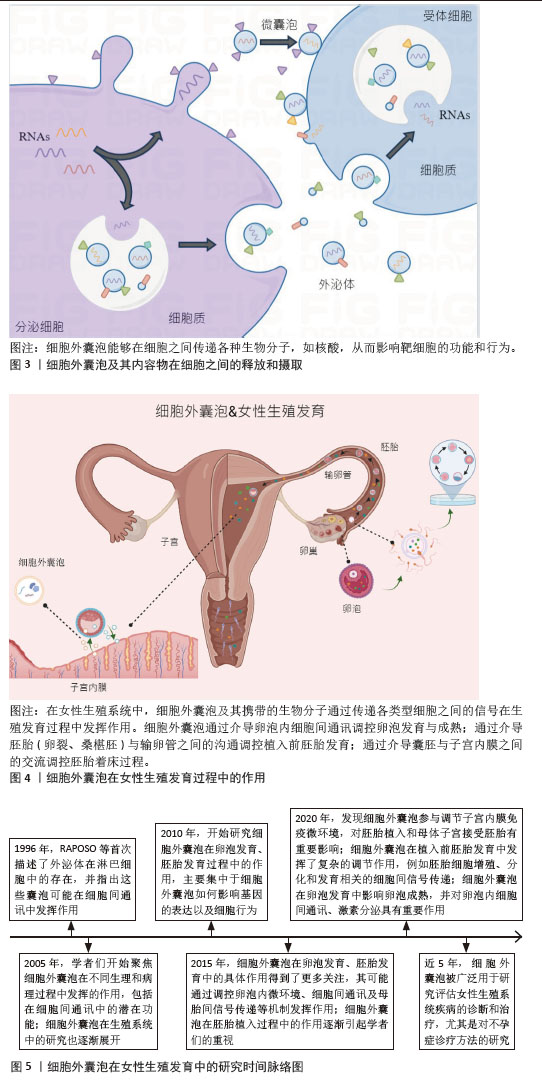
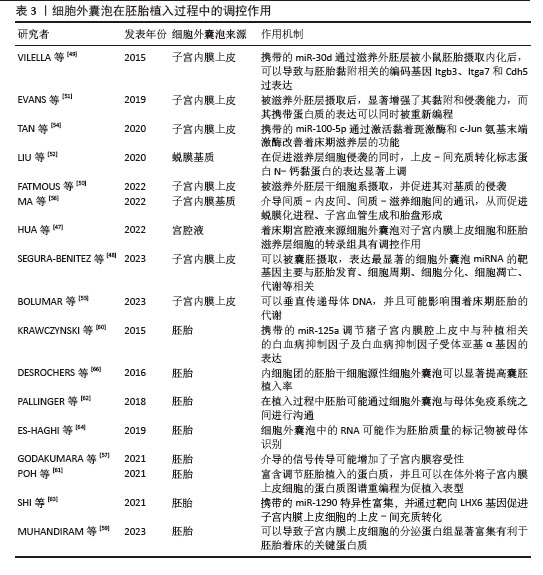
[1] LIU YJ, WANG C. A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication. Cell Commun Signal. 2023;21(1):77.
[2] VAN NIEL G, CARTER DRF, CLAYTON A, et al. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022;23(5):369-382.
[3] POPOWSKI KD, MOATTI A, SCULL G, et al. Inhalable dry powder mRNA vaccines based on extracellular vesicles. Matter. 2022;5(9): 2960-2974.
[4] MARAR C, STARICH B, WIRTZ D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. 2021;22(5): 560-570.
[5] LIANG Y, DUAN L, LU J, et al. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183-3195.
[6] ALEKSEJEVA E, ZAROVNI N, DISSANAYAKE K, et al. Extracellular vesicle research in reproductive science: Paving the way for clinical achievements†. Biol Reprod. 2022;106(3):408-424.
[7] HART AR, KHAN NLA, GODAKUMARA K, et al. The role of extracellular vesicles in endometrial receptivity and their potential in reproductive therapeutics and diagnosis. Reprod Biol. 2022;22(2):100645.
[8] DOMPE C, KULUS M, STEFAŃSKA K, et al. Human Granulosa Cells-Stemness Properties, Molecular Cross-Talk and Follicular Angiogenesis. Cells. 2021;10(6):1396.
[9] KOWALCZYK A, WRZECIŃSKA M, CZERNIAWSKA-PIĄTKOWSKA E, et al. Exosomes - Spectacular role in reproduction. Biomed Pharmacother. 2022;148:112752.
[10] HUNG WT, HONG X, CHRISTENSON LK, et al. Extracellular Vesicles from Bovine Follicular Fluid Support Cumulus Expansion. Biol Reprod. 2015;93(5):117.
