Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (13): 2067-2074.doi: 10.3969/j.issn.2095-4344.1659
Previous Articles Next Articles
Pre-clinical acute toxicity of an immortalized human hepatocyte cell line HepZJ
Lu Yangzhou1, Li Shao1, Jiang Hua2, Li Wei2, Gao Yi1
- 1Department of Hepatobiliary Surgery II, Zhujiang Hospital, Southern Medical University, Guangzhou 510280, Guangdong Province, China; 2National Center for Safety Evaluation of Drugs, National Institutes for Food and Drug Control/The Beijing Key Lab for Pre-clinical Safety Evaluation of Drugs, Beijing 100176, China
-
Revised:2018-12-24Online:2019-05-08Published:2019-05-08 -
Contact:Gao Yi, MD, Chief physician, Professor, Doctoral supervisor, Department of Hepatobiliary Surgery II, Zhujiang Hospital, Southern Medical University, Guangzhou 510280, Guangdong Province, China; Li Wei, Chief pharmacist, National Center for Safety Evaluation of Drugs, National Institutes for Food and Drug Control/The Beijing Key Lab for Pre-clinical Safety Evaluation of Drugs, Beijing 100176, China -
About author:Lu Yangzhou, Master, Department of Hepatobiliary Surgery II, Zhujiang Hospital, Southern Medical University, Guangzhou 510280, Guangdong Province, China -
Supported by:the National High Technology Research and Development Plan of China, No. 2012AA020505 (to GY); the National Natural Science Foundation of China, No. 81470875 (to GY); the Natural Science Foundation of Guangdong Province, No. 2014A030312013 (to GY); Guangdong Provincial Science and Technology Plan Project, No. 2015B020229002 (to GY)
CLC Number:
Cite this article
Lu Yangzhou, Li Shao, Jiang Hua, Li Wei, Gao Yi. Pre-clinical acute toxicity of an immortalized human hepatocyte cell line HepZJ[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(13): 2067-2074.
share this article
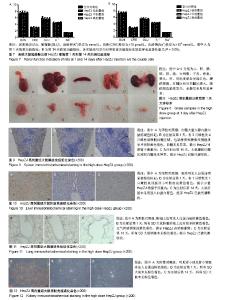
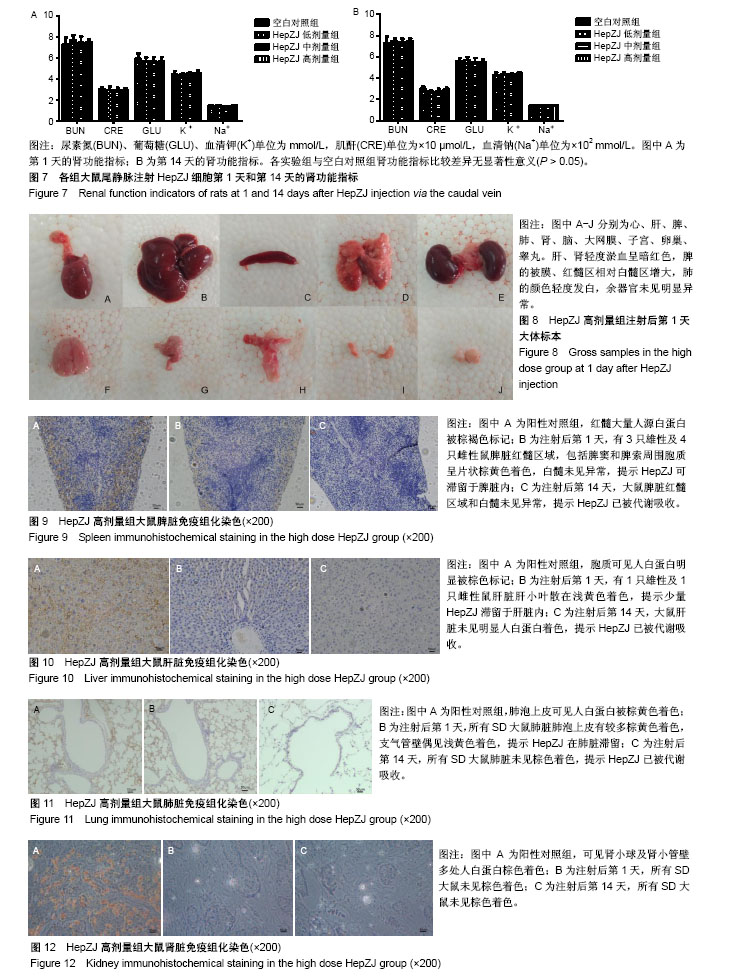
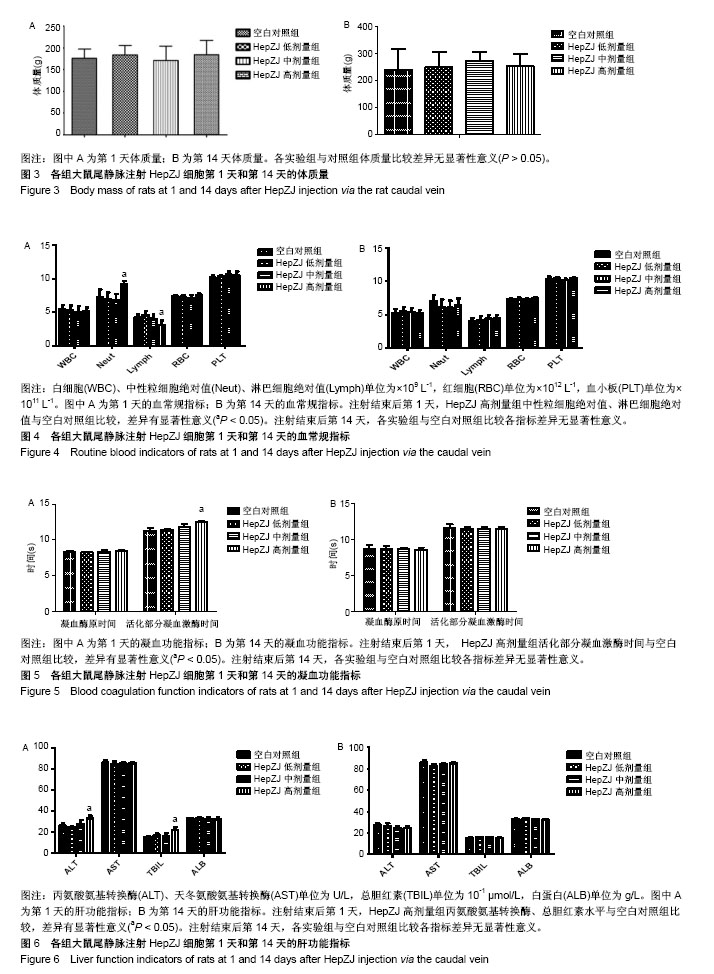
