Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (11): 1659-1668.doi: 10.12307/2023.182
Previous Articles Next Articles
Intervention with platelet-rich fibrin in rabbit models after microvascular anastomosis surgery of the femoral artery: changes in vascular structure and soft tissue
Lu Yangyang, Yan Ruihong, Qiu Zhongpeng, Dai Yi, Wang Zixin, Wang Weishan, Shi Chenhui, Du Xinhui, Li Gang
- Orthopedics Center, the First Affiliated Hospital of Shihezi University Medical School, Shihezi 832000, Xinjiang Uygur Autonomous Region, China
-
Received:2022-01-17Accepted:2022-04-18Online:2023-04-18Published:2022-09-26 -
Contact:Li Gang, Chief physician, Associate professor, Master’s supervisor, Orthopedics Center, the First Affiliated Hospital of Shihezi University Medical School, Shihezi 832000, Xinjiang Uygur Autonomous Region, China Du Xinhui, Chief physician, Master’s supervisor, Orthopedics Center, the First Affiliated Hospital of Shihezi University Medical School, Shihezi 832000, Xinjiang Uygur Autonomous Region, China -
About author:Lu Yangyang, Master candidate, Orthopedics Center, the First Affiliated Hospital of Shihezi University Medical School, Shihezi 832000, Xinjiang Uygur Autonomous Region, China -
Supported by:Science and Technology Program Project of Shihezi University, No. ZZZC 201947A (to DXH)
CLC Number:
Cite this article
Lu Yangyang, Yan Ruihong, Qiu Zhongpeng, Dai Yi, Wang Zixin, Wang Weishan, Shi Chenhui, Du Xinhui, Li Gang. Intervention with platelet-rich fibrin in rabbit models after microvascular anastomosis surgery of the femoral artery: changes in vascular structure and soft tissue[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(11): 1659-1668.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
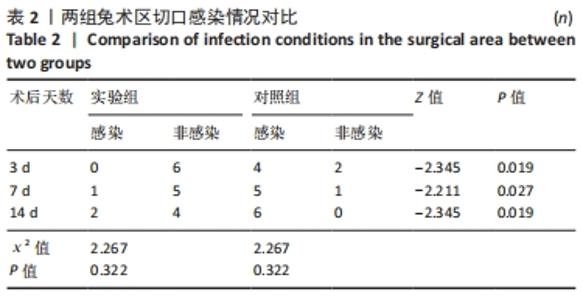
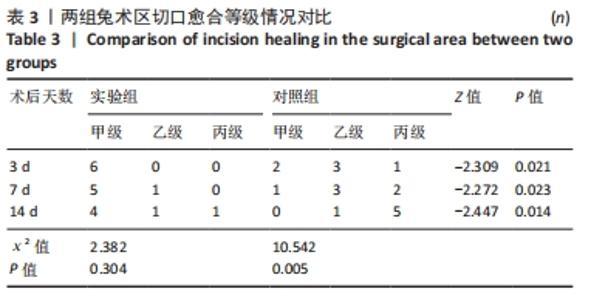
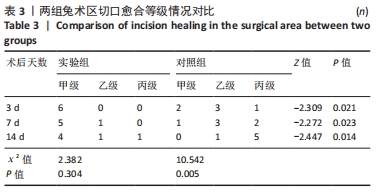
