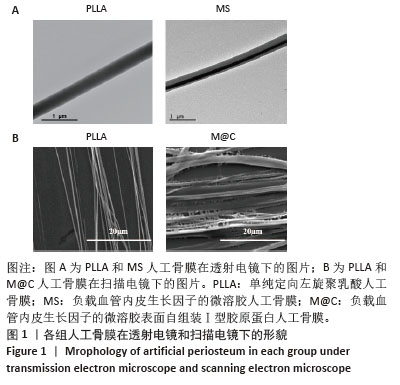
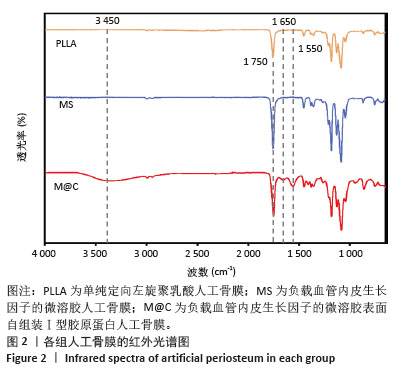
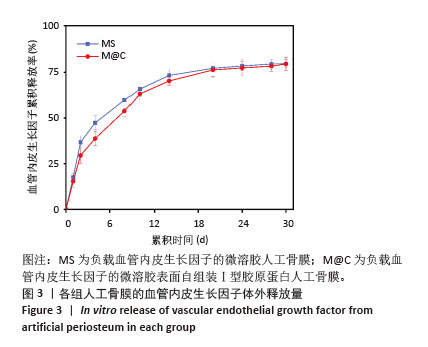
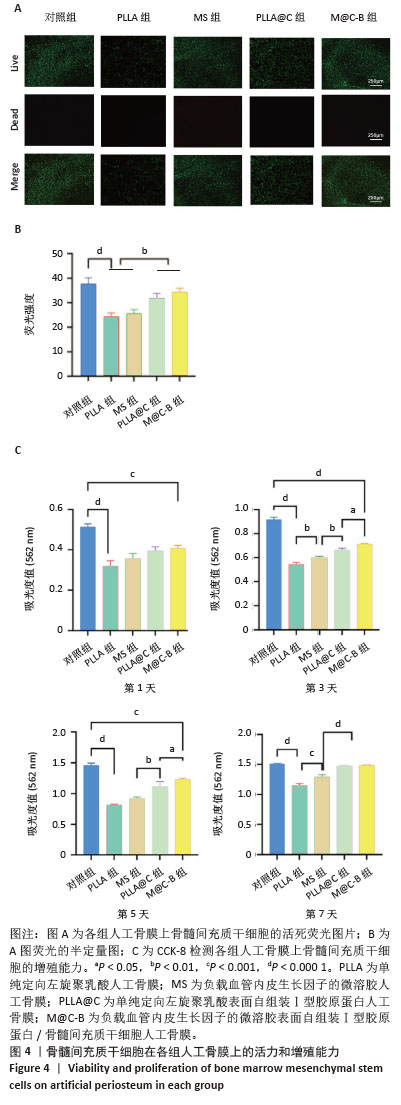
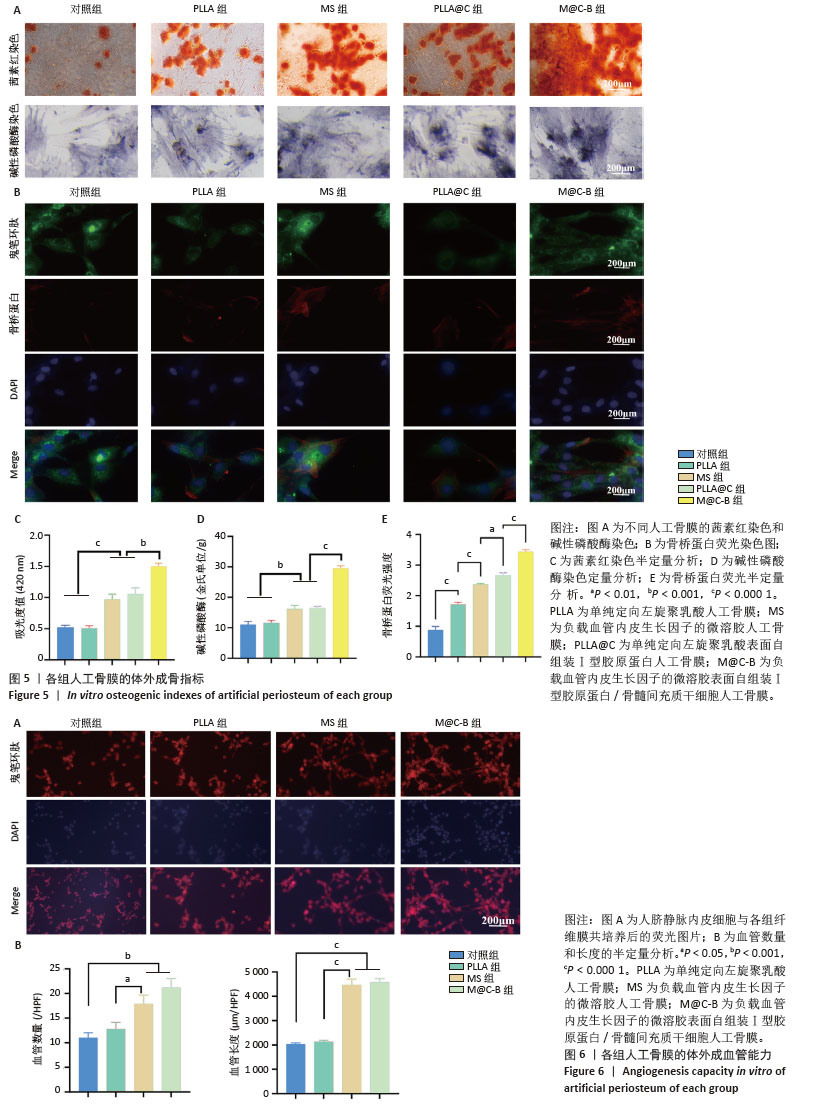
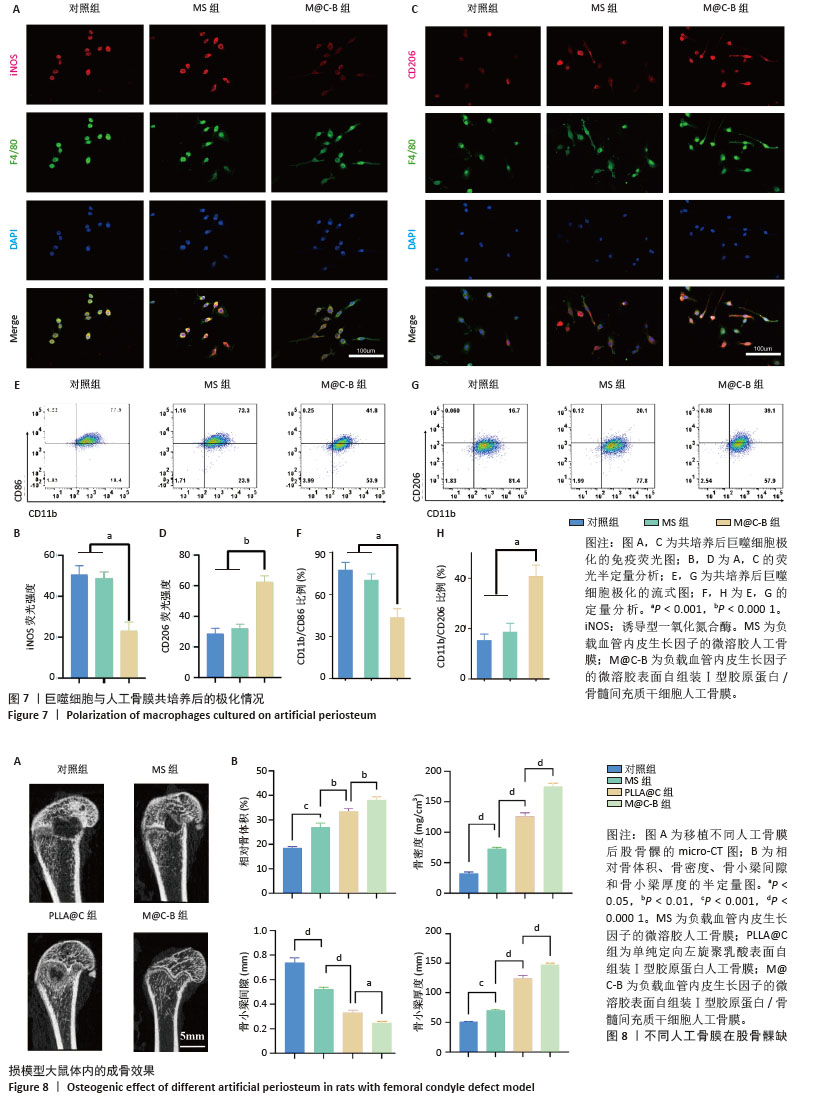
[1] DAI K, DENG S, YU Y, et al. Construction of developmentally inspired periosteum-like tissue for bone regeneration. Bone Res. 2022;10(1):1.
[2] ZHANG X, DENG C, QI S. Periosteum Containing Implicit Stem Cells: A Progressive Source of Inspiration for Bone Tissue Regeneration. Int J Mol Sci. 2024;25(4):2162.
[3] 张伯宇,王建南.天然高分子材料在人工骨膜领域研究进展[J].现代丝绸科学与技术,2024,39(1):28-32.
[4] WU L, GU Y, LIU L, et al. Hierarchical micro/nanofibrous membranes of sustained releasing VEGF for periosteal regeneration. Biomaterials. 2020;227:119555.
