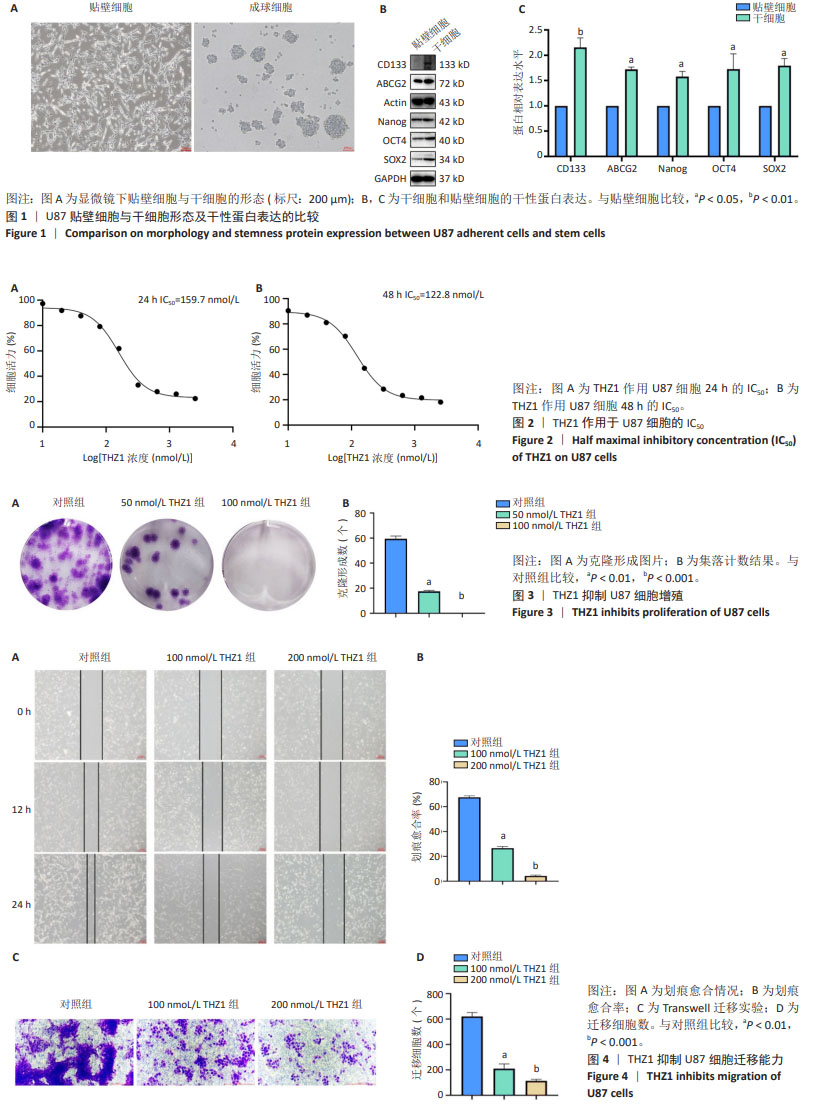
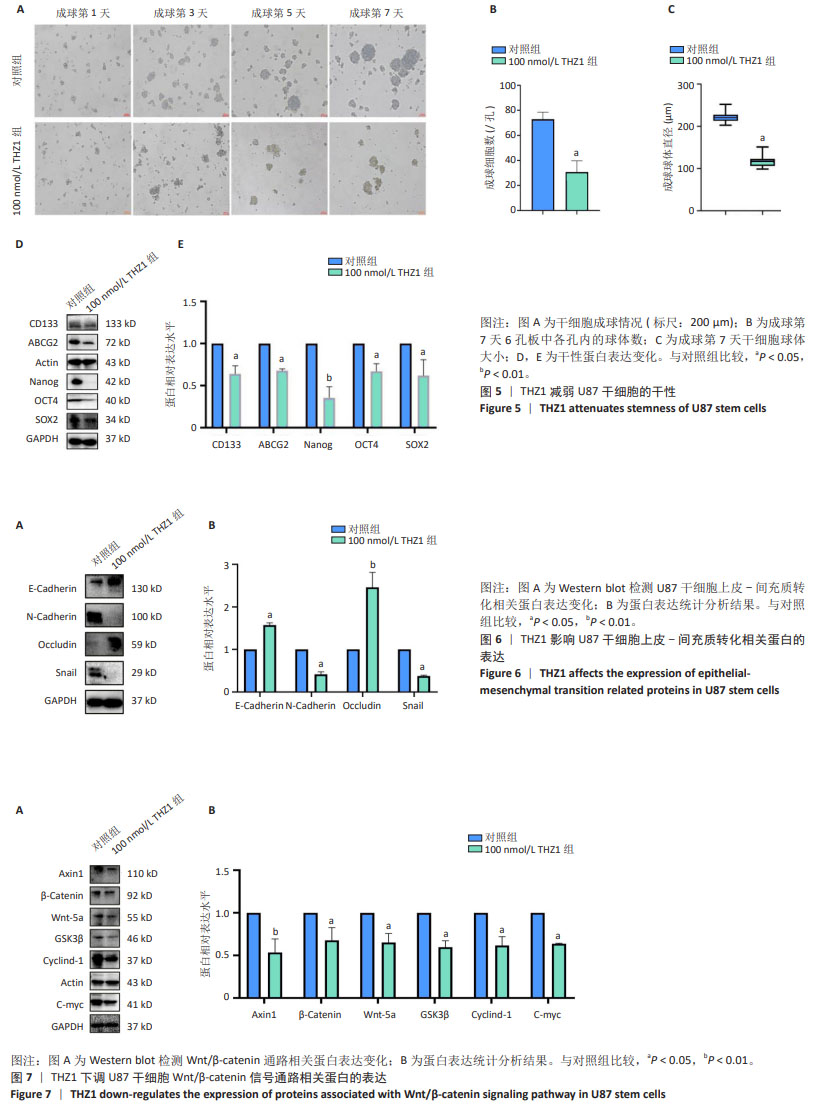
[1] KLEMM F, MAAS RR, BOWMAN RL, et al. Interrogation of the Microenvironmental Landscape in Brain Tumors Reveals Disease-Specific Alterations of Immune Cells. Cell. 2020;181(7):1643-1660.e17.
[2] TAN AC, ASHLEY DM, LÓPEZ GY, et al. Management of glioblastoma: State of the art and future directions. CA Cancer J Clin. 2020;70(4): 299-312.
[3] KARLSSON J, LULY KM, TZENG SY, et al. Nanoparticle designs for delivery of nucleic acid therapeutics as brain cancer therapies. Adv Drug Deliv Rev. 2021;179:113999.
[4] PRASETYANTI PR, MEDEMA JP. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer. 2017;16(1):41.
[5] LI D, WANG L, JIANG B, et al. Improving cancer immunotherapy by preventing cancer stem cell and immune cell linking in the tumor microenvironment. Biomed Pharmacother. 2024;170:116043.
[6] SHEN Q, HILL T, CAI X, et al. Physical confinement during cancer cell migration triggers therapeutic resistance and cancer stem cell-like behavior. Cancer Lett. 2021;506:142-151.
[7] TSAI YT, WU AC, YANG WB, et al. ANGPTL4 Induces TMZ Resistance of Glioblastoma by Promoting Cancer Stemness Enrichment via the EGFR/AKT/4E-BP1 Cascade. Int J Mol Sci. 2019;20(22):5625.
[8] LAMBERT AW, WEINBERG RA. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat Rev Cancer. 2021;21(5):325-338.
[9] HE P, DAI Q, WU X. New insight in urological cancer therapy: From epithelial-mesenchymal transition (EMT) to application of nano-biomaterials. Environ Res. 2023;229:115672.