[11] OWEN CM, JAFFE LA. Luteinizing hormone stimulates ingression of mural granulosa cells within the mouse preovulatory follicle. Biol Reprod. 2024;110(2):288-299.
[12] SHOMALI N, HEMMATZADEH M, YOUSEFZADEH Y, et al. Exosomes: Emerging biomarkers and targets in folliculogenesis and endometriosis. J Reprod Immunol. 2020;142:103181.
[13] GABRYŚ J, KIJ-MITKA B, SAWICKI S, et al. Extracellular vesicles from follicular fluid may improve the nuclear maturation rate of in vitro matured mare oocytes. Theriogenology. 2022;188:116-124.
[14] SOHEL MM, HOELKER M, NOFERESTI SS, et al. Exosomal and Non-Exosomal Transport of Extra-Cellular microRNAs in Follicular Fluid: Implications for Bovine Oocyte Developmental Competence. PLoS One. 2013;8(11):e78505.
[15] HASAN MM, RESHI QUA, LÄTTEKIVI F, et al. Bovine Follicular Fluid Derived Extracellular Vesicles Modulate the Viability, Capacitation and Acrosome Reaction of Bull Spermatozoa. Biology (Basel). 2021;10(11):1154.
[16] NAVAKANITWORAKUL R, HUNG WT, GUNEWARDENA S, et al. Characterization and Small RNA Content of Extracellular Vesicles in Follicular Fluid of Developing Bovine Antral Follicles. Sci Rep. 2016;6:25486.
[17] PU X, ZHANG L, ZHANG P, et al. Human UC-MSC-derived exosomes facilitate ovarian renovation in rats with chemotherapy-induced premature ovarian insufficiency. Front Endocrinol (Lausanne). 2023;14: 1205901.
[18] MACHTINGER R, RODOSTHENOUS RS, ADIR M, et al. Extracellular microRNAs in follicular fluid and their potential association with oocyte fertilization and embryo quality: an exploratory study. J Assist Reprod Genet. 2017;34(4): 525-533.
[19] MARTINEZ RM, LIANG L, RACOWSKY C, et al. Extracellular microRNAs profile in human follicular fluid and IVF outcomes. Sci Rep. 2018;8(1):17036.
[20] DA SILVEIRA JC, ANDRADE GM, DEL COLLADO M, et al. Supplementation with small-extracellular vesicles from ovarian follicular fluid during in vitro production modulates bovine embryo development. PLoS One. 2017; 12(6):e0179451.
[21] WANG Y, WANG X, ZHAO Y, et al. Progress in the effect of microRNA carried by extracellular vesicles in follicular fluid on follicular atresia. Sheng Wu Gong Cheng Xue Bao. 2022;38(8): 2767-2783.
[22] HUNG WT, NAVAKANITWORAKUL R, KHAN T, et al. Stage-specific follicular extracellular vesicle uptake and regulation of bovine granulosa cell proliferation. Biol Reprod. 2017;97(4):644-655.
[23] DA SILVEIRA JC, CARNEVALE EM, WINGER QA, et al. Regulation of ACVR1 and ID2 by cell-secreted exosomes during follicle maturation in the mare. Reprod Biol Endocrinol. 2014; 12:44.
[24] BASTOS NM, FERST JG, GOULART RS, et al. The role of the oviduct and extracellular vesicles during early embryo development in bovine. Anim Reprod. 2022;19(1):e20220015.
[25] CAJAS YN, CAÑÓN-BELTRÁN K, DE LA BLANCA MGM, et al. Role of reproductive fluids and extracellular vesicles in embryo–maternal interaction during early pregnancy in cattle. Reprod Fertil Dev. 2021; 34(2):117-138.
[26] HAMDI M, SÁNCHEZ JM, FERNANDEZ-FUERTES B, et al. Oviductal extracellular vesicles miRNA cargo varies in response to embryos and their quality. BMC Genomics. 2024;25(1):520.
[27] MAZZARELLA R, BASTOS NM, BRIDI A, et al. Changes in Oviductal Cells and Small Extracellular Vesicles miRNAs in Pregnant Cows. Front Vet Sci. 2021;8:639752.
[28] MENJIVAR NG, GAD A, THOMPSON RE, et al. Bovine oviductal organoids: a multi-omics approach to capture the cellular and extracellular molecular response of the oviduct to heat stress. BMC Genomics. 2023;24(1):646.
[29] ALMIÑANA C, TSIKIS G, LABAS V, et al. Deciphering the oviductal extracellular vesicles content across the estrous cycle: implications for the gametes-oviduct interactions and the environment of the potential embryo. BMC Genomics. 2018;19(1):622.
[30] FANG X, TANGA BM, BANG S, et al. Oviduct Epithelial Cell-Derived Extracellular Vesicles Improve Porcine Trophoblast Outgrowth. Vet Sci. 2022;9(11):609.
