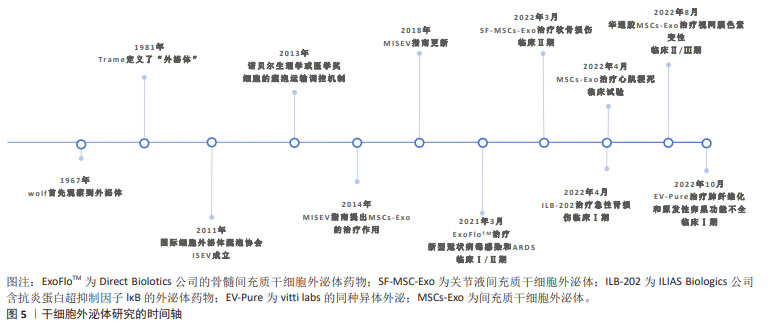
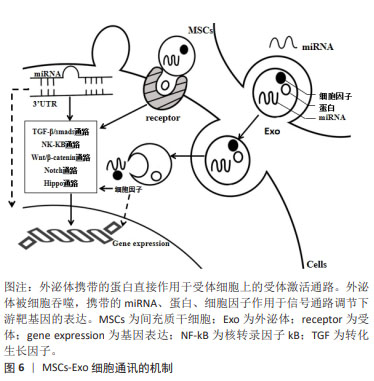
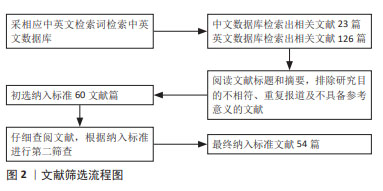
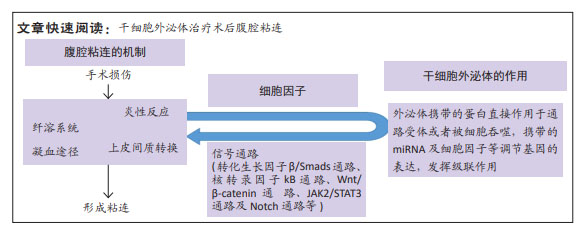
[1] MORIS D, CHAKEDIS J, RAHNEMAI-AZAR AA, et al. Postoperative abdominal adhesions: clinical significance and advances in prevention and management. J Gastrointest Surg. 2017;21(10):1713-1722.
[2] CARMICHAEL SP, SHIN J, VAUGHAN JW, et al. Regenerative medicine therapies for prevention of abdominal adhesions: a scoping review. J Surg Res. 2022;275:252-264.
[3] 朱兰,郎景和,任常,等.妇产科手术后盆腹腔粘连预防中国指南[J].中华妇产科杂志,2023,58(3):161-169.
[4] 张龙龙,樊强,顾越雷,等.术后腹腔粘连的研究进展[J].中国普外基础与临床杂志,2018,25(2):245-249.
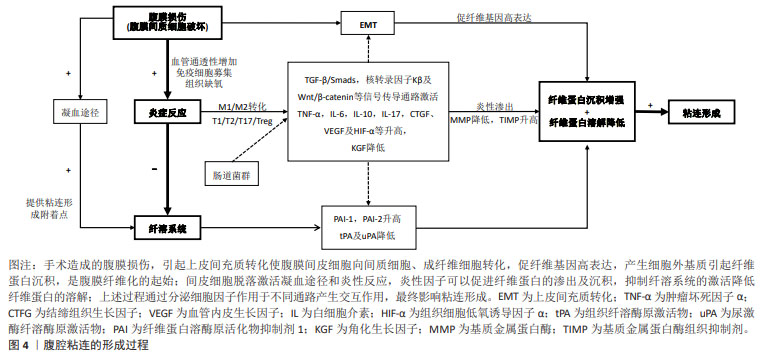
[5] WANG R, GUO T, LI J. Mechanisms of peritoneal mesothelial cells in peritoneal adhesion. Biomolecules. 2022;12(10):1498-1513.