2.1 实验动物数量分析 36只兔均造模成功,无麻醉及手术意外导致实验动物死亡的情况,仅在造模后14 d对照组中有2只兔取材时因术区粘连严重,取材完毕后无条件结扎股动脉断端,导致出血过多而死亡。术后所有兔精神、肌力及饮食状态恢复良好,36只兔全部纳入结果分析。 2.2 两组兔术后大体观察结果 2.2.1 术后切口感染及愈合情况 理想的手术切口愈合期间术区不应有红肿及硬结、局部皮温高于健侧或存在异常分泌物渗出等炎症反应情况。实验组中术后7 d有1只兔子术区切口出现红肿并伴有少量血性积液渗出等情况,定为乙级愈合;术后14 d观察到1只兔子术区切口乙级愈合,1只兔子术区切口化脓,定为丙级愈合;其余15只兔术区切口均愈合良好,属于甲级愈合。实验组术区切口感染情况见表2,术区切口愈合等级情况见表3。实验组组内术区切口感染情况及愈合等级情况对比差异均无显著性意义(P > 0.05)。"
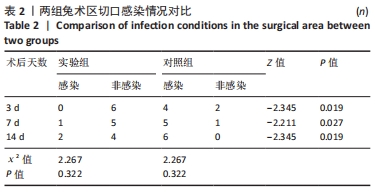
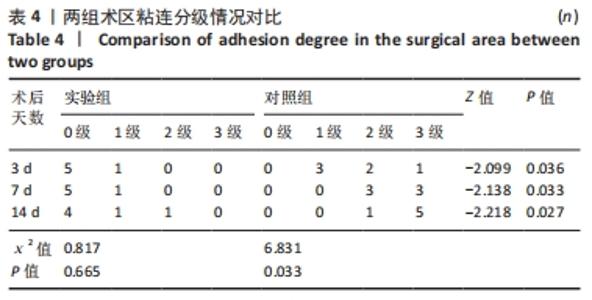
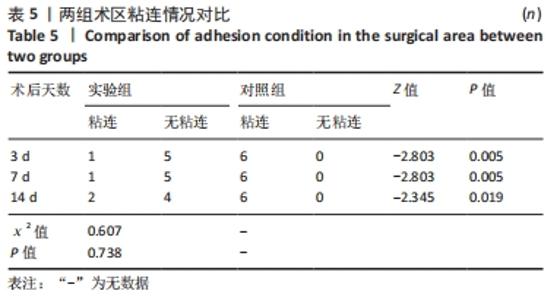
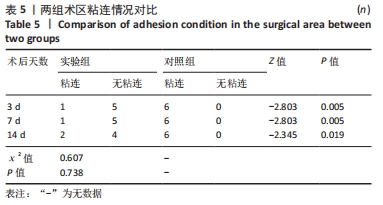
对照组中术后3,7 d分别有2,1只共计3只兔术区切口愈合良好,无任何感染表现,评定为甲级愈合。对照组中术后3,7,14 d时分别有4,5,6只共计15只兔术区切口出现炎症反应,其中7只术区切口出现红肿并伴不同程度的血性分泌物渗出的情况,但均无化脓表现,愈合情况定为乙级愈合,另外8只术区切口发生了明显的化脓,定为丙级愈合。对照组术区切口感染情况见表2,术区切口愈合等级情况见表3。对照组组内术区切口感染情况对比差异无显著性意义(P > 0.05),对照组组内术区切口愈合等级情况对比差异有显著性意义(P < 0.05)。 两组之间术区切口感染的动物数量及术区切口愈合等级情况对比存在明显差异,差异均有显著性意义(P < 0.05),见表2,3。 2.2.2 术后术区股动脉与周围组织粘连情况 结果显示,实验组中术区股动脉组织的粘连程度以0级为主,表现为1级粘连的在术后3个时间节点上各有1只兔,组内无粘连程度表现为3级的兔,见表4。实验组组内粘连程度在术后3,7,14 d上的表现没有明显变化,差异无显著性意义(P > 0.05)。实验组组内术区股动脉组织粘连的兔数量在术后3个时间节点无明显变化,差异无显著性意义(P > 0.05),见表5。 "
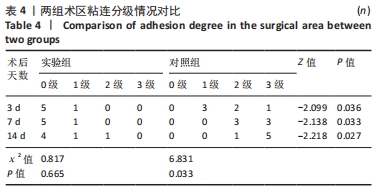
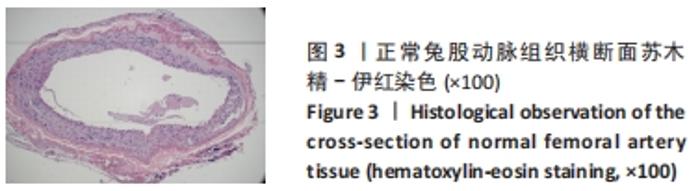
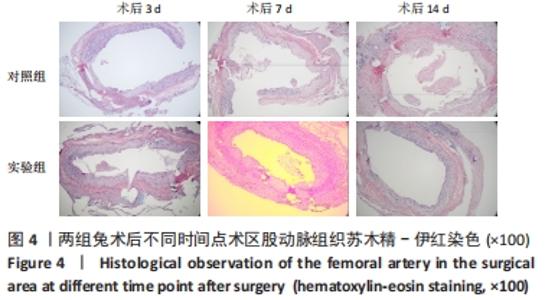
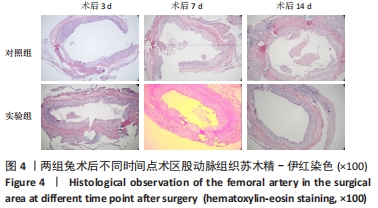
对照组中所有兔术区股动脉组织均有不同程度的粘连,且以2级和3级为主,0级粘连程度的动物数量为0。对照组组内粘连程度于术后3,7,14 d逐渐加重,差异有显著性意义(P < 0.05),见表4。 术后不同时间节点,两组之间术区股动脉与周围组织粘连程度相比差异均有显著性意义(P < 0.05),且实验组整体的粘连程度均轻于对照组,同时实验组中术区粘连的动物数量明显少于对照组(P < 0.05),见表5。 2.3 两组兔术区股动脉组织苏木精-伊红染色结果 每组各时间选取染色结果满意且具有代表性的1张苏木精-伊红染色切片,共计6张切片进入分析。 未损伤的新西兰大白兔股动脉组织横断面的苏木精-伊红染色结果可见血管内皮细胞均匀地散布于血管管腔内侧,血管内弹力膜层连续无中断,血管内膜层及外膜层完好无损,均质红染的血管平滑肌层位于内膜及外膜层之间,见图3。 "

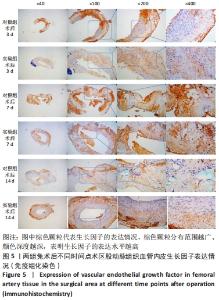
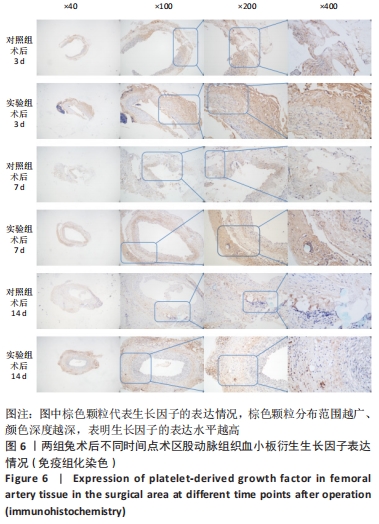
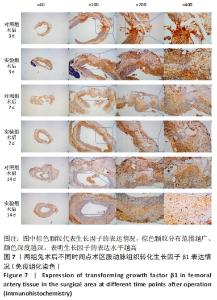
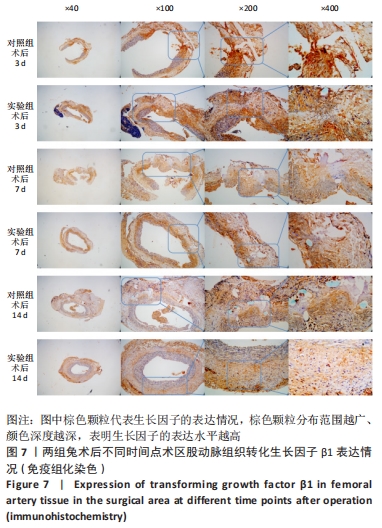
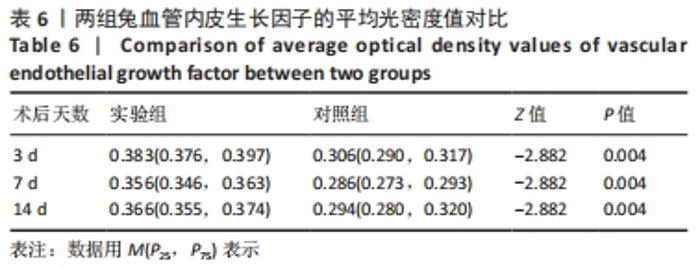
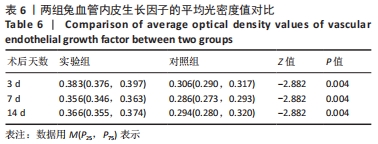
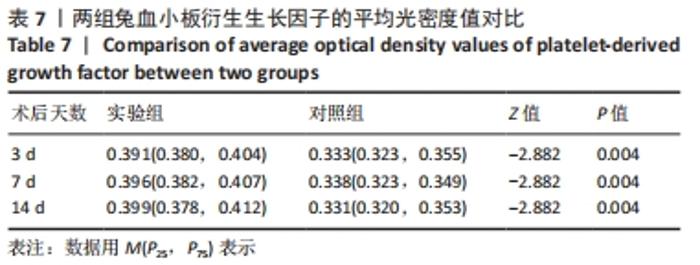
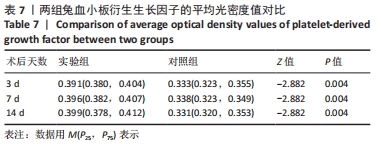
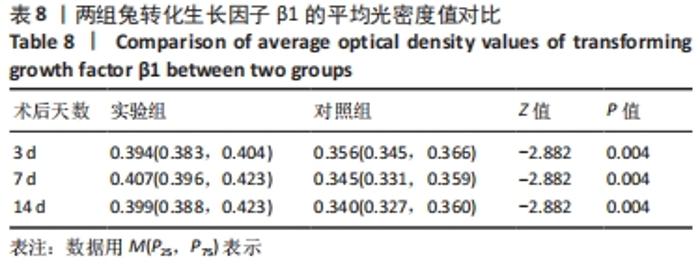
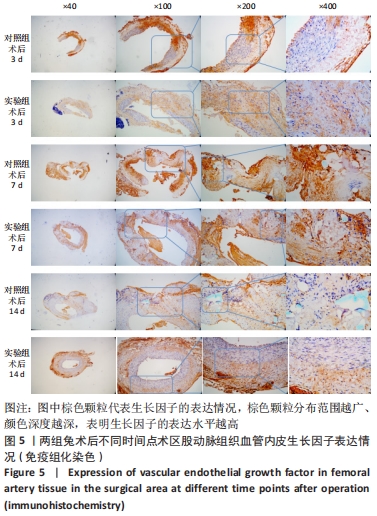
