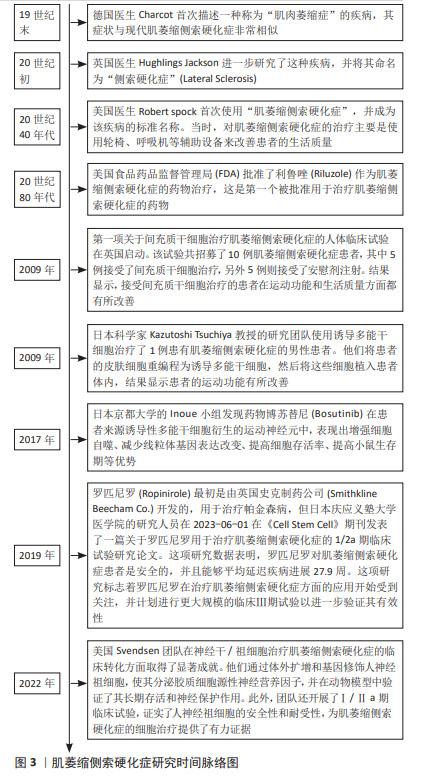
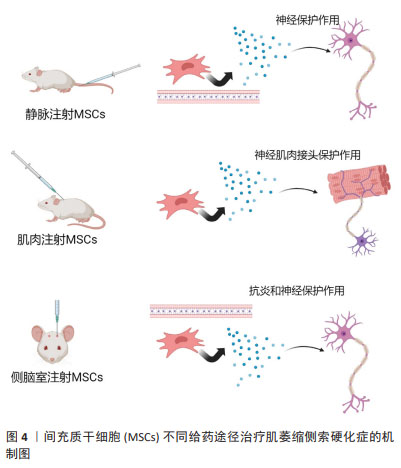
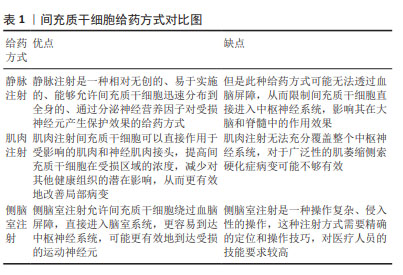
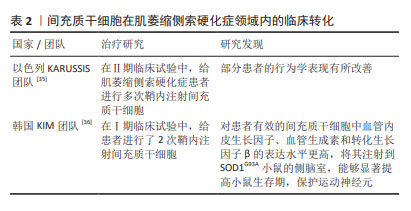
[1] CAMPISI L, CHIZARI S, HO JSY, et al. Clonally expanded CD8 T cells characterize amyotrophic lateral sclerosis-4. Nature. 2022;606(7916): 945-952.
[2] ZONDLER L, MÜLLER K, KHALAJI S, et al. Peripheral monocytes are functionally altered and invade the CNS in ALS patients. Acta Neuropathol. 2016;132(3):391-411.
[3] LIU X, HE J, GAO FB, et al. The epidemiology and genetics of Amyotrophic lateral sclerosis in China. Brain Res. 2018;1693(Pt A): 121-126.
[4] XU L, CHEN L, WANG S, et al. Incidence and prevalence of amyotrophic lateral sclerosis in urban China: a national population-based study. J Neurol Neurosurg Psychiatry. 2020;91(5):520-525.
[5] TENDULKAR S, HEGDE S, GARG L, et al. Caspar, an adapter for VAPB and TER94, modulates the progression of ALS8 by regulating IMD/NFκB-mediated glial inflammation in a Drosophila model of human disease. Hum Mol Genet. 2022;31(17):2857-2875.
[6] YAMAWAKI M, AKIBA M, MATSUMOTO N, et al. Defective neuronal and oligodendroglial differentiation by FTD3- and ALS17-associated Ile29-to-Val mutation of CHMP2B. Mol Genet Metab Rep. 2019;19: 100458.
[7] LIANG D, LIN WJ, REN M, et al. m6A reader YTHDC1 modulates autophagy by targeting SQSTM1 in diabetic skin. Autophagy. 2022; 18(6):1318-1337.