[6] ORECCHIONI M, GHOSHEH Y, PRAMOD AB, et al. Macrophage polarization: different gene signatures in M1(LPS+) vs. Classically and M2(LPS–) vs. alternatively activated macrophages. Front Immunol. 2019;24(10):1084-1096.
[7] BUTCHER MJ, ZHU J. Recent advances in understanding the Th1/Th2 effector choice. Fac Rev. 2021;15(10):10-30.
[8] DONG L, ZHENG X, WANG G. Peritoneal adhesions induce Th17/Treg imbalance in mice. Int J Clin Exp Pathol. 2018;11(9):4352-4362.
[9] 成晶晶.外周血Th17/Treg失衡与卵巢子宫内膜异位囊肿的关系及对其粘连程度的评估价值[J].中国现代医药杂志,2022,24(5):35-38.
[10] ASCHER S, WILMS E, PONTAROLLO G, et al. Gut microbiota restricts NETosis in acute mesenteric ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2020;40(9):2279-2292.
[11] 杨丽丽,卞尧尧,赵敏,等.细胞因子与术后腹腔粘连关系及中医药防治[J].中华中医药学刊,2018,36(12):2935-2939.
[12] TANG J, XIANG Z, BERNARDS MT, et al. Peritoneal adhesions: occurrence, prevention and experimental models. Acta Biomater. 2020;116(15):84-104.
[13] FU M, PENG D, LAN T, et al. Multifunctional regulatory protein connective tissue growth factor (CTGF): a potential therapeutic target for diverse diseases. Acta Pharm Sin B. 2022;12(4):1740-1760.
[14] WEI G, WANG Z, LIU R, et al. A combination of hybrid polydopamine-human keratinocyte growth factor nanoparticles and sodium hyaluronate for the efficient prevention of postoperative abdominal adhesion formation. Acta Biomater. 2022;138(15):155-167.
[15] 谢中,杨标顺,钱亮.达芙通联合补佳乐对腔镜粘连术后患者TNF-α、VEGF、TGF-β的影响及疗效观察[J].中国计划生育学杂志, 2019,27(3):342-345.
[16] SHENTU Y, JIANG H, LIU X, et al. Nestin promotes peritoneal fibrosis by protecting hif1-αfrom proteasomal degradation. Front Physiol. 2020;16(11):517912.
[17] 吴云桦,杨妮,王星傑,等.Nrf2激动剂t-BHQ在预防术后腹腔粘连形成中的价值及其可能的机制[J].临床医学研究与实践,2021, 6(13):1-3, 15.
[18] ZENG X, LU B, WANG F, et al. The effect of Smad2- and Smad3-targeting RNA interference on extracellular matrix synthesis in rat fibroblasts of peritoneal adhesion tissues. Am J Transl Res. 2020;12(11):7420-7429.
[19] LI H, WANG L, SHAO M, et al. Pirfenidone attenuates the EMT process and the secretion of VEGF in TGF-β2-induced ARPE-19 cells via inhibiting the activation of the NF-κB/snail signaling pathway. J Ophthalmol. 2023;303:4798071-47980720.
[20] TIAN L, SUN T, FAN M, et al. Novel silk protein/hyaluronic acid hydrogel loaded with azithromycin as an immunomodulatory barrier to prevent postoperative adhesions. Int J Biol Macromol. 2023; 235(30):123811.
[21] ALBRECHT LV, TEJEDA-MUÑOZ N, DE ROBERTIS EM. Cell biology of canonical wnt signaling. Annu Rev Cell Dev Biol. 2021;37(6):369-389.
[22] 于晓猛,冯仁蕊,王宏,等.Wnt/β-catenin信号通路与大鼠腹膜纤维化-EMT相关研究[J].生物医学工程与临床,2022,26(4):486-490.
[23] 赵敏.活血通腑方调控巨噬细胞极化和SOCS/JAK2/STAT/PPAR-γ通路防治腹腔粘连的机制研究[D].南京:南京中医药大学,2020.
