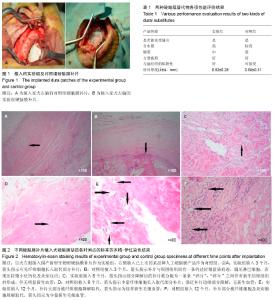
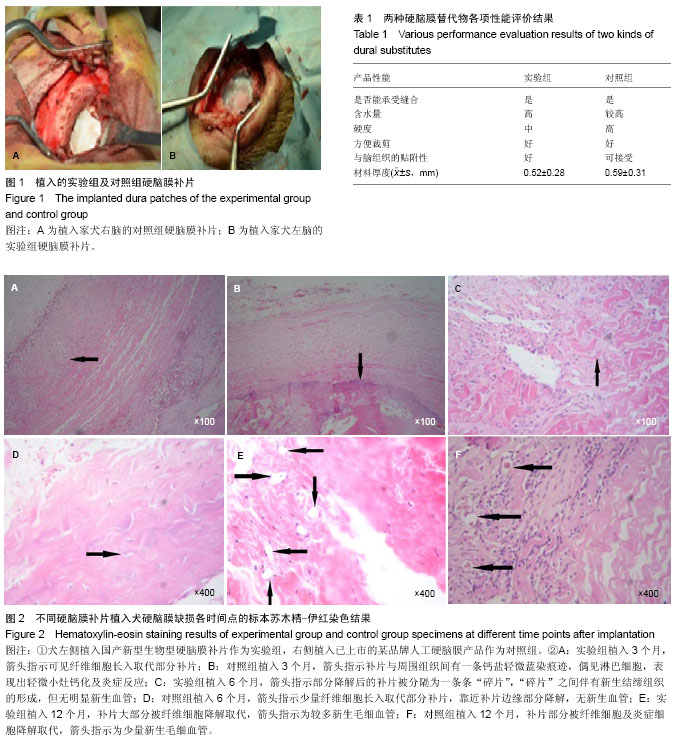
Chinese Journal of Tissue Engineering Research ›› 2014, Vol. 18 ›› Issue (25): 3947-3952.doi: 10.3969/j.issn.2095-4344.2014.25.003
Previous Articles Next Articles
Safety and validity of a new-type biological dura patch
Chang Hong-bo1, Pan Teng-fei2, Lu Wang-sheng1, Wang Peng1, Cui Kai2, Zhang Jian-ning1
- 1Institute of Neurosurgery, Navy General Hospital of PLA, Beijing 100048, China; 2Lepu Medical Technology (Beijing) Co., Ltd., Beijing 102200, China
-
Received:2014-04-30Online:2014-06-18Published:2014-06-18 -
Contact:Zhang Jian-ning, M.D., Ph.D., Doctoral supervisor, Institute of Neurosurgery, Navy General Hospital of PLA, Beijing 100048, China -
About author:Chang Hong-bo, Master, Attending physician, Institute of Neurosurgery, Navy General Hospital of PLA, Beijing 100048, China
CLC Number:
Cite this article
Chang Hong-bo, Pan Teng-fei, Lu Wang-sheng, Wang Peng, Cui Kai, Zhang Jian-ning. Safety and validity of a new-type biological dura patch[J]. Chinese Journal of Tissue Engineering Research, 2014, 18(25): 3947-3952.