2.1 临床症状 在急性毒性实验中,HepZJ高剂量组尾静脉注射细胞悬液后即时有1只雌性大鼠出现蜷缩俯卧、呼吸困难、口唇发绀,数分钟后死亡,立即予剖检处理(因剖检后血液凝固而未能行相关指标检测,于注射后第1天统一观察大体标本)。另有1只雌鼠和1只雄鼠出现静止俯卧、焦躁不安,数分钟后恢复正常。余各组SD大鼠在注射后14 d内饮食正常、被毛均匀分布、活动正常,无异常临床症状。 2.2 各组大鼠体质量比较 在急性毒性实验中,注射后第1天和第14天时,各实验组与对照组体质量比较差异无显著性意义(P > 0.05),见图3,提示HepZJ细胞对动物体质量无不良影响。 2.3 各组大鼠血常规指标比较 注射结束后第1天,HepZJ低剂量组、HepZJ中剂量组白细胞、红细胞、血小板计数与空白对照组比较差异无显著性意义,HepZJ高剂量组与空白对照组中性粒细胞绝对值分别为(9.2±0.8)× 109 L-1,(7.0±1.5) ×109 L-1,差异有显著性意义(P < 0.05),淋巴细胞绝对值分别为(3.1±1.1)×109 L-1,(4.3±0.8)×109 L-1,差异有显著性意义(P < 0.05),见图4A。注射结束后第14天,各实验组与空白对照组比较各指标差异无显著性意义,见图4B。结果提示HepZJ单次注射后短时间内可在动物体内诱发炎症反应,14 d(恢复期)后炎症反应消失。 2.4 各组大鼠凝血功能指标比较 注射结束后第1天,HepZJ低剂量组、HepZJ中剂量组各指标与空白对照组比较差异无显著性意义,HepZJ高剂量组与空白对照组活化部分凝血激酶时间分别为(12.5±0.5) s,(11.3±0.6) s,差异有显著性意义(P < 0.05),见图5A。注射结束后第14天,各实验组与空白对照组比较各指标差异无显著性意义,见图5B。结果提示HepZJ单次注射短时间内在动物体内可致凝血功能异常,14 d(恢复期)后凝血功能恢复正常。 2.5 各组大鼠血清生化指标比较 对于肝功能检测,注射后第1天HepZJ低剂量组、HepZJ中剂量组各指标与空白对照组比较差异无显著性意义,HepZJ高剂量组与空白对照组的丙氨酸氨基转换酶分别为(32.7±4.6) U/L、(26.0±3.6) U/L,差异有显著性意义(P < 0.05),总胆红素分别为(22.0±4.4)× 10-1 μmol/L、(15.1±1.9)×10-1 μmol/L,差异有显著性意义(P < 0.05),见图6A。第14天(恢复期)结束时各指标差异无显著性意义,见图6B。对于肾功能检测,注射后第1天与第14天各指标差异无显著性意义,见图7。结果提示HepZJ单次注射短时间内在动物体内可致肝功能异常,14 d后肝功能恢复正常,但是对肾功能及内环境电解质无不良影响。 2.6 各组大鼠大体病理及免疫组化检测结果 在急性毒性实验中,除HepZJ高剂量组1只大鼠死亡外,HepZJ低剂量组和HepZJ中剂量组大鼠在注射后第1天和第14天,剖检观察心、肝、脾、肺、肾、脑、睾丸、卵巢、子宫和大网膜,其体积大小、形态、颜色、质地等未见明显异常,未行免疫组化检查。HepZJ高剂量组注射后第1天(包括死亡大鼠)大体标本,见图8,其中肝、肾轻度淤血呈暗红色,脾的被膜、红髓区相对白髓区增大,肺的颜色轻度发白,余器官未见明显异常。因此,只对脾、肝、肺、肾行免疫组化检查,镜下模糊着色为可疑染色,浅黄色为弱染色,棕黄色为中度染色,棕褐色为强染色,无着色判定为阴性结果,综合分析判定可疑染色、弱染色、中度染色和强染色是非特异性着色还是阳性结果。 脾脏免疫组化染色:注射后第1天有3只雄性及4只雌性鼠脾脏红髓区域,包括脾窦和脾索周围胞质呈片状棕黄色着色,白髓未见异常。第14天剖检所有大鼠未见明显异常,见图9,提示HepZJ进入体内后短时间内可被脾脏摄取,但随时间延长可被代谢吸收。 肝脏免疫组化染色:注射后第1天有1只雄性及1只雌性鼠肝脏肝小叶散在浅黄色着色,偶尔可见中央静脉浅黄色着色。第14天剖检所有大鼠未见明显异常,见图10,提示HepZJ进入体内后少数细胞短时间内可被肝脏摄取,但随时间延长可被代谢吸收。 肺脏免疫组化染色:注射后第1天所有SD大鼠肺脏肺泡上皮有较多细胞质棕黄色着色,支气管壁偶见浅黄色着色。第14天剖检所有大鼠未见明显异常,见图11,提示HepZJ进入体内后短时间内可聚集在肺部,但随时间延长可被代谢吸收。 肾脏免疫组化染色:未见明显异常,见图12。 以上结果提示HepZJ经尾静脉注射进入动物体内后的主要靶器官是脾脏、肝脏和肺脏,剂量过高时可引起HepZJ细胞栓塞,出现严重肺栓塞时可引起死亡。"