[24] ARDALAN KHALES S, AARABI A, ABBASZADEGAN MR, et al. INPP5A/HLA-G1/IL-10/MMP-21 axis in progression of esophageal squamous cell carcinoma. Iran Biomed J. 2022;26(6):440-453.
[25] 郭嘉,丁琼桦,刘泽,等.间充质干细胞来源外泌体的生物学特性及免疫调控作用[J].中国组织工程研究,2022,26(7):1093-1101.
[26] 王含必,邓成艳.外泌体的生物功能及临床治疗应用潜能[J].生殖医学杂志,2021,30(7):966-970.
[27] 陈妍锜,赵敏,杨丽丽,等.中医药防治间皮细胞参与术后腹腔粘连形成[J].中华中医药学刊,2021,39(4):32-35.
[28] SHI M, LIU H, ZHANG T, et al. Extracellular vesicles derived from adipose mesenchymal stem cells promote peritoneal healing by activating MAPK-ERK1/2 and PI3K-Akt to alleviate postoperative abdominal adhesion. Stem Cells Int. 2022;2022:1940761.
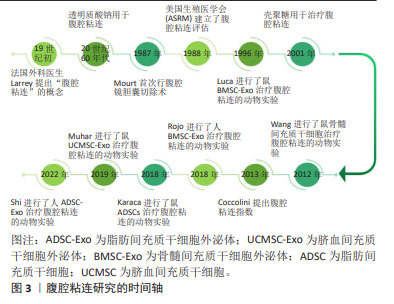
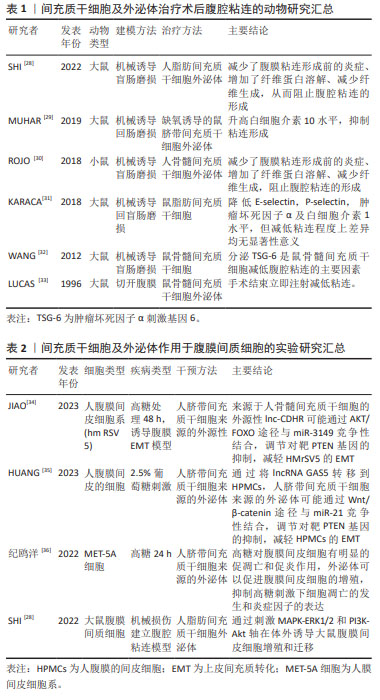
[29] MUHAR AM, PUTRA A, WARLI SM, et al. Hypoxia-mesenchymal stem cells inhibit intra-peritoneal adhesions formation by upregulation of the IL-10 expression. Open Access Maced J Med Sci. 2019;7(23): 3937-3943.
[30] ROJO D, CONGET P. Acellular derivatives of mesenchymal stem cells prevent peritoneal adhesions in an animal model. J Surg Res. 2018; 223:198-206.
[31] KARACA G, PEHLIVANLI F, AYDIN O, et al. The effect of mesenchymal stem cell use on intra-abdominal adhesions in a rat model. Ann Surg Treat Res. 2018;94(2):57-62.
[32] WANG N, LI Q, ZHANG L, LIN H, et al. Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6. PLoS One. 2012;7(8):e43768.
[33] LUCAS PA, WAREJCKA DJ, ZHANG LM, et al. Effect of rat mesenchymal stem cells on development of abdominal adhesions after surgery. J Surg Res. 1996;62(2):229-232.
[34] JIAO T, HUANG Y, SUN H, et al. Exosomal lnc-CDHR derived from human umbilical cord mesenchymal stem cells attenuates peritoneal epithelial-mesenchymal transition through AKT/FOXO pathway. Aging (Albany NY). 2023;15(14):6921-6932.
[35] HUANG Y, MA J, FAN Y, et al. Mechanisms of human umbilical cord mesenchymal stem cells-derived exosomal lncRNA GAS5 in alleviating EMT of HPMCs via Wnt/β-catenin signaling pathway. Aging (Albany NY). 2023;15(10):4144-4158.
