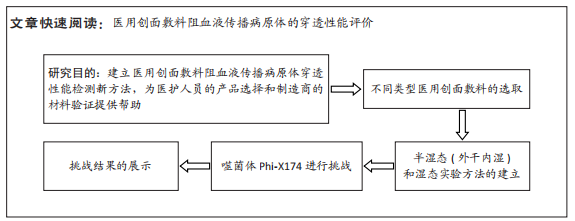
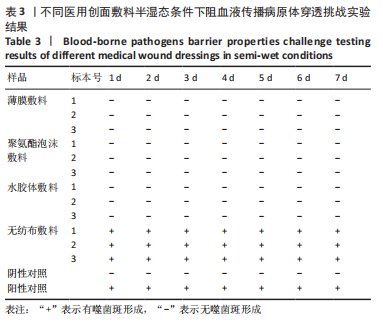
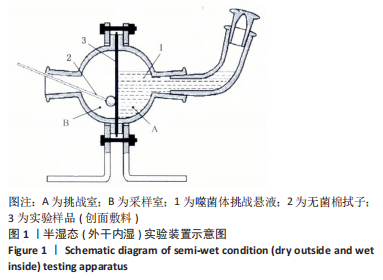
[1] 孙永合,傅继华.常见的血源性传播疾病的流行病学现状[J].预防医学论坛,2006,12(2):210-215.
[2] 黄东兰,卢永娟.人类免疫缺陷病毒阳性患者手术的防护措施[J].检验医学与临床,2009,6(4):317-318.
[3] HARVEY L, TAYLOR JL, ASSOUMOU SA, et al. Sexually transmitted and blood-borne infections among patients presenting to a low-barrier substance use disorder medication clinic. J Addict Med. 2021; 15(6):461-467.
[4] KRASNOSELSKIKH TV, SHABOLTAS AV. Multidisciplinary approach to the prevention of sexually transmitted infections and blood-borne infections. HIV Infection and Immunosuppressive Disorders. 2019; 10(4):100-112.
[5] HASHEMI-SHAHRI SM, SHARIFI-MOOD B, METANAT M, et al. Blood-Borne Infections in Tattooed People. Int J Infect. 2016;3(2):1-4.
[6] 王滨琳,李映兰.锐器伤所致医务人员血源性传播疾病的现状与对策[J].伤害医学,2014,3(4):42-46.
[7] 张咏梅,江智霞,酒井顺子,等.医务人员血源性职业暴露防护措施的应用研究[J].中华医院感染学杂志,2009,19(11):1400-1402.
[8] DOWNIE F, EGDELL S, BIELBY A, et al. Barrier dressings in surgical site infection prevention strategies. Br J Nurs. 2010;19(20):S42-46.
[9] AUGUSTINE R, KALARIKKAL N, THOMAS S. An in vitro method for the determination of microbial barrier property (MBP) of porous polymeric membranes for skin substitute and wound dressing applications. Tissue Eng Regen Med. 2015;12(1):12-19.
[10] KACZMAREK B, NADOLNA K, OWCZAREK A, et al. The characterization of thin films based on chitosan and tannic acid mixture for potential applications as wound dressings. Polym Test. 2019;78:106007.
[11] NUUTILA K, ERIKSSON E. Moist wound healing with commonly available dressings. Adv Wound Care. 2021;10(12):685-698.
[12] Lei J, Sun L, Li P, et al. The wound dressings and their applications in wound healing and management. Health Sci J. 2019;13(4):662.
[13] AMEEN H, MOORE K, LAWRENCE JC, et al. Investigating the bacterial barrier properties of four contemporary wound dressings. J Wound Care. 2000;9(8):385-388.
[14] ZENG Z, ZHU M, CHEN L, et al. Design the molecule structures to achieve functional advantages of hydrogel wound dressings: Advances and strategies. Compos B Eng. 2022;247:110313.
[15] NORTON R, FINLEY PJ. Clinically isolated bacteria resistance to silver-based wound dressings. J Wound Care. 2021;30(3):238-247.
[16] 高仪轩,周彪,巴特,等.新型敷料在创面修复中的应用与进展[J].中华损伤与修复杂志,2022,17(1):68-71.
[17] 杜天乐,刘东林,景春晖,等.创伤敷料对促进创面愈合的研究进展[J].医学综述,2015,21(6):969-971.
[18] 李晓明,刘苹,张波.创面敷料的研究现状[J].重庆医学,2017,46(20): 2851-2853.
[19] 彭尹玲,黄雪萍,彭喜玲.新型敷料在骨科手术开放性感染伤口患者中的应用效果[J].中国当代医药,2021,28(25):273-276.
[20] XU B, LI A, WANG R, et al. Elastic janus film for wound dressings: unidirectional biofluid transport and effectively promoting wound healing. Adv Funct Mater. 2021;31(41):2105265.
[21] ZENG QK, QI XL, SHI GY, et al. Wound dressing: from nanomaterials to diagnostic dressings and healing evaluations. ACS Nano. 2022;16(2): 1708-1733.
[22] 张称,王惠子,王琳,等.银离子敷料联合湿性换药护理在术后感染性伤口患者中的应用[J].齐鲁护理杂志,2021,27(18):153-155.
[23] 王丽,周晓玲,林陶玉.银离子抗菌敷料对感染性压疮患者临床应用效果研究[J].中华医院感染学杂志,2017,27(23):5512-5515.
[24] GABRIEL A, BARRETT C, CULLEN B, et al. Infection and inflammation in the wound environment: addressing issues of delayed wound healing with advanced wound dressings. Wounds. 2020;32(1 Suppl):S1-S17.
[25] ALJGHAMI ME, SABOOR S, AMINI-NIK S. Emerging innovative wound dressings. Ann Biomed Eng. 2019;47(3):659-675.
[26] 张娟丽,张丹丹,魏聪,等.膜敷料类医疗器械产品阻菌性能检验新方法研究[J].河南科学,2016,34(8):1274-1277.
[27] 栾同青,王文庆,张萌萌,等.抗菌敷料抗菌功效体外评价研究[J].中国药事,2021,35(10):1149-1156.
[28] 国家食品药品监督管理局.血液和体液防护装备 防护服材料抗血液传播病原体穿透性能测试 Phi-X174噬菌体试验方法,YY/T 0689-2008[S].2016.
[29] VAUGHAN J, BENSON R, VAUGHAN K. Assessing the effectiveness of antimicrobial wound dressings in vitro. Advanced Wound Repair Therapies. 2011:227-246.
[30] ZHANG J, WANG N, HE L, et al. Short Communication: Discordance of HIV-1 Viral Load from Paired Blood and Seminal Plasma Samples in a Chinese Men Who Have Sex with Men Population. AIDS Res Hum Retroviruses. 2019;35(4):393-395.
[31] 陆珍珍,蓝光华,张景云,等.艾滋病患者精液和血液中HIV释放和序列差异的研究进展[J].传染病信息,2021,34(5):468-472. |