share this article
| [1] Miltyk W,Palka JA.Potential role of pyrroline5-carboxylate in Regulation of collagen biosynthesis in cultured human skin fibroblasts.Comp Biochem Physiol A Mol Integr Physiol. 2000; 125(2):265-271. [2] Esposito F,Grimod G,Cavallo LM,et al.Collagen-only biomatrix as dural substitute: What happened after a 5-year observational follow-up study.Clin Neurol Neurosurg. 2013; 115(9):1735-1737. [3] Parlato C,di Nuzzo G,Luongo M,et al.Use of a collagen biomatrix (TissuDura) for dura repair: a long-term neuroradiological and neuropathological evaluation. Acta Neurochir (Wien).2011;153(1):142-147. [4] Stendel R,Danne M,Fiss I,et al.Efficacy and safety of a collagen matrix for cranial and spinal dural reconstruction using different fixation techniques. J Neurosurg. 2008; 109(2):215-221. [5] Esposito F,Cappabianca P,Fusco M,et al.Collagen-only biomatrix as a novel dural substitute. Examination of the efficacy, safety and outcome: clinical experience on a series of 208 patients.Clin Neurol Neurosurg. 2008;110(4): 343-351. [6] 史志东,郭英,邓美海,等.自制生物型人工硬脑膜与美国进口同类产品的比较[J].中华神经医学杂志,2006,5(10):997-1000. [7] van Wachem PB,Brouwer LA,Zeeman R,et al.Tissue reactions to epoxy-crosslinked porcine heart valves post-treated with detergents or a dicarboxylic acid. J Biomed Mater Res.2001;55(3):415-423. [8] Papakostas JC,Avgos S,Arnaoutoglou E,et al.The use of Vascu-Guard bovine pericardium patch for arteriotomy closure in carotid endarterectomy: Early and long term results.Ann Vasc Surg.2013;23(13):890-896. [9] Coleman S,Kerr H,Krishnamurthi V,et al.The use of bovine pericardium for complex urologic venous reconstruction. Urology.2013;22(13):4290-4295. [10] Costa BS,Cavalcanti-Mendes Gde A,Abreu MS,et al.Clinical experience with a novel bovine collagen dura mater substitute.Arq Neuropsiquiatr.2011;69(2A):217-220. [11] Weidner N.Intratumor microvessel density as a prognostic factor in cancer.Am J Pathol.1995;147(1):33-41. [12] 陶小虎.人工硬脑膜修补材料在神经外科的应用[J].中国组织工程研究,2012, 16(12):2217-2220. [13] 李文辉,吴日乐,岑莲.人工硬脑膜修补材料的研究及其临床应用[J].组织工程与重建外科杂志,2013,9(2):113-115. [14] Tomita T,Hayashi N,Okabe M,et al.New dried human amniotic membrane is useful as a substitute for dural repair after skull base surgery.J Neurol Surg B Skull Base. 2012;73(5): 302-307. [15] Kim DW,Eum WS,Jang SH,et al.A transparent artificial dura mater made of silk fibroin as an inhibitor of inflammation in craniotomized rats.J Neurosurg.2011;114(2):485-490. [16] Yamada K,Miyamoto S,Takayama M,et al.Clinical application of a new bioabsorbable artificial dura mater.J Neurosurg. 2002; 96(4):731-735. [17] Matsumoto Y,Aikawa H,Tsutsumi M,et al.Histological examination of expanded polytetrafluoroethylene artificial dura mater at 14 years after craniotomy: case report.Neurol Med Chir (Tokyo).2013;53(1):43-46. [18] Vakis A,Koutentakis D,Karabetsos D,et al.Use of polytetrafluoroethylene dural substitute as adhesion preventive material during craniectomies.Clin Neurol Neurosurg. 2006;108(8):798-802. [19] Sherman JH,Pouratian N,Okonkwo DO,et al. Reconstruction of the sellar dura in transsphenoidal surgery using an expanded polytetrafluoroethylene dural substitute.Surg Neurol. 2008;69(1):73-76. [20] 李文生,黄瑞宏,周希汉,等.人工硬脑膜补片在标准大骨瓣减压术中的应用[J].中国临床神经外科杂志,2013,18(4):234-235. [21] Huang YH,Lee TC,Chen WF,et al.Safety of the nonabsorbable dural substitute in decompressive craniectomy for severe traumatic brain injury .J Trauma. 2011;71(3):533-537. [22] Malliti M,Page P,Gury C,et al.Comparison of deep wound infection rates using a synthetic dural substitute (neuro-patch) or pericranium graft for dural closure: a clinical review of 1 year.Neurosurgery.2004;54(3):599-603. [23] Biroli F,Esposito F,Fusco M,et al.Novel equine collagen-only dural substitute.Neurosurgery.2008;62(3):273-274. [24] Bejjani GK,Zabramski J.Safety and efficacy of the porcine small intestinal submucosa dural substitute: results of a prospective multicenter study and literature review.J Neurosurg.2007;106(6):1028-1033. [25] 贾锋,陆兆丰,邱永明,等.