[8] ASSONI AF, GUERRERO EN, WARDENAAR R, et al. IFNγ protects motor neurons from oxidative stress via enhanced global protein synthesis in FUS-associated amyotrophic lateral sclerosis. Brain Pathol. 2024;34(1):e13206.
[9] JIN S, SUN Z, FANG X, et al. A patient carrying a heterozygous p.Asn267Ser TARDBP missense mutation diagnosed as ALS and only involving lower motor neurons. Neurol Sci. 2023;44(2):777-782.
[10] MCCAULEY ME, O’ROURKE JG, YÁÑEZ A, et al. C9orf72 in myeloid cells suppresses STING-induced inflammation. Nature. 2020;585(7823): 96-101.
[11] SHAMMAS MK, HUANG X, WU BP, et al. OMA1 mediates local and global stress responses against protein misfolding in CHCHD10 mitochondrial myopathy. J Clin Invest. 2022;132(14):e157504.
[12] YUAN T, WANG T, ZHANG J, et al. Robust and Multifunctional Nanoparticles Assembled from Natural Polyphenols and Metformin for Efficient Spinal Cord Regeneration. ACS Nano. 2023;17(18): 18562-18575.
[13] MURPHY S, SCHMITT-JOHN T, DOWLING P, et al. Proteomic profiling of the brain from the wobbler mouse model of amyotrophic lateral sclerosis reveals elevated levels of the astrogliosis marker glial fibrillary acidic protein. Eur J Transl Myol. 2023;33(3):11555.
[14] ABE K, ITOYAMA Y, SOBUE G, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7-8):610-617.
[15] YU CH, DAVIDSON S, HARAPAS CR, et al. TDP-43 Triggers Mitochondrial DNA Release via mPTP to Activate cGAS/STING in ALS. Cell. 2020; 183(3):636-649.e18.
[16] OKANO H, MORIMOTO S. iPSC-based disease modeling and drug discovery in cardinal neurodegenerative disorders. Cell Stem Cell. 2022;29(2):189-208.
[17] WALD-ALTMAN S, PICHINUK E, KAKHLON O, et al. A differential autophagy-dependent response to DNA double-strand breaks in bone marrow mesenchymal stem cells from sporadic ALS patients. Dis Model Mech. 2017;10(5):645-654.
[18] SUNOHARA T, MORIZANE A, MATSUURA S, et al. MicroRNA-Based Separation of Cortico-Fugal Projection Neuron-Like Cells Derived From Embryonic Stem Cells. Front Neurosci. 2019;13:1141.
[19] BOILLÉE S, YAMANAKA K, LOBSIGER CS, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312(5778):1389-1392.
[20] GIUNTI D, MARINI C, PARODI B, et al. Role of miRNAs shuttled by mesenchymal stem cell-derived small extracellular vesicles in modulating neuroinflammation. Sci Rep. 2021;11(1):1740.
[21] MARCONI S, BONACONSA M, SCAMBI I, et al. Systemic treatment with adipose-derived mesenchymal stem cells ameliorates clinical and pathological features in the amyotrophic lateral sclerosis murine model. Neuroscience. 2013;248:333-343.
[22] MAGOTA H, SASAKI M, KATAOKA-SASAKI Y, et al. Intravenous infusion of mesenchymal stem cells delays disease progression in the SOD1G93A transgenic amyotrophic lateral sclerosis rat model. Brain Res. 2021; 1757:147296.
[23] CHIAROTTO GB, CARTAROZZI LP, PEREZ M, et al. Delayed onset, immunomodulation, and lifespan improvement of SOD1G93A mice after intravenous injection of human mesenchymal stem cells derived from adipose tissue. Brain Res Bull. 2022;186:153-164.
[24] KOOK MG, LEE S, SHIN N, et al. Repeated intramuscular transplantations of hUCB-MSCs improves motor function and survival in the SOD1 G93A mice through activation of AMPK. Sci Rep. 2020;10(1):1572.