[5] 杨雨晴,陈志宇.早期短暂M1巨噬细胞在骨组织工程中的作用及应用[J].中国组织工程研究,2024,28(4):594-601.
[6] SCHLUNDT C, FISCHER H, BUCHER CH, et al. The multifaceted roles of macrophages in bone regeneration: A story of polarization, activation and time. Acta Biomater. 2021;133:46-57.
[7] LU Y, LIAN X, CAO Y, et al. An enhanced tri-layer bionic periosteum with gradient structure loaded by mineralized collagen for guided bone regeneration and in-situ repair. Int J Biol Macromol. 2024; 277(Pt 1):134148.
[8] CHEN X, YU B, WANG Z, et al. Progress of Periosteal Osteogenesis: The Prospect of In Vivo Bioreactor. Orthop Surg. 2022;14(9):1930-1939.
[9] ZHANG W, WANG N, YANG M, et al. Periosteum and development of the tissue-engineered periosteum for guided bone regeneration. J Orthop Translat. 2022;33:41-54.
[10] WANG J, CHEN G, CHEN ZM, et al. Current strategies in biomaterial-based periosteum scaffolds to promote bone regeneration: A review. J Biomater Appl. 2023;37(7):1259-1270.
[11] WANG YH, ZHAO CZ, WANG RY, et al. Correction: The crosstalk between macrophages and bone marrow mesenchymal stem cells in bone healing. Stem Cell Res Ther. 2022;13(1):524.
[12] 张怡凡,陈悦,周硕,等.HIF-1α通过调控H型血管生成参与牙周炎的发展[J].山西医科大学学报,2024,55(6):746-752.
[13] HU Y, HUANG J, CHEN C, et al. Strategies of Macrophages to Maintain Bone Homeostasis and Promote Bone Repair: A Narrative Review. J Funct Biomater. 2022;14(1):18.
[14] GOU M, WANG H, XIE H, et al. Macrophages in guided bone regeneration: potential roles and future directions. Front Immunol. 2024;15:1396759.
[15] 王寒,邢辉.3D打印生物工程骨支架的相关研究进展[J].中国矫形外科杂志,2024,32(11):1001-1006.