人工硬脑膜Dura-GenTM植入转归的实验研究[J].中国微侵袭神经外科杂志,2006,11(9):407-409. [26] Rabinowitz L,Monnerie H,Shashidhara S,et al. Growth of rat cortical neurons on DuraGen, a collagen-based dural graft matrix.Neurol Res.2005;27(8):887-894. [27] 韦鹏翔,李丹青,周玉嘉,等.人工硬脑膜材料在神经外科手术中的应用:附100 例分析[J].中国组织工程研究, 2013,17(29): 5415-5420. [28] Ström JO,Boström S,Bobinski L,et al.Low-grade infection complicating silastic dural substitute 32 years post-operatively. Brain Inj.2011;25(2):250-254. [29] Esmaeili A,Nejat F,Nouri M,et al.Unusual dural substitute complications in pediatric patients.Childs Nerv Syst. 2012; 28(3):485-487. [30] Wang YF,Guo HF,Ying DJ.Multilayer scaffold of electrospun PLA-PCL-collagen nanofibers as a dural substitute.J Biomed Mater Res B Appl Biomater.2013;101(8):1359-1366. [31] Pettorini BL,Tamburrini G,Massimi L,et al.The use of a reconstituted collagen foil dura mater substitute in paediatric neurosurgical procedures--experience in patients.Br J Neurosurg.2010;24(1):51-54. [32] Kurpinski K,Patel S.Dura mater regeneration with a novel synthetic, bilayered nanofibrous dural substitute: an experimental study.Nanomedicine (Lond).2011;6(2): 325-337. [33] Sandoval-Sánchez JH,Ramos-Zúñiga R,de Anda SL,et al.A new bilayer chitosan scaffolding as a dural substitute: experimental evaluation.World Neurosurg. 2012;77(3-4): 577-582. [34] Cole PD,Stal D,Sharabi SE,et al.A comparative, long-term assessment of four soft tissue substitutes.Aesthet Surg J. 2011;31(6):674-681. [35] Terasaka S,Iwasaki Y,Kuroda S,et al.A novel method of dural repair using polyglycolic acid non-woven fabric and fibrin glue: clinical results of 140 cases.No Shinkei Geka. 2006;34(11): 1109-1117. [36] Hoell T,Hohaus C,Huschak G,et al.Total dura substitute in the spine: double layer dural substitute made from polylactide layer and bovine pericardium.Acta Neurochir (Wien). 2007; 149(12):1259-1262. [37] Shimada Y,Hongo M,Miyakoshi N,et al.Dural substitute with polyglycolic acid mesh and fibrin glue for dural repair: technical note and preliminary results. J Orthop Sci. 2006; 11(5): 454-458. [38] Wang H,Dong H,Kang CG,et al.Preliminary exploration of the development of a collagenous artificial dura mater for sustained antibiotic release.Chin Med J (Engl). 2013;126(17): 3329-3333. [39] Shah AR,Pearlman AN,O'Grady KM,et al.Combined use of fibrin tissue adhesive and acellular dermis in dural repair.Am J Rhinol.2007;21(5):619-621. [40] Jackson N,Muthuswamy J.Artificial dural sealant that allows multiple penetrations of implantable brain probes.J Neurosci Methods.2008;171(1):147-152. [41] Horta R,Costa J,Valença-Filipe R,et al.ALT chimeric flap associated to a dura mater biomatrix substitute for severe desfigurative mandible osteoradionecrosis and deficient bone consolidation after a free fibula flap.Br J Oral Maxillofac Surg. 2014. pii: S0266-4356(14)00197-1. doi: 10.1016/j.bjoms.2014.01.024. [Epub ahead of print] [42] Cavallo LM,Solari D,Somma T,et al.Use of equine pericardium sheet (LYOMESH®) as dura mater substitute in endoscopic endonasal transsphenoidal surgery.Transl Med UniSa.2013;7:23-28. [43] Costa BS,Cavalcanti-Mendes Gde A,Abreu MS,et al.Clinical experience with a novel bovine collagen dura mater substitute. Arq Neuropsiquiatr. 2011;69(2A):217-220. [44] Pettorini BL,Tamburrini G,Massimi L,et al.The use of a reconstituted collagen foil dura mater substitute in paediatric neurosurgical procedures--experience in 47 patients.Br J Neurosurg.2010;24(1):51-54. [45] He D,Genecov DG,Herbert M,et al.Effect of recombinant human bone morphogenetic protein-2 on bone regeneration in large defects of the growing canine skull after dura mater replacement with a dura mater substitute.J Neurosurg. 2010;112(2):319-328. [46] Chappell ET,Pare L,Salehpour M,et al.GORE PRECLUDE MVP dura substitute applied as a nonwatertight "underlay" graft for craniotomies: product and technique evaluation.Surg Neurol.2009;71(1):126-128; discussion 128-9. [47] Attenello FJ,McGirt MJ,Garcés-Ambrossi GL,et al.Suboccipital decompression for Chiari I malformation: outcome comparison of duraplasty with expanded polytetrafluoroethylene dural substitute versus pericranial autograft.Childs Nerv Syst.2009;25(2):183-190. |
