Chinese Journal of Tissue Engineering Research ›› 2015, Vol. 19 ›› Issue (15): 2376-2381.doi: 10.3969/j.issn.2095-4344.2015.15.016
Previous Articles Next Articles
Effects of different cryopreservation methods on the ultrastructure and viability of amniotic membrane
Liu Dai1, Jin Jie2, Xie Fang1, Zhang Chao1, Lu Jian-jian1, Xu Jia-jie1, Xu Jun3, Teng Li1
- 1 Fifth Department of Plastic Surgery, Plastic Surgery Hospital of Peking Union Medical College & Chinese Academy of Medical Science, Beijing 100144, China
2 Department of Histology and Embryology, Yanjing Medical College, Capital Medical University, Beijing 101300, China
3 Department of Plastic and Reconstructive Surgery, General Hospital of PLA, Beijing 100853, China
-
Revised:2015-03-02Online:2015-04-09Published:2015-04-09 -
Contact:Teng Li, Chief physician, Fifth Department of Plastic Surgery, Plastic Surgery Hospital of Peking Union Medical College & Chinese Academy of Medical Science, Beijing 100144, China -
About author:Liu Dai, Studying for doctorate, Attending physician, Fifth Department of Plastic Surgery, Plastic Surgery Hospital of Peking Union Medical College & Chinese Academy of Medical Science, Beijing 100144, China Jin Jie, Lecturer, Department of Histology and Embryology, Yanjing Medical College, Capital Medical University, Beijing 101300, China Liu Dai and Jin Jie contributed equally to this work. -
Supported by:the National Natural Science Foundation of China, No. 30672188
CLC Number:
Cite this article
Liu Dai, Jin Jie, Xie Fang, Zhang Chao, Lu Jian-jian, Xu Jia-jie, Xu Jun, Teng Li. Effects of different cryopreservation methods on the ultrastructure and viability of amniotic membrane[J]. Chinese Journal of Tissue Engineering Research, 2015, 19(15): 2376-2381.
share this article
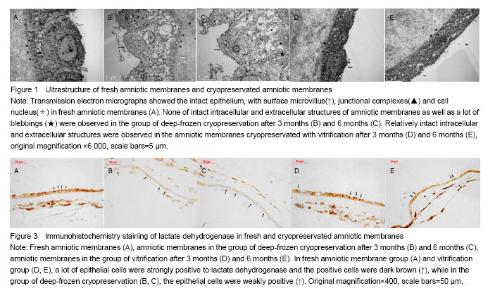
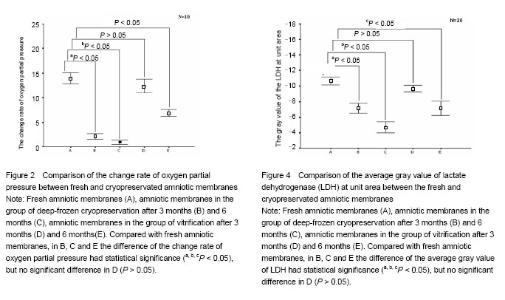
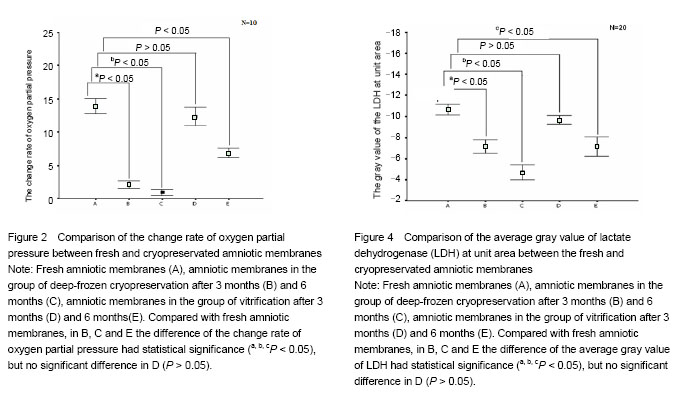
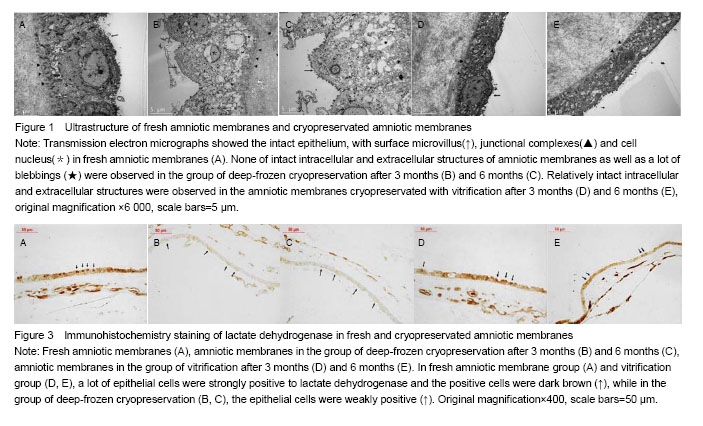
Effect of different cryopreservation methods on ultrastructure changes of amniotic membranes As observed with transmission electron microscopy, fresh amniotic membranes showed intact epithelium, with surface microvillus and junctional complexes between the cells and the basal membrane. Very few cytoplasmic extrusions (blebbing) were observed on the fresh membranes (Figure 1A). Whereas no intact intracellular and extracellular structures were identified in the amnion specimens preserved in the group of deep-frozen cryopreservation, especially after 6 months, presenting no microvillus, loose cytoplasm and nucleus, and absent junctional complexes (Figures 1B, C). No matter after 3 months and 6 months, the amniotic membranes cryopreserved by vitrification showed intact epithelium with thick chromatin and dense microvillus, junctional complexes and a few blebbings (Figures 1D, E). Effect of different cryopreservation methods on oxygen partial pressure of amniotic membranes Oxygen partial pressure of amniotic membranes is referred to the pressure of the soluble oxygen in the amniotic epithelium. The pressure variety in 60 seconds examined by microcomputer analysis system for biological oxygen consumption is positive correlated with viability of the cells. Figure 2 represents the change rate of oxygen partial pressure of amniotic membranes in different conditions. The change rate of oxygen partial pressure of fresh amniotic membranes was (13.9±1.62)%, and it decreased in preserved amniotic membranes in the group of deep-frozen cryopreservation after 3 months [(2.12±0.74)%, P < 0.05] and 6 months [(0.93±0.65)%, P < 0.05], and also in the group of vitrification after 6 months [(6.85±0.99)%, P < 0.05]. But there was no statistical significance between the rate of fresh amniotic membranes and ones cryopreservated by vitrification after 3 months [(12.34±1.90)%, P > 0.05]. Effect of cryopreservations on the lactate dehydrogenase viability of amniotic membranes As shown by the immunohistochemical staining, the amniotic epithelium and extracellular matrix of fresh amniotic membranes and cryopreserved amniotic membranes by vitrification after 3 months and 6 months expressed lactate dehydrogenase with dark brown, but there was not much expression of lactate dehydrogenase in the amniotic membranes in the group of deep-frozen cryopreservation (Figure 3). Twenty positive epithelial cells of each sample were collected randomly by using Leica QWin image analysis system, and the average gray value of lactate dehydrogenase at unit area was measured (Figure 4). The average gray value of the lactate dehydrogenase in fresh amniotic membranes was 0.106±0.011, and it decreased in cryopreserved amniotic membranes: the group of deep-frozen cryopreservation after 3 months was 0.071±0.014 (P < 0.05) and 6 months was 0.047±0.015 (P < 0.05); in the group of vitrification after 3 months was 0.097±0.088 (P > 0.05) and 6 months was 0.072±0.019 (P < 0.05). There was no statistical significance between the fresh amniotic membranes and amniotic membranes in the group of vitrification after 3 months. "
| [1] Davis JW. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J. 1910; 15:307-396. [2] Shimmura S, Shimazaki J, Ohashi Y, et al. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea. 2001;20:408-413. [3] Kim JC, Tseng SC. The effects on inhibition of corneal neovascularization after human amniotic membrane transplantation in severely damaged rabbit corneas. Korean J Ophthalmol. 1995;9:32-46. [4] Ricci E, Vanosi G, Lindenmair A, et al. Anti-fibrotic effects of fresh and cryopreserved human amniotic membrane in a rat liver fibrosis model. Cell Tissue Bank. 2013;14(3):475-488. [5] Gomes JA, Romano A, Santos MS, et al. Amniotic membrane use in ophthalmology. Curr Opin Ophthalmol. 2005;16:233-240. [6] Dua HS, Gomes JA, King AJ, et al. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51-77. [7] Singh R, Gupta P Kumar P, et al. Properties of air dried radiation processed amniotic membranes under different storage conditions. Cell Tissue Bank. 2003;4:95-100. [8] Okabe M, Kitagawa K, Yoshida T, et al. Hyperdry human amniotic membrane is useful material for tissue engineering: physical, morphological properties, and safety as the new biological material.J Biomed Mater Res A. 2014;102(3): 862-870. [9] Gao J, Liu L, Liu W, et al. Study of process optimization on freeze drying of human amniotic membrane. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2012;29(4):705-709. [10] Kruse FE, Joussen AM, Rohrschneider K, et al. Cryopreserved human amniotic membrane for ocular surface reconstruction. Graefes Arch Clin Exp Ophthalmol. 2000;238:68-75. [11] Burgos H, Faulk WP. The maintenance of human amniotic membranes in culture. Br J Obstet Gynaecol. 1981;88: 294-300. [12] Zhang H, Zhou Y, Li Y, et al. Prediction of clinical pregnancy in vitrified-warmed single blastocyst transfer cycles by pre-freeze morphology. Iran J Reprod Med. 2014;12(8):567-572. [13] Ganatra MA, Durrani KM. Method of obtaining and preparation of fresh human amniotic membrane for clinical use. J Pak Med Assoc. 