术后7 d时,对照组兔股动脉吻合口区域断裂的血管开始修复,可见部分血管内皮细胞生长,内弹力膜层部分恢复,血管内膜层大部分中断,血管中膜平滑肌细胞坏死开始增殖,纤维结缔组织和血管外膜层结缔组织生长明显。而实验组兔股动脉吻合口区域断裂的血管基本修复,血管内皮细胞生长活跃,内弹力膜层大部分恢复,血管内膜层基本连续,仅少部分中断,血管中膜平滑肌细胞逐渐修复,血管中膜、外膜结构恢复良好,界线基本清晰,见图4。 术后14 d时,对照组兔股动脉吻合口区域断裂的血管修复基本完整,血管腔内可见血栓形成,血管腔较为狭窄,血管内皮细胞生长活跃,内弹力膜层大部分恢复,血管内膜层部分是由平滑肌细胞增殖迁移构成,血管中膜平滑肌细胞过度增殖,纤维结缔组织和血管外膜层结缔组织生长明显,血管内膜、中膜、外膜结构紊乱,界线不清。实验组中观察到兔股动脉吻合口区域断裂的血管修复完整,无原有结构异位出现的现象,血管腔内未见血栓形成,无明显狭窄,血管内皮细胞生长良好,血管内弹力膜层基本恢复正常,血管内膜层连续且修复完整,血管中膜平滑肌细胞无过度增殖现象,血管中膜层及外膜层结构原位修复完好且界线清晰,见图4。 总之,苏木精-伊红染色结果显示:对照组中血管内皮细胞开始生长的时间为术后第7天,而实验组中术后第3天就观察到了血管内皮细胞生长现象;同样内弹力膜层开始出现的时间在对照组和实验组中分别为术后第7天和第3天;实验组中血管内皮细胞整体生长情况较对照组更为活跃,实验组血管内膜层的重建更为完善,对照组血管中膜平滑肌细胞坏死、迁移及创伤性过度增殖情况比实验组严重,实验组术后3,7,14 d时血管组织各层结构的恢复情况均优于对照组。 2.4 两组兔术区股动脉组织免疫组织化学染色结果 富血小板纤维蛋白成分中的VEGF、PDGF及转化生长因子β1在组织损伤的修复过程中起着重要作用。实验组与对照组在术后3,7,14 d分别进行VEGF、PDGF及转化生长因子β1的免疫组化实验,结果发现实验组的血管组织管腔结构大体上更加完整,于3个时间节点上均比对照组修复得完好。同时,实验组中3种生长因子所对应的棕色颗粒数量及表达面积均多于对照组,见图5-7。 "
| [1] PAKALA R, WILLERSON JT, BENEDICT CR. Effect of serotonin, thromboxane A2, and specific receptor antagonists on vascular smooth muscle cell proliferation. Circulation. 1997;96(7):2280-2286. [2] 张铁慧, 梁武, 任远飞, 等. 含血管内皮生长因子缓释微粒显微缝线促进大鼠小血管吻合后的内皮再生[J]. 中国组织工程研究,2018,22(6):877-882. [3] ZHANG L, SI T, FISCHER AJ, et al. Coaxial Electrospray of Ranibizumab-Loaded Microparticles for Sustained Release of Anti-VEGF Therapies. PLoS One. 2015; 10(8):e0135608. [4] 贾亚超, 康庆林, 柴益民. 显微血管吻合术后血栓形成的防治进展[J]. 中华显微外科杂志,2015,38(2):205-208. [5] HANASONO MM, BUTLER CE. Prevention and treatment of thrombosis in microvascular surgery. J Reconstr Microsurg. 2008;24(5):305-314. [6] 肖得力, 孙崟喆, 崔城, 等. 浓缩生长因子纤维蛋白膜的制备及降解性能[J]. 中国组织工程研究,2021,25(34):5413-5419. [7] KUBESCH A, BARBECK M, AL-MAAWI S, et al. A low-speed centrifugation concept leads to cell accumulation and vascularization of solid platelet-rich fibrin: an experimental study in vivo. Platelets. 2019;30(3):329-340. [8] DOHAN EHRENFEST DM, PINTO NR, PEREDA A, et al. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets. 2018;29(2):171-184. [9] CHIARAVALLOTI AJ, ZUBKOV B, ZUBKOV A. Treatment of a Chronic Cutaneous Surgical Wound With Platelet-Rich Fibrin. Dermatol Surg. 2018;44(3):449-452. [10] BIELECKI T, DOHAN EHRENFEST DM. Platelet-rich plasma (PRP) and Platelet-Rich Fibrin (PRF): surgical adjuvants, preparations for in situ regenerative medicine and tools for tissue engineering. Curr Pharm Biotechnol. 2012;13(7):1121-1130. [11] PHILLIPS R, DUDLEY H. The effect of tetracyline lavage and trauma on visceral and parietal peritoneal ultrastructure and adhesion formation. Br J Surg. 1984; 71(7):537-539. [12] 刘洋波. 几丁聚糖对显微血管吻合后血管壁愈合过程的影响研究[D]. 衡阳:南华大学,2007. [13] MARUCCIA M, FATIGATO G, ELIA R, et al. Microvascular coupler device versus hand‐sewn venous anastomosis: A systematic review of the literature and data meta‐analysis. Microsurgery. 2020;40(5):608-617. [14] 侯彪. 一种新型光敏粘合剂联合温敏材料泊洛沙姆行血管吻合的实验研究[D]. 衡阳:南华大学,2016. [15] 查选平. 微型钛血管吻合夹吻合血管的实验研究[D]. 上海:第二军医大学,2002. [16] 侯毅, 顾立强. 显微血管吻合技术的现状与展望[J]. 中华显微外科杂志,2014, 37(2):201-204. [17] ZHANG SM, ZHU LH, CHEN HZ, et al. Interferon regulatory factor 9 is critical for neointima formation following vascular injury. Nat Commun. 2014;5(1):1-17. [18] WEINTRAUB WS. The pathophysiology and burden of restenosis. Am J Cardiol. 2007;100(5A):3K-9K. [19] SONG SH, KIM K, JO EK, et al. Fibroblast growth factor 12 is a novel regulator of vascular smooth muscle cell plasticity and fate. Arterioscler Thromb Vasc Biol. 2016;36(9):1928-1936. [20] SHI N, CHEN SY. Mechanisms simultaneously regulate smooth muscle proliferation and differentiation. J Biomed Res. 2014;28(1):40-46. [21] 何凌锋, 李学渊, 王欣, 等. 断指再植术后不同用药方案的临床病例对照研究[J]. 中华手外科杂志,2014,30(3):230-231. [22] 贾亚超. 内皮祖细胞在血管吻合术后血栓预防及血管修复中的作用[D]. 上海:上海交通大学,2016. [23] KNAFL D, THALHAMMER F, VOSSEN MG. In-vitro release pharmacokinetics of amikacin, teicoplanin and polyhexanide in a platelet rich fibrin-layer (PRF)-a laboratory evaluation of a modern, autologous wound treatment. PLoS One. 2017;12(7):e0181090. [24] CHOUKROUN J, ADDA F, SCHOEFFER C, et al. PRF: an opportunity in perio-implantology. Implantodontie, 