| [1] Meirelles Júnior RF, Salvalaggio P, Rezende MB, et al. Liver transplantation: history, outcomes and perspectives. Einstein (Sao Paulo). 2015;13(1): 149-152.[2] 丁易涛,施晓雷.生物人工肝脏临床研究现状[J].中国研究型医院, 2017,4(5): 17-25.[3] Sakiyama R, Blau BJ, Miki T. Clinical translation of bioartificial liver support systems with human pluripotent stem cell-derived hepatic cells. World J Gastroenterol. 2017;23(11):1974-1979.[4] Forbes SJ, Gupta S, Dhawan A. Cell therapy for liver disease: From liver transplantation to cell factory. J Hepatol. 2015;62(1 Suppl):S157-169.[5] Kim Y, Kang K, Yoon S, et al. Prolongation of liver-specific function for primary hepatocytes maintenance in 3D printed architectures. Organogenesis. 2018;14(1):1-12.[6] Lancett P, Williamson B, Barton P, et al. Development and Characterization of a Human Hepatocyte Low Intrinsic Clearance Assay for Use in Drug Discovery. Drug Metab Dispos. 2018;46(8):1169-1178.[7] Fang ZP, Jiang BG, Gu XF, et al. P21-activated kinase 5 plays essential roles in the proliferation and tumorigenicity of human hepatocellular carcinoma. Acta Pharmacol Sin. 2014;35(1):82-88.[8] van Wenum M, Treskes P, Tang CY, et al. Scaling-up of a HepaRG progenitor cell based bioartificial liver: optimization for clinical application and transport. Biofabrication. 2017;9(3):035001. [9] Layton DS, Bean AG, Dodge NM, et al. Differential cytokine expression and regulation of human anti-pig xenogeneic responses by modified porcine dendritic cells. Xenotransplantation. 2008;15(4):257-267.[10] 石伟,蔡端,张群华,等. 异种肝细胞移植缓解大鼠急性肝功能衰竭及其排斥反应观察[J]. 中华肝胆外科杂志, 2005,11(4):265-268.[11] Yang G, Hong H, Torres A, et al. Standards for Deriving Nonhuman Primate-Induced Pluripotent Stem Cells, Neural Stem Cells and Dopaminergic Lineage. Int J Mol Sci. 2018;19(9): E2788.[12] Steens J, Klein D. Current Strategies to Generate Human Mesenchymal Stem Cells In Vitro. Stem Cells Int. 2018;2018:6726185.[13] Hansel MC, Gramignoli R, Blake W, et al. Increased reprogramming of human fetal hepatocytes compared with adult hepatocytes in feeder-free conditions. Cell Transplant. 2014;23(1):27-38.[14] Crombleholme TM, Langer JC, Harrison MR, et al. Transplantation of fetal cells. Am J Obstet Gynecol. 1991;164(1 Pt 1):218-230.[15] Zhang Y, Shi J, Liu S. Establishment and Characterization of a Telomerase-Immortalized Sheep Trophoblast Cell Line. Biomed Res Int. 2016;2016:5808575.