1996;46:126-128. [14] Kesting MR, Loeffelbein DJ, Steinstraesser L, et al. Cryopreserved human amniotic membrane for soft tissue repair in rats. Ann Plast Surg. 2008;60(6):684-691. [15] Gholipourmalekabadi M, Mozafari M, Salehi M, et al. Development of a cost-effective and simple protocol for decellularization and preservation of human amniotic membrane as a soft tissue replacement and delivery system for bone marrow stromal cells. Adv Healthc Mater. 2015 doi: 10.1002/adhm. 201400704 [16] Koike C, Zhou K, Takeda Y, et al. Characterization of amniotic stem cells. Cell Reprogram. 2014;16(4):298-305. [17] Hennerbichler S, Reichl B, Pleiner D, et al. The influence of various storage conditions on cell viability in amniotic membrane. Cell Tissue Bank. 2007;8(1):1-8. [18] Lee SH, Tseng SCG. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997;123:303-312. [19] Deolinda de Oliveira Pena J, Melo GB, Gomes JA, et al. Ultrastructural and growth factor analysis of amniotic membrane preserved by different methods for ocular surgery. Arq Bras Oftalmol. 2007;70(5):756-762. [20] Thomasen H, Pauklin M, Noelle B, et al. The effect of long-term storage on the biological and histological properties of cryopreserved amniotic membrane. Curr Eye Res. 2011;36(3):247-255. [21] Rama P, Giannini R, Bruni A, et al. Further evaluation of amniotic membrane banking for transplantation in ocular surface diseases. Cell Tissue Bank. 2001;2:155-163. [22] Dohrmann P, Fory R, Rupp K, et al. Animal experiment studies of amnion transplantation as peritoneal replacement. Langenbecks Arch Chir Suppl II Verh Dtsch Ges Chir. 1990;1055-1059. [23] Rall WF, Fahy GM. Ice-free of mouse embryos at -196 ℃ by vitrification. Nature. 1985;313:573-575. [24] Song YG, Hagen PO, Lighfoot FG. In vivo evaluation of the effects of a new ice-free cryopreservation process on autologoeus cascular grafts. J Incest Surg. 2000; 13(5): 279-288. [25] Roy TK, Brandi S, Tappe NM, et al. Embryo vitrification using a novel semi-automated closed system yields in vitro outcomes equivalent to the manual Cryotop method. Hum Reprod. 2014;29(11):2431-2438. [26] Choi WJ, Lee JH, Park MH, et al. Influence of the vitrification solution on the angiogenic factors in vitrificated mouse ovarian tissue. Obstet Gynecol Sci. 2013;56(6): 382-388. [27] Fujita T, Takami Y, Ezoe K. et al. Successful preservation of human skin by vitrification. J Burn Care Rehabil. 2000;21(4): 304-309. [28] Brockbank KG1, Chen Z, Greene ED, et al. Vitrification of heart valve tissues. Methods Mol Biol. 2015;1257:399-421. [29] Kartberg AJ, Hambiliki F, Arvidsson T, et al. Vitrification with DMSO protects embryo membrane integrity better than solutions without DMSO. Reprod Biomed Online. 2008; 17(3): 378-384. [30] Krabcova I, Jirsova K, Bednar J. Rapid cooling of the amniotic membrane as a model system for the vitrification of posterior corneal lamellae. Cell Tissue Bank. 2014;15(1): 165-173. |
| [1] | Li Jing, Xie Jianshan, Cui Huilin, Cao Ximei, Yang Yanping, Li Hairong. Expression and localization of diacylglycerol kinase zeta and protein kinase C beta II in mouse back skin with different coat colors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1196-1200. |
| [2] | Yang Caihui, Liu Qicheng, Dong Ming, Wang Lina, Zuo Meina, Lu Ying, Niu Weidong. Serine/threonine protein kinases can promote bone destruction in mouse models of chronic periapical periodontitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3654-3659. |