2000;42:55-62. [25] KARIMI K, BSC HR. The Benefits of Platelet-Rich Fibrin - ScienceDirect. Facial Plast Surg Clin North Am. 2019;27(3):331-340. [26] UTOMO DN, MAHYUDIN F, HERNUGRAHANTO KD, et al. Implantation of platelet rich fibrin and allogenic mesenchymal stem cells facilitate the healing of muscle injury: An experimental study on animal. Int J Surg. 2018;11:4-9. [27] MIRON RJ, FUJIOKA-KOBAYASHI M, BISHARA M, et al. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng Part B Rev. 2017;23(1):83-99. [28] ANITUA E, NURDEN P, PRADO R, et al. Autologous fibrin scaffolds: When platelet- and plasma-derived biomolecules meet fibrin. Biomaterials. 2019;192:440-460. [29] TALBOT S, FOSTER SL, WOOLF CJ. Neuroimmunity: Physiology and Pathology. Annu Rev Immunol. 2016;34(1):421-447. [30] MEDZHITOV R. Origin and physiological roles of inflammation. Nature. 2008; 454(7203):428-435. [31] NASIRZADE J, KARGARPOUR Z, HASANNIA S, et al. Platelet Rich Fibrin Elicits an Anti inflammatory Response in Macrophages In Vitro. J Periodontol. 2020; 91(2):244-252. [32] CIESLIK-BIELECKA A, DOHAN EHRENFEST DM, LUBKOWSKA A, et al. Microbicidal properties of Leukocyte- and Platelet-Rich Plasma/Fibrin (L-PRP/L-PRF): new perspectives. J Biol Regul Homeost Agents. 2012;26(2 Suppl 1):43S-52S. [33] 王煜慧, 吕慧欣, 张明锐, 等. 富血小板纤维蛋白促进牙龈组织修复和再生的研究[J]. 口腔医学研究,2022,38(2):144-149. [34] 徐海燕, 刘斌, 戴太强, 等. PRF 促进小鼠全层皮肤损伤修复的实验研究[J]. 口腔医学研究,2017,33(8):816-819. [35] NURDEN AT. The biology of the platelet with special reference to inflammation, wound healing and immunity. Front Biosci (Landmark Ed). 2018;23(4):726-751. [36] BAI MY, WANG CW, WANG JY, et al. Three-dimensional structure and cytokine distribution of platelet-rich fibrin. Clinics (Sao Paulo). 2017; 72(2):116-124. [37] MARTÍNEZ CE, SMITH PC, PALMA ALVARADO VA. The influence of platelet-derived products on angiogenesis and tissue repair: a concise update. Front Physiol. 2015;6:290. [38] MELINCOVICI CS, BOSCA AB, SUSMAN S, et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59(2):455-467. [39] 冒慧敏, 史大卓, 刘秀华. 