in the Pathogenesis of Pulmonary Fibrosis. Am J Respir Crit Care Med. 2013;188(7):820-830. [32] 胡广志,卢红艳.肺周细胞与肺发育及肺部疾病研究进展[J].国际儿科学杂志,2022,49(3):197-201. [33] SHAMMOUT B, JOHNSON JR. Pericytes in Chronic Lung Disease. Adv Exp Med Biol. 2019;1147:299-317. [34] YUAN K, AGARWAL S, CHAKRABORTY A, et al. Lung Pericytes in Pulmonary Vascular Physiology and Pathophysiology. Compr Physiol. 2021;11(3):2227-2247. [35] ZHANG Y, DONG X, SHIRAZI J, et al. Pulmonary endothelial cells exhibit sexual dimorphism in their response to hyperoxia. Am J Physiol Heart Circ Physiol. 2018;315(5):H1287-H1292. [36] 王思思, 伍金林.高氧诱导肺血管内皮细胞损伤与支气管肺发育不良的研究进展[J]中华妇幼临床医学杂志(电子版),2021,17(3): 368-372. [37] WU CF, CHIANG WC, LAI CF, et al. Transforming Growth Factor β-1 Stimulates Profibrotic Epithelial Signaling to Activate Pericyte-Myofibroblast Transition in Obstructive Kidney Fibrosis. Am J Pathol. 2013;182(1):118-131. [38] YAO F, LUO Y, LIU YC, et al. Imatinib inhibits pericyte-fibroblast transition and inflammation and promotes axon regeneration by blocking the PDGF-BB/PDGFRβ pathway in spinal cord injury. Inflamm Regen. 2022;42(1):44. [39] YANG T, GUO L, FANG Y, et al. Pericytes of Indirect Contact Coculture Decrease Integrity of Inner Blood-Retina Barrier Model In Vitro by Upgrading MMP-2/9 Activity. Dis Markers. 2021;2021:7124835. [40] DONOGHUE L, TYBURSKI JG, STEFFES CP, et al. Vascular endothelial growth factor modulates contractile response in microvascular lung pericytes. Am J Surg. 2006;191(3):349-352. [41] EDELMAN DA, JIANG Y, TYBURSKI J, et al. Pericytes and Their Role in Microvasculature Homeostasis. J Surg Res. 2006;135(2):305-311. |
| [1] | Pan Yujun, Shi Changjiang, Cao Sheng, Mu Huaizhao, Zhang Yilong, Wang Kaihua. An injectable dextran/gelatin composite hydrogel loaded with exosomes for repair of rat intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5477-5482. |
| [2] | Liu Huan, Li Han, Ma Yunhao, Zhong Weijian, Ma Guowu. Osteogenic capacity of partially demineralized dentin particles in the maxillary sinus lift [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 354-359. |
| [3] | Dong Shiwu, Zhou Lanxi, Shao Lu, Yu Zhengwen. Effect of magnesium alloy biomaterial degradation on endothelialized cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(21): 3398-3406. |
| [4] | Liang Hanying, Ma Yunhao, Li Han, Li Dongyang, Zhong Weijian, Ma Guowu. Osteogenic effects of partially demineralized autogenous dentin particles in implant site preservation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(16): 2488-2492. |
| [5] | Li Chuanhong, Yu Xing, Yang Yongdong, Yang Kaitan, Zhao He. Subarachnoid injection via the posterior atlanto-occipital interspace is an alternative way of administration in a rat model of spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(33): 5376-5383. |
| [6] | Guo Yangyan, Yu Zhengwen, Zhang Jian. Research hotspots of magnesium alloy biomaterials in an in vivo animal [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3556-3565. |
| [7] | Wang Shuyun, Xie Junhui, Yu Xuefeng. Effect and mechanism of mesenchymal stem cells in the treatment of diabetic nephropathy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 148-152. |
| [8] | Zhang Chao, Lü Xin. Heterotopic ossification after acetabular fracture fixation: risk factors, prevention and treatment progress [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1434-1439. |
| [9] | Zhao Binbin, Zhong Weijian, Ma Guowu, Li Yongqi, Wang Ning. Comparison of the osteogenic effect of three different bone graft materials [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1507-1510. |
| [10] | Huo Shaochuan, Wang Haibin, Tang Hongyu, Wang Yueqi, Chen Qunqun, Feng Ziyu, Li Yikai. Treatment of knee osteoarthritis by tonifying kidney and spleen and activating blood circulation herbs via chondrocyte IL-1beta/ERR-alpha/SOX9/Col2alpha1 signaling pathway in a rat model [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(35): 5577-5581. |
| [11] | Cui Qingda, Wang Haige, Zhao Haijun, Liu Wei, Bi Zhenggang. Removal of transepiphyseal plate after fixation for a certain period of time: growth inhibition of epiphyseal plate after a period of observation [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(3): 372-377. |
| [12] |
Shang Zhizhong, Jiang Yanbiao, Yao Lan, Wang Anan, Wang Hongxia, Tian Yuanxin, Liu Dengrui, Ma Bin .
Feasibility of stem cell therapy for renal ischemia/reperfusion injury: a systematic review based on animal experiments [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(25): 4094-4100. |
| [13] | Lin Yunzhi, Fang Guofang, Li Xiuwang, Wu Jiachang, Wu Mingjie, Tan Liang, Lai Guohua, Ye Zhuofeng, Sang Hongxun. Application of semi-automatic spinal surgery robot system in spinal surgery [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(24): 3792-3796. |
| [14] | Cheng Lei, Jin Jian, Hu Jingguo, Lu Yusong. Inflammatory reaction and lactic acid concentration after implantation of polylactic acid rib nail versus pure titanium embracing fixator in animals#br# [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(16): 2567-2571. |
| [15] | Cao Yang, Hu Ping, Tian Min, Wei Fang, Gu Qing, Lü Hongbin. Effects of 6-phosphofructokinase-2/fructose-2,6-bisphosphatase 3 on tubule formation of human umbilical vein endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(14): 2217-2222. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||