| [1] | Yuan Bo, Wang Zhiwei, Tang Yifan, Zhou Shengyuan, Chen Xiongsheng, Jia Lianshun. Construction of polycaprolactone-tricalcium phosphate with different mixture ratios using three-dimensional printing technology and its osteoinductivity in vitro [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(6): 821-826. |
| [2] | Wei Wei, Liu Yanfei, Zhang Ling, Xiong Na . Self-assembling peptide hydrogel: hemostatic effect and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(2): 310-316. |
| [3] | Zhang Minbo, Peng Qifeng, Ma Yaping, Kong Weijun, Liao Wenbo. Physical properties and biocompatibility of 3D printed bone microparticle/poly(lactic-co-glycolic acid) scaffold [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(14): 2215-2222. |
| [4] | Li Zhi, Tan Chunhua, Cai Xianhua, Wang Huasong, Ding Xiaoming, Zhao Yanhong. Fabrication and biocompatibility assessment of the scaffold with biomimetic interconnected macropore structure [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(14): 2223-2227. |
| [5] | Bai Yulong, Gao Yufeng, Zhong Hongbin, Zhao Yantao, Guo Ruizhou, Li Li. Allogeneic and xenogeneic tissue repair materials: how to choose a suitable virus inactivation process [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(14): 2261-2268. |
| [6] | Luo Kai, Yang Yafeng, Ma Teng, Xia Bing, Huang Liangliang, Huang Jinghui, Luo Zhuojing. Effects of perfluorotributylamine/alginate/bioglass biomaterials on viability and osteogenic differentiation of adipose-derived stem cells [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(13): 1995-2001. |
| [7] | Wang Yuanyuan, Song Wenshan, Yu Dejun, Dai Yuankun, Li Bafang. Preparation and evaluation of fish skin acellular dermal matrix for oral guided tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(10): 1526-1532. |
| [8] | Liu Shaoyang, Zou Hanlin . Biocompatibility of anodic titanium oxide: an experimental research [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(10): 1575-1580. |
| [9] | Cai Yuhui, Hu Kesu, Zhang Yi. Biosafety evaluation of chitosan lactate/hyaluronate sponge [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(10): 1558-1563. |
| [10] | Huang Chang-jin, Song Wei-ming. Skin soft tissue expansion: history, development, technical revolution and application innovation [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(8): 1267-1274. |
| [11] | Zhang Lei, Zhou Song, Cheng Bao-chang. Actuality and challenge of biomaterials in annulus fibrosus repair [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(6): 958-963. |
| [12] | Li Zhi-yue, Zhu Zhi-bo, Zhao Qun, Xiang Si-yu, Zhao Peng, Xu Zhe-wei. Biocompatibility of basic fibroblast growth factor-poly(lactic-co-glycolic acid) microspheres/ hydroxyapatite/poly(l-lactic acid) porous materialsBiocompatibility of basic fibroblast growth factor-poly(lactic-co-glycolic acid) microspheres/ hydroxyapatite/poly(l-lactic acid) porous materials [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(6): 914-920. |
| [13] | Meng Ai, Wang Jian, Sui Lei. Effect of mixed acid reflux time on purification and biocompatibility of large-inner-diameter multi-walled carbon nanotubes [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(6): 896-901. |
| [14] | Yang Ji-bin, Zhu Xi-zhong, Xiong Hua-zhang, Li Yu-wan, Jin Ying, Liu Yi. Biocompatibility of human amniotic mesenchymal stem cells with human acellular amniotic membrane scaffold [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(5): 742-747. |
| [15] | Wang Jing, Wang Mengyang, Feng Qiaoqiao, Sun Jijun, Ci Haosu. Perforation of the pulp chamber floor: repair with mineral trioxide aggregate, light-cured glass ionomer cement and light-cured calcium hydroxide [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(34): 5458-5463. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||