血小板源性生长因子对血管平滑肌细胞效应的机制研究进展[J]. 生理科学进展,2015,46(5):359-364. [40] PARK ES, PA LEE K, JUNG SH, et al. Compound K, an intestinal metabolite of ginsenosides, inhibits PDGF-BB-induced VSMC proliferation and migration through G1 arrest and attenuates neointimal hyperplasia after arterial injury. Atherosclerosis. 2013;228(1):53-60. [41] KIM JY, KIM KH, LEE WR, et al. Apamin inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and migration through suppressions of activated Akt and Erk signaling pathway. Vasc Pharmacol. 2015;70:8-14. [42] CHEN Z, CAI Y, ZHANG W, et al. Astragaloside IV inhibits platelet-derived growth factor-BB-stimulated proliferation and migration of vascular smooth muscle cells via the inhibition of p38 MAPK signaling. Exp Ther Med. 2014;8(4):1253-1258. [43] 朱晋坤, 毛华, 尹扬光, 等. 血小板源性生长因子和血小板源性内皮细胞生长因子在内皮细胞和血管平滑肌细胞中的作用研究[J]. 中国全科医学,2015, 18(9):1023-1028. [44] DAVIS VL, ABUKABDA AB, RADIO NM, et al. Platelet-rich preparations to improve healing. Part I: workable options for every size practice. J Oral Implantol. 2014; 40(4):500-510. [45] BARRIENTOS S, STOJADINOVIC O, GOLINKO MS, et al. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585-601. [46] WU LM, WANG JK, LIU J, et al. Gait analysis combined with the expression of TGF-β1, TGF-β3 and CREB during Achilles tendon healing in rat. Chin J Traumatol. 2021;24(6):360-367. [47] LIU X, JOSHI SK, RAVISHANKAR B, et al. Upregulation of transforming growth factor-β signaling in a rat model of rotator cuff tears. J Shoulder Elbow Surg. 2014;23(11):1709-1716. [48] 周蓉芳. 自体纤维蛋白凝胶在甲状腺手术中的应用探索[D]. 衡阳:南华大学, 2019. [49] 宋建星, 郭恩覃. 微型血管吻合夹与针线吻合方法的比较研究[J]. 中国修复重建外科杂志,2001,15(5):312-314. [50] 苏铁柱,宗双乐,齐巍,等.动脉硬化患者动静脉内瘘术不同血管吻合方法的临床效果比较[J]. 中国医药,2018,13(10):1544-1546. [51] 郑守华,张水军,宋燕,等.人造血管动静脉端侧吻合移植手术71例体会[J].中华显微外科杂志,2017,40(2):185-187. [52] 杨鹏鹏,李柯柯. 动静脉内瘘术中不同血管吻合方法在尿毒症并动脉硬化患者中的应用效果比较[J].河南医学研究,2021,30(5):867-869. [53] 王思夏,战杰,石强,等. 应用微血管吻合器吻合动脉与静脉的临床体会[J]. 中华显微外科杂志,2015,38(1):84-85. |