[25] MAGOTA H, SASAKI M, KATAOKA-SASAKI Y, et al. Repeated infusion of mesenchymal stem cells maintain the condition to inhibit deteriorated motor function, leading to an extended lifespan in the SOD1G93A rat model of amyotrophic lateral sclerosis. Mol Brain. 2021;14(1):76.
[26] SUZUKI M, SVENDSEN CN. Ex Vivo Gene Therapy Using Human Mesenchymal Stem Cells to Deliver Growth Factors in the Skeletal Muscle of a Familial ALS Rat Model. Methods Mol Biol. 2016;1382: 325-336.
[27] GOTKINE M, CARACO Y, LERNER Y, et al. Safety and efficacy of first-in-man intrathecal injection of human astrocytes (AstroRx®) in ALS patients: phase I/IIa clinical trial results. J Transl Med. 2023;21(1):122.
[28] SIRONI F, VALLAROLA A, VIOLATTO MB, et al. Multiple intracerebroventricular injections of human umbilical cord mesenchymal stem cells delay motor neurons loss but not disease progression of SOD1G93A mice. Stem Cell Res. 2017;25:166-178.
[29] IZRAEL M, SLUTSKY SG, ADMONI T, et al. Safety and efficacy of human embryonic stem cell-derived astrocytes following intrathecal transplantation in SOD1G93A and NSG animal models. Stem Cell Res Ther. 2018;9(1):152.
[30] TERASHIMA T, KOBASHI S, WATANABE Y, et al. Enhancing the Therapeutic Efficacy of Bone Marrow-Derived Mononuclear Cells with Growth Factor-Expressing Mesenchymal Stem Cells for ALS in Mice. iScience. 2020;23(11):101764.
[31] BOHACIAKOVA D, HRUSKA-PLOCHAN M, TSUNEMOTO R, et al. A scalable solution for isolating human multipotent clinical-grade neural stem cells from ES precursors. Stem Cell Res Ther. 2019;10(1):83.
[32] JAVORKOVA E, MATEJCKOVA N, ZAJICOVA A, et al. Immunomodulatory Properties of Bone Marrow Mesenchymal Stem Cells from Patients with Amyotrophic Lateral Sclerosis and Healthy Donors. J Neuroimmune Pharmacol. 2019;14(2):215-225.
[33] YUN YC, JEONG SG, KIM SH, et al. Reduced sirtuin 1/adenosine monophosphate-activated protein kinase in amyotrophic lateral sclerosis patient-derived mesenchymal stem cells can be restored by resveratrol. J Tissue Eng Regen Med. 2019;13(1):110-115.
[34] OH YS, KIM SH, CHO GW. Functional Restoration of Amyotrophic Lateral Sclerosis Patient-Derived Mesenchymal Stromal Cells Through Inhibition of DNA Methyltransferase. Cell Mol Neurobiol. 2016;36(4):613-620.
[35] PETROU P, KASSIS I, YAGHMOUR NE, et al. A phase II clinical trial with repeated intrathecal injections of autologous mesenchymal stem cells in patients with amyotrophic lateral sclerosis. Front Biosci (Landmark Ed). 2021;26(10):693-706.
[36] OH KW, MOON C, KIM HY, et al. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl Med. 2015;4(6): 590-597.
[37] RUEGSEGGER C, MAHARJAN N, GOSWAMI A, et al. Aberrant association of misfolded SOD1 with Na(+)/K(+)ATPase-α3 impairs its activity and contributes to motor neuron vulnerability in ALS. Acta Neuropathol. 2016;131(3):427-451.
[38] KIM HY, KIM H, OH KW, et al. Biological markers of mesenchymal stromal cells as predictors of response to autologous stem cell transplantation in patients with amyotrophic lateral sclerosis: an investigator-initiated trial and in vivo study. Stem Cells. 2014;32(10):2724-2731.
[39] AN D, FUJIKI R, IANNITELLI DE, et al. Stem cell-derived cranial and spinal motor neurons reveal proteostatic differences between ALS resistant and sensitive motor neurons. Elife. 2019;8:e44423.