[16] WANG J, LIU M, YANG C, et al. Biomaterials for bone defect repair: Types, mechanisms and effects. Int J Artif Organs. 2024;47(2):75-84.
[17] SHAW P, DWIVEDI SKD, BHATTACHARYA R, et al. VEGF signaling: Role in angiogenesis and beyond. Biochim Biophys Acta Rev Cancer. 2024; 1879(2):189079.
[18] PATEL SA, NILSSON MB, LE X, et al. Molecular Mechanisms and Future Implications of VEGF/VEGFR in Cancer Therapy. Clin Cancer Res. 2023;29(1):30-39.
[19] QIN Q, LEE S, PATEL N, et al. Neurovascular coupling in bone regeneration. Exp Mol Med. 2022;54(11):1844-1849.
[20] ZHANG J, TONG D, SONG H, et al. Osteoimmunity-Regulating Biomimetically Hierarchical Scaffold for Augmented Bone Regeneration. Adv Mater. 2022;34(36):e2202044.
[21] MA S, WANG C, DONG Y, et al. Microsphere-Gel Composite System with Mesenchymal Stem Cell Recruitment, Antibacterial, and Immunomodulatory Properties Promote Bone Regeneration via Sequential Release of LL37 and W9 Peptides. ACS Appl Mater Interfaces. 2022;14(34):38525-38540.
[22] 李华德,时强,史晨丽.骨髓干细胞聚乳酸羟基乙酸修复大鼠软骨缺损[J].中国矫形外科杂志,2024,32(13):1222-1228.
[23] GAO Y, ZOU Y, SOKOLOWSKEI D, et al. Nr4a1 enhances Wnt4 transcription to promote mesenchymal stem cell osteogenesis and alleviates inflammation-inhibited bone regeneration. Mol Ther. 2024; 32(5):1479-1496.
[24] XU Z, WU L, TANG Y, et al. Spatiotemporal Regulation of the Bone Immune Microenvironment via Dam-Like Biphasic Bionic Periosteum for Bone Regeneration. Adv Healthc Mater. 2023;12(1):e2201661.
[25] 张洁,田艾.M2巨噬细胞参与骨再生相关信号通路的作用与机制[J].中国组织工程研究,2023,27(2):314-321.
[26] LIU Z, ZHU J, LI Z, et al. Biomaterial scaffolds regulate macrophage activity to accelerate bone regeneration. Front Bioeng Biotechnol. 2023;11:1140393.
[27] 赵月鑫,陈滨.巨噬细胞极化在骨组织工程免疫研究中的进展[J].中国组织工程研究,2022,26(13):2120-2126.
[28] HU K, SHANG Z, YANG X, et al. Macrophage Polarization and the Regulation of Bone Immunity in Bone Homeostasis. J Inflamm Res. 2023;16:3563-3580.
[29] WU M, LIU H, ZHU Y, et al. Bioinspired soft-hard combined system with mild photothermal therapeutic activity promotes diabetic bone defect healing via synergetic effects of immune activation and angiogenesis. Theranostics. 2024;14(10):4014-4057.
[30] ZHAO Q, LIU X, YU C, et al. Macrophages and Bone Marrow-Derived Mesenchymal Stem Cells Work in Concert to Promote Fracture Healing: A Brief Review. DNA Cell Biol. 2022;41(3):276-284.
[31] YOON SJ, KIM SH, CHOI JW, et al. Guided cortical and cancellous bone formation using a minimally invasive technique of BMSC- and BMP-2-laden visible light-cured carboxymethyl chitosan hydrogels. Int J Biol Macromol. 2023;227:641-653.
[32] ARTHUR A, GRONTHOS S. Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue. Int J Mol Sci. 2020;21(24):9759.
[33] ZHENG ZW, CHEN YH, WU DY, et al. Development of an Accurate and Proactive Immunomodulatory Strategy to Improve Bone Substitute Material-Mediated Osteogenesis and Angiogenesis. Theranostics. 2018;8(19):5482-5500.