[31] QU P, ZHAO Y, WANG R, et al. Extracellular vesicles derived from donor oviduct fluid improved birth rates after embryo transfer in mice. Reprod Fertil Dev. 2019;31(2):324-332.
[32] LOPERA-VASQUEZ R, HAMDI M, MAILLO V, et al. Effect of bovine oviductal extracellular vesicles on embryo development and quality in vitro. Reproduction. 2017;153(4):461-470.
[33] ALMIÑANA C, CORBIN E, TSIKIS G, et al. Oviduct extracellular vesicles protein content and their role during oviduct-embryo cross-talk. Reproduction. 2017; 154(3):153-168.
[34] BAUERSACHS S, MERMILLOD P, ALMIÑANA C. The Oviductal Extracellular Vesicles’ RNA Cargo Regulates the Bovine Embryonic Transcriptome. Int J Mol Sci. 2020;21(4): 1303.
[35] FU B, MA H, ZHANG DJ, et al. Porcine oviductal extracellular vesicles facilitate early embryonic development via relief of endoplasmic reticulum stress. Cell Biol Int. 2022;46(2):300-310.
[36] BOUTOURLINSKY K, ALLÈGRE N, CHAZAUD C. Ex Vivo Culture for Preimplantation Mouse Embryo to Analyze Pluripotency. Methods Mol Biol. 2021;2214:1-10.
[37] KOBANAWA M. Fertilization, embryo culture, and clinical results using low lactate embryo culture medium for pre-culture, insemination, and beyond. Reprod Med Biol. 2022;21(1):e12458.
[38] SIDRAT T, KHAN AA, JOO MD, et al. Bovine Oviduct Epithelial Cell-Derived Culture Media and Exosomes Improve Mitochondrial Health by Restoring Metabolic Flux during Pre-Implantation Development. Int J Mol Sci. 2020;21(20):7589.
[39] RODRÍGUEZ-ALONSO B, MAILLO V, ACUÑA OS, et al. Spatial and Pregnancy-Related Changes in the Protein, Amino Acid, and Carbohydrate Composition of Bovine Oviduct Fluid. Int J Mol Sci. 2020;21(5):1681.
[40] DISSANAYAKE K, NÕMM M, LÄTTEKIVI F, et al. Oviduct as a sensor of embryo quality: deciphering the extracellular vesicle (EV)-mediated embryo-maternal dialogue. J Mol Med (Berl). 2021;99(5):685-697.
[41] PENNAROSSA G, ARCURI S, ZMIJEWSKA A, et al. Bioengineering-tissue strategies to model mammalian implantation in vitro. Front Bioeng Biotechnol. 2024;12:1430235.
[42] NG YH, ROME S, JALABERT A, et al. Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantation. PLoS One. 2013;8(3):e58502.
[43] TAN Q, SHI S, LIANG J, et al. MicroRNAs in Small Extracellular Vesicles Indicate Successful Embryo Implantation during Early Pregnancy. Cells. 2020;9(3):645.
[44] GREENING DW, NGUYEN HP, ELGASS K, et al. Human Endometrial Exosomes Contain Hormone-Specific Cargo Modulating Trophoblast Adhesive Capacity: Insights into Endometrial-Embryo Interactions. Biol Reprod. 2016;94(2):38.
[45] SEGURA-BENÍTEZ M, CARBAJO-GARCÍA MC, CORACHÁN A, et al. Proteomic analysis of extracellular vesicles secreted by primary human epithelial endometrial cells reveals key proteins related to embryo implantation. Reprod Biol Endocrinol. 2022; 20(1):3.
[46] HART AR, KHAN NLA, DISSANAYAKE K, et al. The Extracellular Vesicles Proteome of Endometrial Cells Simulating the Receptive Menstrual Phase Differs from That of Endometrial Cells Simulating the Non-Receptive Menstrual Phase. Biomolecules. 2023;13(2):279.
[47] HUA R, LIU Q, LIAN W, et al. Transcriptome regulation of extracellular vesicles derived from porcine uterine flushing fluids during peri-implantation on endometrial epithelial cells and embryonic trophoblast cells. Gene. 2022;822:146337.
[48] SEGURA-BENÍTEZ M, BAS-RIVAS A, JUÁREZ-BARBER E, et al. Human blastocysts uptake extracellular vesicles secreted by endometrial cells containing miRNAs related to implantation. Hum Reprod. 2023;38(8): 1547-1559.
[49] VILELLA F, MORENO-MOYA JM, BALAGUER N, et al. Hsa-miR-30d, secreted by the human endometrium, is taken up by the pre-implantation embryo and might modify its transcriptome. Development. 2015;142(18):3210-3221.