[40] LEE HJ, KIM KS, AHN J, et al. Human motor neurons generated from neural stem cells delay clinical onset and prolong life in ALS mouse model. PLoS One. 2014;9(5):e97518.
[41] GUTTENPLAN KA, WEIGEL MK, ADLER DI, et al. Knockout of reactive astrocyte activating factors slows disease progression in an ALS mouse model. Nat Commun. 2020;11(1):3753.
[42] LEPORE AC, RAUCK B, DEJEA C, et al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11(11):1294-1301.
[43] KNIPPENBERG S, RATH KJ, BÖSELT S, et al. Intraspinal administration of human spinal cord-derived neural progenitor cells in the G93A-SOD1 mouse model of ALS delays symptom progression, prolongs survival and increases expression of endogenous neurotrophic factors. J Tissue Eng Regen Med. 2017;11(3):751-764.
[44] KLEIN SM, BEHRSTOCK S, MCHUGH J, et al. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 2005;16(4):509-521.
[45] SUZUKI M, MCHUGH J, TORK C, et al. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS One. 2007;2(8):e689.
[46] GOWING G, SHELLEY B, STAGGENBORG K, et al. Glial cell line-derived neurotrophic factor-secreting human neural progenitors show long-term survival, maturation into astrocytes, and no tumor formation following transplantation into the spinal cord of immunocompromised rats. Neuroreport. 2014;25(6):367-372.
[47] CHENNAMPALLY P, SAYED-ZAHID A, SOUNDARARAJAN P, et al. Author Correction: A microfluidic approach to rescue ALS motor neuron degeneration using rapamycin. Sci Rep. 2021;11(1):19743.
[48] MELAMED Z, LÓPEZ-ERAUSKIN J, BAUGHN MW, et al. Premature polyadenylation-mediated loss of stathmin-2 is a hallmark of TDP-43-dependent neurodegeneration. Nat Neurosci. 2019;22(2): 180-190.
[49] GUERRA SAN JUAN I, NASH LA, SMITH KS, et al. Loss of mouse Stmn2 function causes motor neuropathy. Neuron. 2022;110(10):1671-1688.e6.
[50] IIDA M, MIYAZAKI I, TANAKA K, et al. Dopamine D2 receptor-mediated antioxidant and neuroprotective effects of ropinirole, a dopamine agonist. Brain Res. 1999;838(1-2):51-59.
[51] TANAKA K, MIYAZAKI I, FUJITA N, et al. Molecular mechanism in activation of glutathione system by ropinirole, a selective dopamine D2 agonist. Neurochem Res. 2001;26(1):31-36.
[52] DU F, LI R, HUANG Y, et al. Dopamine D3 receptor-preferring agonists induce neurotrophic effects on mesencephalic dopamine neurons. Eur J Neurosci. 2005;22(10):2422-2430.
[53] HÖGLINGER GU, RIZK P, MURIEL MP, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7(7):726-735.
[54] FUJIMORI K, ISHIKAWA M, OTOMO A, et al. Modeling sporadic ALS in iPSC-derived motor neurons identifies a potential therapeutic agent. Nat Med. 2018;24(10):1579-1589.
[55] IMAMURA K, IZUMI Y, WATANABE A, et al. The Src/c-Abl pathway is a potential therapeutic target in amyotrophic lateral sclerosis. Sci Transl Med. 2017;9(391):eaaf3962.
[56] GOTO K, IMAMURA K, KOMATSU K, et al. Simple Derivation of Spinal Motor Neurons from ESCs/iPSCs Using Sendai Virus Vectors. Mol Ther Methods Clin Dev. 2017;4:115-125.
[57] COOK CN, WU Y, ODEH HM, et al. C9orf72 poly(GR) aggregation induces TDP-43 proteinopathy. Sci Transl Med. 2020;12(559): eabb3774.
[58] ESHIMA J, O’CONNOR SA, MARSCHALL E, et al. Molecular subtypes of ALS are associated with differences in patient prognosis. Nat Commun. 2023;14(1):95. |