[34] MAO Y, CHEN Y, LI W, et al. Physiology-Inspired Multilayer Nanofibrous Membranes Modulating Endogenous Stem Cell Recruitment and Osteo-Differentiation for Staged Bone Regeneration. Adv Healthc Mater. 2022; 11(21):e2201457.
[35] JIN H, ZHU X, LIU H, et al. Type-I Collagen Polypeptide-Based Composite Nanofiber Membranes for Fast and Efficient Bone Regeneration. ACS Biomater Sci Eng. 2024;10(9):5632-5640.
[36] JOVANOVIC M, GUTERMAN-RAM G, MARINI JC. Osteogenesis Imperfecta: Mechanisms and Signaling Pathways Connecting Classical and Rare OI Types. Endocr Rev. 2022;43(1):61-90.
[37] CHENG L, CHEN Z, CAI Z, et al. Bioinspired Functional Black Phosphorus Electrospun Fibers Achieving Recruitment and Biomineralization for Staged Bone Regeneration. Small. 2020;16(50):e2005433.
[38] HUA X, HOU M, DENG L, et al. Irisin-loaded electrospun core-shell nanofibers as calvarial periosteum accelerate vascularized bone regeneration by activating the mitochondrial SIRT3 pathway. Regen Biomater. 2023;11:rbad096.
[39] ZHAO L, LAN W, DONG X, et al. Enhenced cell adhesion on collagen I treated parylene-C microplates. J Biomater Sci Polym Ed. 2021;32(17):2195-2209.
[40] LE D, MCMILLAN S. A Novel Distal Biceps Rupture Repair Technique Utilizing a Biocomposite Scaffold. Surg Technol Int. 2023. doi: 10.52198/23.STI.42.OS1656.
[41] DONG C, TAN G, ZHANG G, et al. The function of immunomodulation and biomaterials for scaffold in the process of bone defect repair: A review. Front Bioeng Biotechnol. 2023;11:1133995.
[42] WANG G, LV Z, WANG T, et al. Surface Functionalization of Hydroxyapatite Scaffolds with MgAlEu-LDH Nanosheets for High-Performance Bone Regeneration. Adv Sci (Weinh). 2022;10(1): e2204234.
[43] KIM T, HONG J, KIM J, et al. Two-Dimensional Peptide Assembly via Arene-Perfluoroarene Interactions for Proliferation and Differentiation of Myoblasts. J Am Chem Soc. 2023;145(3):
1793-1802.
[44] SUZAWA M, TAMURA Y, FUKUMOTO S, et al. Stimulation of Smad1 transcriptional activity by Ras-extracellular signal-regulated kinase pathway: a possible mechanism for collagen-dependent osteoblastic differentiation. J Bone Miner Res. 2002;17(2):240-248.
[45] HUANG J, HAN Q, CAI M, et al. Effect of Angiogenesis in Bone Tissue Engineering. Ann Biomed Eng. 2022;50(8):898-913.
[46] 过丽强,赵世天,舒冰.Notch信号通路在骨折愈合过程中作用的研究进展[J].上海交通大学学报(医学版),2023,43(2):222-229.
[47] STREET J, BAO M, DEGUZMAN L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99(15):9656-9661.
[48] WEIVODA MM, BRADLEY EW. Macrophages and Bone Remodeling. J Bone Miner Res. 2023;38(3):359-369.
[49] VERMEULEN S, TAHMASEBI BIRGANI Z, HABIBOVIC P. Biomaterial-induced pathway modulation for bone regeneration. Biomaterials. 2022;283:121431.
[50] XUE N, DING X, HUANG R, et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals (Basel). 2022; 15(7):879.
[51] 杜锦洲,钱运,范存义.β-磷酸三钙的骨诱导性和组织工程应用研究进展[J].中华骨科杂志,2024,44(13):906-914.
[52] ZHANG FF, HAO Y, ZHANG KX, et al. Interplay between mesenchymal stem cells and macrophages: Promoting bone tissue repair. World J Stem Cells. 2024;16(4):375-388.
[53] LOCATI M, CURTALE G, MANTOVANI A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol. 2020;15: 123-147.
|