| [1] | Yang Zhishan, Tang Zhenglong. YAP/TAZ, a core factor of the Hippo signaling pathway, is involved in bone formation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1264-1271. |
| [2] | Xu Cong, Zhao He, Sun Yan. Regeneration of facial nerve injury repaired by biomaterial nerve conduits [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1089-1095. |
| [3] | Ke Weiqiang, Chen Xianghui, Chen Xiaoling, Meng Jie, Ma Yanlin. Rituximab combined with autologous peripheral blood stem cell transplantation in the treatment of diffuse large B-cell lymphoma and the expression of related factors [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 915-920. |
| [4] | Xu Qijing, Yang Yichun, Lei Wei, Yang Ying, Yu Jiang, Xia Tingting, Zhang Meng, Zhang Tao, Zhang Qian. Advances and problems in cell-free treatment of diabetic skin chronic wounds [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 962-969. |
| [5] | Liu Hongwen, Li Jiao, Xu Wenhao, Nie Hua, Liu Shaojiang, Xu Jie, Yin Li. Differential expression profiles of microRNAs in muscle tissue of denervated skeletal muscle atrophy rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 732-737. |
| [6] | Zhang Jinbiao, Li Xiaoming, Xing Wanlin, Ma Fei, Yu Qiaoya, Dai Rongqin. Early warning efficacy of thrombus molecular markers after total knee arthroplasty in patients complicated with venous thromboembolism [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(4): 572-577. |
| [7] | Chen Jingqiao, Li Ying, Meng Maohua, Xu Xingxing, Wang Qinying, Wang Huan, Lu Jing, Shu Jiayu, Dong Qiang. Research progress in platelet-rich fibrin in stomatology [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 441-446. |
| [8] | Xie Pingjin, Luo Zhen, Lu Qigui, Guo Yanxing, Chen Qunqun, Li Feilong. Effect of ligustrazine and overexpression of miR-20b-5p on synovial, cartilage and subchondral bone angiogenesis in rats with early-stage knee osteoarthritis: a histological observation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 237-245. |