[10] RAMESH V, BRABLETZ T, CEPPI P. Targeting EMT in Cancer with Repurposed Metabolic Inhibitors. Trends Cancer. 2020;6(11):942-950.
[11] NUSSE R, CLEVERS H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169(6):985-999.
[12] NAJAFI M, FARHOOD B, MORTEZAEE K. Cancer stem cells (CSCs) in cancer progression and therapy. J Cell Physiol. 2019;234(6):8381-8395.
[13] LI Q, LAI Q, HE C, et al. RUNX1 promotes tumour metastasis by activating the Wnt/β-catenin signalling pathway and EMT in colorectal cancer. J Exp Clin Cancer Res. 2019;38(1):334.
[14] SUN J, ZHANG Q, SUN X, et al. THZ1 targeting CDK7 suppresses c-KIT transcriptional activity in gastrointestinal stromal tumours. Cell Commun Signal. 2022;20(1):138.
[15] CHOW PM, CHANG YW, KUO KL, et al. CDK7 inhibition by THZ1 suppresses cancer stemness in both chemonaïve and chemoresistant urothelial carcinoma via the hedgehog signaling pathway. Cancer Lett. 2021;507:70-79.
[16] ATTIA YM, SALAMA SA, SHOUMAN SA, et al. Targeting CDK7 reverses tamoxifen resistance through regulating stemness in ER+ breast cancer. Pharmacol Rep. 2022;74(2):366-378.
[17] HORBINSKI C, BERGER T, PACKER RJ, et al. Clinical implications of the 2021 edition of the WHO classification of central nervous system tumours. Nat Rev Neurol. 2022;18(9):515-529.
[18] BARTHEL L, HADAMITZKY M, DAMMANN P, et al. Glioma: molecular signature and crossroads with tumor microenvironment. Cancer Metastasis Rev. 2022;41(1):53-75.