[16] 卢扬洲,黎少,姜华,等. 新型永生化人肝细胞系HepZJ的安全性研究[J]. 药物评价研究,2018,41(6):1062-1067.[17] Ra JC, Shin IS, Kim SH, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20(8):1297-1308.[18] 王琼,汤钱球,张小云,等.炎症和凝血指标在脓毒血症诊断和预后评估中价值分析[J].临床血液学杂志(输血与检验), 2018,31(5):750-753.[19] Thorell SE, Nash MJ, Thachil J. Clinical implications of clotting screens. Int J Lab Hematol. 2015;37(1):8-13.[20] Eschbach D, Horst K, Sassen M, et al. Hypothermia does not influence liver damage and function in a porcine polytrauma model. Technol Health Care. 2018;26(2):209-221.[21] Long C, Yang J, Yang H, et al. Attenuation of renal ischemia/reperfusion injury by oleanolic acid preconditioning via its antioxidant, anti?inflammatory, and anti?apoptotic activities. Mol Med Rep. 2016;13(6): 4697-4704.[22] 国家食品药品监督管理局药品审评中心. 治疗用生物制品非临床安全性评价指导原则[EB/OL]. http://www.cde.org.cn/zdyz.do?method= largePage&id=100, 2010-05-06/2018-10-29.[23] Stacey GN, Coecke S, Price AB, et al. Ensuring the Quality of Stem Cell-Derived In Vitro Models for Toxicity Testing. Adv Exp Med Biol. 2016; 856:259-297.[24] Van Meer PJ, Graham ML, Schuurman HJ. The safety, efficacy and regulatory triangle in drug development: Impact for animal models and the use of animals. Eur J Pharmacol. 2015;759:3-13.[25] Misik J, Pavlikova R, Cabal J, et al. Acute toxicity of some nerve agents and pesticides in rats. Drug Chem Toxicol. 2015;38(1):32-36.[26] Christapher PV, Parasuraman S, Asmawi MZ, et al. Acute and subchronic toxicity studies of methanol extract of Polygonum minus leaves in Sprague Dawley rats. Regul Toxicol Pharmacol. 2017;86:33-41. [27] Xu S, Zhang Z, Chu M. Long-term toxicity of reduced graphene oxide nanosheets: Effects on female mouse reproductive ability and offspring development. Biomaterials. 2015;54:188-200.[28] Kuroda T, Yasuda S, Sato Y. Tumorigenicity studies for human pluripotent stem cell-derived products. Biol Pharm Bull. 2013;36(2):189-192.[29] De Matteis V. Exposure to Inorganic Nanoparticles: Routes of Entry, Immune Response, Biodistribution and In Vitro/In Vivo Toxicity Evaluation.Toxics. 