| [9] | Zhang Luyao, Yang Kang. Effects of different exercises on renal interstitial fibrosis in type 2 diabetic mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 200-207. |
| [10] | Niu Zihan, Yu Yang, Ai Jiang, Bu Panpan, Li Wenbo, Suriye·Reheman, Ma Shaolin. Total flavonoids of Hippophae rhamnoides L. interfere with the regression of hypertrophic scar tissue blocks in a rabbit ear model [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 258-263. |
| [11] | Peng Kun. Properties of force growth factor E peptide bionic bone matrix with polyethylene glycol derivative hydrogel as a carrier [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(12): 1811-1816. |
| [12] | Ma Hongfeng, Ren Qing, Cheng Wanmin, Wang Jiguo, Meng Qingyan. Effects of Sanhuang gel on wound healing, basic fibroblast growth factor and interleukin 17 levels of auricular skin defect in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(12): 1842-1847. |
| [13] | He Ruya, Liu Yunling, Nie Minhai, Liu Xuqian. Repairing equivalent injury of oral mucosa with concentrated growth factor fibrin membrane combined with recombinant human epidermal growth factor active protein polypeptide complex [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(12): 1848-1855. |
| [14] | Feng Junming, Xiong Xianmei, Ma Liqiong, Zhang Yan, Chen Zijie, Li Shijie, Chen Baixing, Jiang Ziwei, Zeng Zhanpeng, Gao Yijia . Comparison of platelet-rich plasma, concentrated growth factor and 3D micro-nanostructure composite scaffolds in repair of rabbit radius defects [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(12): 1906-1913. |
| [15] | Zhu Zhoujun, Zhang Jiahui, Gao Jian, Xu Bin, Wang Chao, Xiang Wei, Jia Haoruo, Wang Guosheng. Effects of epidermal growth factor receptor on proliferation, differentiation and apoptosis of mouse articular cartilage surface cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(11): 1728-1732. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||