[19] CAYROL F, PRADITSUKTAVORN P, FERNANDO TM, et al. THZ1 targeting CDK7 suppresses STAT transcriptional activity and sensitizes T-cell lymphomas to BCL2 inhibitors. Nat Commun. 2017;8:14290.
[20] CHEN HD, HUANG CS, XU QC, et al. Therapeutic Targeting of CDK7 Suppresses Tumor Progression in Intrahepatic Cholangiocarcinoma. Int J Biol Sci. 2020;16(7):1207-1217.
[21] TANG L, JIN J, XU K, et al. SOX9 interacts with FOXC1 to activate MYC and regulate CDK7 inhibitor sensitivity in triple-negative breast cancer. Oncogenesis. 2020;9(5):47.
[22] CHENG ZJ, MIAO DL, SU QY, et al. THZ1 suppresses human non-small-cell lung cancer cells in vitro through interference with cancer metabolism. Acta Pharmacol Sin. 2019;40(6):814-822.
[23] ABUDUREHEMAN T, XIA J, LI MH, et al. CDK7 Inhibitor THZ1 Induces the Cell Apoptosis of B-Cell Acute Lymphocytic Leukemia by Perturbing Cellular Metabolism. Front Oncol. 2021;11:663360.
[24] HUANG T, DING X, XU G, et al. CDK7 inhibitor THZ1 inhibits MCL1 synthesis and drives cholangiocarcinoma apoptosis in combination with BCL2/BCL-XL inhibitor ABT-263. Cell Death Dis. 2019;10(8):602.
[25] ZHANG T, LI J, YANG M, et al. CDK7/GRP78 signaling axis contributes to tumor growth and metastasis in osteosarcoma. Oncogene. 2022; 41(40):4524-4536.
[26] XIA L, ZHENG Z, LIU JY, et al. Targeting Triple-Negative Breast Cancer with Combination Therapy of EGFR CAR T Cells and CDK7 Inhibition. Cancer Immunol Res. 2021;9(6):707-722.
[27] HUANG T, SONG X, XU D, et al. Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics. 2020;10(19): 8721-8743.
[28] SUVÀ ML, TIROSH I. The Glioma Stem Cell Model in the Era of Single-Cell Genomics. Cancer Cell. 2020;37(5):630-636.
[29] BOYD NH, TRAN AN, BERNSTOCK JD, et al. Glioma stem cells and their roles within the hypoxic tumor microenvironment. Theranostics. 2021;11(2):665-683.
[30] CHEN X, NIU W, FAN X, et al. Oct4A palmitoylation modulates tumorigenicity and stemness in human glioblastoma cells. Neuro Oncol. 2023;25(1):82-96.
[31] AKHMETKALIYEV A, ALIBRAHIM N, SHAFIEE D, et al. EMT/MET plasticity in cancer and Go-or-Grow decisions in quiescence: the two sides of the same coin? Mol Cancer. 2023;22(1):90.
[32] LAMBERT AW, FIORE C, CHUTAKE Y, et al. ΔNp63/p73 drive metastatic colonization by controlling a regenerative epithelial stem cell program in quasi-mesenchymal cancer stem cells. Dev Cell. 2022;57(24): 2714-2730.e8.
[33] BAI X, NI J, BERETOV J, et al. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat Rev. 2018;69:152-163.
[34] MCCABE EM, RASMUSSEN TP. lncRNA involvement in cancer stem cell function and epithelial-mesenchymal transitions. Semin Cancer Biol. 2021;75:38-48.
[35] BABU D, MUDIRAJ A, YADAV N, et al. Rabeprazole has efficacy per se and reduces resistance to temozolomide in glioma via EMT inhibition. Cell Oncol (Dordr). 2021;44(4):889-905.
[36] ZHANG J, CAI H, SUN L, et al. LGR5, a novel functional glioma stem cell marker, promotes EMT by activating the Wnt/β-catenin pathway and predicts poor survival of glioma patients. J Exp Clin Cancer Res. 2018;37(1):225.
[37] LIU J, XIAO Q, XIAO J, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7(1):3.
[38] YU F, YU C, LI F, et al. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct Target Ther. 2021;6(1):307.
[39] WEI B, CAO J, TIAN JH, et al. Mortalin maintains breast cancer stem cells stemness via activation of Wnt/GSK3β/β-catenin signaling pathway. Am J Cancer Res. 2021;11(6):2696-2716.
[40] GRINAT J, HEUBERGER J, VIDAL RO, et al. The epigenetic regulator Mll1 is required for Wnt-driven intestinal tumorigenesis and cancer stemness. Nat Commun. 2020;11(1):6422.
[41] YIN J, DING F, CHENG Z, et al. METTL3-mediated m6A modification of LINC00839 maintains glioma stem cells and radiation resistance by activating Wnt/β-catenin signaling. Cell Death Dis. 2023;14(7):417. |