2017;5(4): E29.[30] 曾颖星,郑永霞,徐艳雯,等. 肝细胞移植治疗肝病的研究进展[J]. 医学综述, 2018, 24(7): 1344-1348.[31] You S, Zhu B, Liu H, et al. Safety of Human Hepatoma Cell-Line Constructing Bioartificial Liver Supporting System Treating Patients with Liver Failure. Hepatogastroenterology. 2014;61(132):933-936.[32] Yun JW, Ahn JH, Kwon E, et al. Human umbilical cord-derived mesenchymal stem cells in acute liver injury: Hepatoprotective efficacy, subchronic toxicity, tumorigenicity, and biodistribution. Regul Toxicol Pharmacol. 2016;81:437-447.[33] 国家食品药品监督管理总局药品审评中心.人体细胞治疗研究和制剂质量控制技术指导原则[EB/OL]. http://www.cde.org.cn/zdyz.do?method= largePage&id=38, 2008-09-04/2018-10-29.[34] Food and Drug Administration, HHS. International Conference on Harmonisation; addendum to International Conference on Harmonisation Guidance on S6 Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals; availability. Notice. Fed Regist. 2012;77(97): 29665-29666.[35] 国家食品药品监督管理总局药品审评中心.细胞制品研究与评价技术指导原则(征求意见稿)[EB/OL]. http://www.cde.org.cn/news.do?method= largeInfo&id=313749, 2016-12-19/2018-10-29.[36] 国家食品药品监督管理总局.《药物非临床研究质量管理规范》(国家食品药品监督管理总局令第34号)[EB/OL]. http://www.gov.cn/gongbao/content/ 2017/content_5241929.htm, 2017-08-02/2018-10-29.[37] He J, Ruan GP, Yao X, et al. Chronic Toxicity Test in Cynomolgus Monkeys For 98 Days with Repeated Intravenous Infusion of Cynomolgus Umbilical Cord Mesenchymal Stem Cells. Cell Physiol Biochem. 2017;43(3):891-904.[38] Mäkelä T, Takalo R, Arvola O, et al. Safety and biodistribution study of bone marrow-derived mesenchymal stromal cells and mononuclear cells and the impact of the administration route in an intact porcine model. Cytotherapy. 2015;17(4):392-402.[39] Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54-63.[40] 汪溪洁,马璟. 药物安全性评价新技术和新方法研究进展[J]. 中国医药工业杂志,2017,48(3):341-350.[41] Kandhare AD, Bodhankar SL, Mohan V, et al. Acute and repeated doses (28 days) oral toxicity study of Vicenin-1, a flavonoid glycoside isolated from fenugreek seeds in laboratory mice. Regul Toxicol Pharmacol. 2016; 81:522-531. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||