[50] FATMOUS M, RAI A, POH QH, et al. Endometrial small extracellular vesicles regulate human trophectodermal cell invasion by reprogramming the phosphoproteome landscape. Front Cell Dev Biol. 2022;10: 1078096.
[51] EVANS J, RAI A, NGUYEN HPT, et al. Human Endometrial Extracellular Vesicles Functionally Prepare Human Trophectoderm Model for Implantation: Understanding Bidirectional Maternal-Embryo Communication. Proteomics. 2019;19(23):e1800423.
[52] LIU M, CHEN X, CHANG QX, et al. Decidual small extracellular vesicles induce trophoblast invasion by upregulating N-cadherin. Reproduction. 2020;159(2):171-180.
[53] OGHBAEI F, ZAREZADEH R, JAFARI-GHARABAGHLOU D, et al. Epithelial-mesenchymal transition process during embryo implantation. Cell Tissue Res. 2022; 388(1):1-17.
[54] TAN Q, SHI S, LIANG J, et al. Endometrial cell-derived small extracellular vesicle miR-100-5p promotes functions of trophoblast during embryo implantation. Mol Ther Nucleic Acids. 2020;23:217-231.
[55] BOLUMAR D, MONCAYO-ARLANDI J, GONZALEZ-FERNANDEZ J, et al. Vertical transmission of maternal DNA through extracellular vesicles associates with altered embryo bioenergetics during the periconception period. Elife. 2023;12: RP88008.
[56] MA Q, BEAL JR, BHURKE A, et al. Extracellular vesicles secreted by human uterine stromal cells regulate decidualization, angiogenesis, and trophoblast differentiation. Proc Natl Acad Sci U S A. 2022;119(38):e2200252119.
[57] GODAKUMARA K, ORD J, LÄTTEKIVI F, et al. Trophoblast derived extracellular vesicles specifically alter the transcriptome of endometrial cells and may constitute a critical component of embryo-maternal communication. Reprod Biol Endocrinol. 2021;19(1):115.
[58] GODAKUMARA K, HEATH PR, FAZELI A. Rhythm of the First Language: Dynamics of Extracellular Vesicle-Based Embryo-Maternal Communication in the Pre-Implantation Microenvironment. Int J Mol Sci. 2023;24(7):6811.
[59] MUHANDIRAM S, DISSANAYAKE K, ORRO T, et al. Secretory Proteomic Responses of Endometrial Epithelial Cells to Trophoblast-Derived Extracellular Vesicles. Int J Mol Sci. 2023;24(15):11924.
[60] KRAWCZYNSKI K, NAJMULA J, BAUERSACHS S, et al. MicroRNAome of porcine conceptuses and trophoblasts: expression profile of micrornas and their potential to regulate genes crucial for establishment of pregnancy. Biol Reprod. 2015;92(1):21.
[61] POH QH, RAI A, CARMICHAEL II, et al. Proteome reprogramming of endometrial epithelial cells by human trophectodermal small extracellular vesicles reveals key insights into embryo implantation. Proteomics. 2021; 21(13-14):e2000210.
[62] PALLINGER E, BOGNAR Z, BOGDAN A, et al. PIBF+ extracellular vesicles from mouse embryos affect IL-10 production by CD8+ cells. Sci Rep. 2018;8(1):4662.
[63] SHI S, TAN Q, LIANG J, et al. Placental trophoblast cell-derived exosomal microRNA-1290 promotes the interaction between endometrium and embryo by targeting LHX6. Mol Ther Nucleic Acids. 2021;26:760-772.
[64] ES-HAGHI M, GODAKUMARA K, HÄLING A, et al. Specific trophoblast transcripts transferred by extracellular vesicles affect gene expression in endometrial epithelial cells and may have a role in embryo-maternal crosstalk. Cell Commun Signal. 2019;17(1):146.
[65] HAWKE DC, WATSON AJ, BETTS DH. Extracellular vesicles, microRNA and the preimplantation embryo: non-invasive clues of embryo well-being. Reprod Biomed Online. 2021;42(1):39-54.
[66] DESROCHERS LM, BORDELEAU F, REINHART-KING CA, et al. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat Commun. 2016;7:11958.
[67] GIACOMINI E, VAGO R, SANCHEZ AM, et al. Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Sci Rep. 2017;7(1):5210.
[68] LAL A, ROUDEBUSH WE, CHOSED RJ. Embryo Biopsy Can Offer More Information Than Just Ploidy Status. Front Cell Dev Biol. 2020;8:78.
[69] SUN Y, ZHANG Y, MA X, et al. Determining Diagnostic Criteria of Unexplained Recurrent Implantation Failure: A Retrospective Study of Two vs Three or More Implantation Failure. Front Endocrinol (Lausanne). 2021; 12:619437.
|