| [3] | Yan Peng, Ma Yufei, Cui Jingfu, Hao Shaofei, Liu Jinhui, Guan Chunlei, Wang Xiaoran, Yang Xiaoyu. Mechanism of anodic block electrical stimulation of sacral nerve root to reconstruct bladder function [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3684-3689. |
| [4] | Wu Dalei, Zhou Shouheng, Yan Jianwei, Li Bo, Xu Nuo, Shi Chun, Gao Yang. Alcohol extract of Eucommia ulmoides Oliv. promotes bone healing in rats with periapical periodontitis [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(23): 3685-3689. |
| [5] | Zhang Yuan, Zeng Min, Zhai Bo. Vitrification-based cryopreservation of tissues: strengths and existing problems [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(23): 3751-3755. |
| [6] | Qiu Xiaoyang, Wang Yuanyuan, Liu Chunpeng, Chen Hongcai, Wu Xuan, Zhan Xiaofen. Application of environment-friendly bio-tissue sample preparation kit in fluorescence in situ hybridization detection of HER2 protein 2-positive invasive breast cancer [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(16): 2572-2577. |
| [7] | Wang Liudi, Liu Wei, Xie Yuanyuan, Gao Tianyun, Huang Feifei, Wang Bin. Biological characteristics of three kinds of human placenta-derived mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(9): 1377-1383. |
| [8] | Cao Peng, Wang Haonan, Tian Weifeng, Sun Naichao, Bai Jiangbo, Yu Kunlun, Tian Dehu. Human amniotic membrane repairs acute sciatic nerve injury in rat models [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(7): 1046-1051. |
| [9] | Long Huidong1, 2, Long Lingli1, Mai Qingyun1, Zhao Wen1, Li Yubin1. Protection of reduced glutathione and ulinastatin on frozen-thawed ovarian tissues after xenotransplantation [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(7): 1084-1089. |
| [10] | Bai Qiushi1, 2, Su Sheng1, 2, Wang Liangjia1, 2, Zheng Yangyang2, Kang Juanjuan2, Xu Jinying2, Chi Guangfan2. Immunofluorescence staining of adeno-associated virus type 2 after subpial injection in rat models of spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(31): 4979-4985. |
| [11] | Li Dongmei, Liu Xinhui,Li Qingxing. Changes of vascular endothelial growth factor after repair of pig mandibular defects by nano-hydroxyapatite/collagen composite [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(26): 4148-4153. |
| [12] | Jin Wenhu, Zhou Aiting, Wu Zhonghuan, Fan Zhenhai, Wang Dali, Wei Zairong. Vascular endothelial growth factor gene modified human amnion-derived mesenchymal stem cells promote the survival of ultra-long random skin flap [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(21): 3349-3356. |
| [13] | Zheng Wanling1, Zhang Aijun2, Wang Hao1, Wang Pingping1, Li Jianhua1, Wen Minmin1, Jin Peisheng2. Feasibility of fat particles cryopreserved for different time for use as subcutaneous fillers [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(11): 1706-1710. |
| [14] | Liu Lei, Yong Qi, Li Na, Yang Duo, Zhang Guo-ying, Cui Lei. Vitreous cryopreservation and thawing of adipose-derived stem cells/demineralized bone matri [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(9): 1357-1363. |
| [15] | Gao Ming-long, Shi Shao-xia, Zhang Kun, Zhang Ying-dong, Li Na, Yu Ming, Wang Yong-liang. Effects of brain-derived neurotrophic factor-modified human amniotic membrane-derived mesenchymal stem cell transplantation on learning and memory abilities of Alzheimer's disease rats [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(9): 1419-1424. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||