[36] 纪鸥洋,方均燕,宋阿会,等.间充质干细胞外泌体对腹膜间皮细胞高糖损伤的作用研究[J].组织工程与重建外科,2022,18(4):294-299.
[37] 胡珍艳,尕永梅,麦迪乃姆·努尔麦麦提.间充质干细胞来源的外泌体中miR-143-3p抑制自噬并逆转心肌缺血再灌注损伤的机制研究[J].实用临床医药杂志,2022,26(17):46-52.
[38] 蔡黎黎,咸娴,胡维佳,等.人脐带干细胞外泌体对抑郁模型小鼠海马小胶质细胞极化和神经元凋亡的影响[J].中华行为医学与脑科学杂志,2023,32(5):399-406.
[39] 杨静,胡华钟,张书勤,等.脐带间充质干细胞来源的外泌体通过抑制上皮间质转化缓解肺纤维化[J].南方医科大学学报,2020, 40(7):988-994.
[40] 沈括,王许杰,刘开拓,等.人脂肪间充质干细胞外泌体对小鼠RAW264.7细胞的炎症反应和小鼠全层皮肤缺损创面愈合的影响[J].中华烧伤与创面修复杂志,2022,38(3):215-226.
[41] 赵海波,赵夏,高甲科,等.间充质干细胞来源的外泌体对肌腱细胞损伤修复的影响及其机制[J].中华创伤杂志,2021,37(7):653-661.
[42] 王振刚,付塬,张闻达,等.间充质干细胞来源的外泌体对2型糖尿病小鼠胰岛功能和炎症因子的影响[J].中国实验诊断学,2023, 27(9):1077-1082.
[43] 赵莹莹,陈莉莎,闫丽,等.脐带间充质干细胞外泌体对大鼠卵巢功能作用及机制研究[J].临床军医杂志,2022,50(11):1155-1158.
[44] HORITA M, FARQUHARSON C, STEPHEN LA. The role of miR-29 family in disease. J Cell Biochem. 2021;122(7):696-715.
[45] 张旭东,谭季春.间充质干细胞及外泌体治疗子宫内膜损伤的研究进展[J].中国实用妇科与产科杂志,2023,39(2):236-239.
[46] YANG J, LIU XX, FAN H, et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One. 2015;10(10):e0140551.
[47] YANG J, ZHOU CZ, ZHU R, et al. miR-200b-containing microvesicles attenuate experimental colitis associated intestinal fibrosis by inhibiting epithelial-mesenchymal transition. J Gastroenterol Hepatol. 2017;32(12):1966-1974.
[48] 刘文涛,王新月,杨毅等.骨髓间充质干细胞来源的外泌体诱导巨噬细胞向M型极化[J].中国组织化学与细胞化学杂志,2022,31(3): 232-238.
[49] 林颖,胡锦章,颛孙永勋,等.骨髓间充质干细胞外泌体调节哮喘小鼠Foxp3+ Treg/Th17的平衡[J].中国组织工程研究,2018,22(17): 2637-2643.
[50] LI X, LIU L, YANG J, et al. Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181c attenuating burn-induce-d excessive inflammation. EBioMedicine. 2016;8(10):72-82.
[51] QU Y, ZHANG Q, CAI X, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21(10):2491-2502.
[52] LI L, WANG Y, YU X, et al. Bone marrow mesenchymal stem cell-derived exosomes promote plasminogen activator inhibitor 1 expression in vascular cells in the local microenvironment during rabbit osteonecrosis of the femoral head. Stem Cell Res. 2020;11(1):480-493.
[53] AN Y, ZHAO J, NIE F, et al. Exosomes from adipose-derived stem cells (ADSCs) overexpressing miR-21 promote vascularization of endothelial cells. Sci Rep. 2019;9(1):12861.
[54] ZHE C, WANG H, YANG X, et al. Therapeutic potential of mesenchymal cell–derived miRNA-150-5p–expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J Immunol. 2018:2472-2482. |