Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (22): 5716-5727.doi: 10.12307/2026.160
Previous Articles Next Articles
Prostaglandin E1 pretreatment inhibits ferroptosis in endothelial cells in a rat model of spinal cord ischemia-reperfusion injury
Huang Yushan1, Wang Rongrong1, Li Xiangmiao1, Bai Jinzhu1, 2, 3
- 1School of Rehabilitation Medicine, Capital Medical University, Beijing 100068, China; 2 Department of Spine and Spinal Cord Surgery, Beijing Boai Hospital, China Rehabilitation Research Center, Beijing 100068, China; 3 College of Orthopedics, Capital Medical University, Beijing 100069, China
-
Received:2025-04-09Accepted:2025-08-04Online:2026-08-08Published:2025-12-26 -
Contact:Bai Jinzhu, Professor, Chief physician, School of Rehabilitation Medicine, Capital Medical University, Beijing 100068, China; Department of Spine and Spinal Cord Surgery, Beijing Boai Hospital, China Rehabilitation Research Center, Beijing 100068, China; College of Orthopedics, Capital Medical University, Beijing 100069, China -
About author:Huang Yushan, MS candidate, School of Rehabilitation Medicine, Capital Medical University, Beijing 100068, China -
Supported by:Key Project of China Rehabilitation Research Center, Nos. 2022ZX-05 and 2018ZX-08 (to BJZ)
CLC Number:
Cite this article
Huang Yushan, Wang Rongrong, Li Xiangmiao, Bai Jinzhu. Prostaglandin E1 pretreatment inhibits ferroptosis in endothelial cells in a rat model of spinal cord ischemia-reperfusion injury[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5716-5727.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
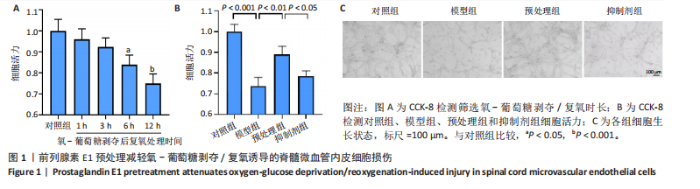
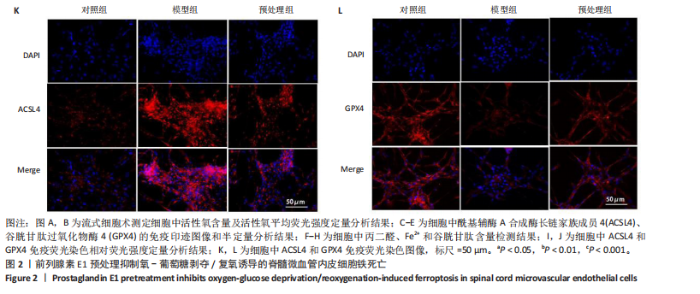
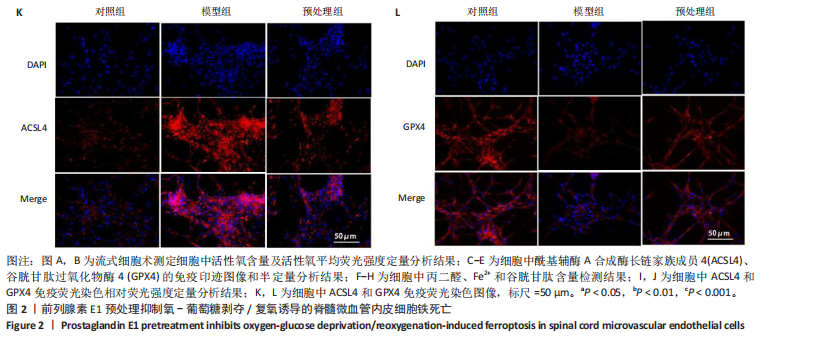
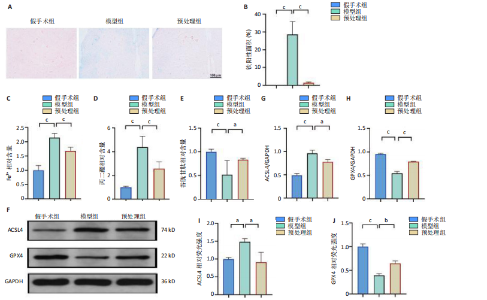
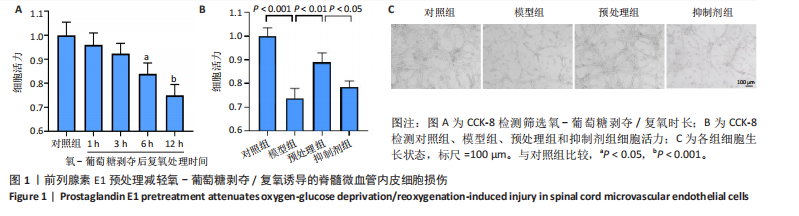
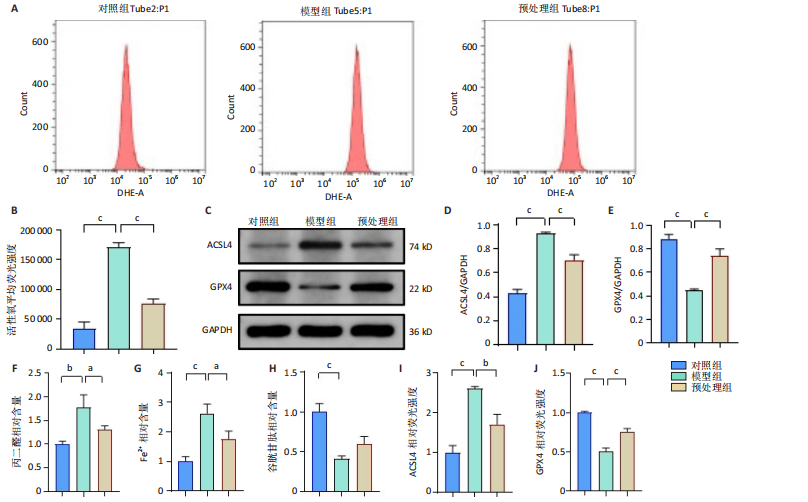
2.1 细胞实验结果 2.1.1 前列腺素E1预处理减轻氧-葡萄糖剥夺/复氧诱导的脊髓微血管内皮细胞损伤 CCK-8检测结果显示,与对照组相比,氧-葡萄糖剥夺/复氧1,3 h后的细胞活力无明显变化(P > 0.05),氧-葡萄糖剥夺/复氧6,12 h的细胞活力显著下降(P < 0.05,P < 0.001),见图1A。因此,此次研究选择12 h作为合适的复氧时长用于后续实验。 CCK-8检测结果显示,模型组细胞活力显著低于对照组(P < 0.001),预处理组细胞活力高于模型组(P < 0.01),抑制剂组细胞活力低于预处理组(P < 0.05),见图1B,表明前列腺素E1预处理可以改善氧-葡萄糖剥夺/复氧模型的细胞活力,而ML385可逆转前列腺素E1的保护作用。不同处理方式的细胞生长状态见图1C。对照组细胞生长状态良好,紧密排列;模型组细胞数量减少,细胞变疏;预处理组细胞数量增加,抑制剂组细胞数量减少。 2.1.2 前列腺素E1预处理抑制氧-葡萄糖剥夺/复氧诱导的脊髓微血管内皮细胞铁死亡 流式细胞术检测结果显示,模型组细胞内活性氧水平高于对照组,预处理组细胞内活性氧水平低于模型组,见图2A,B。 Western blot检测结果显示,与对照组比较,模型组酰基辅酶A合成酶长链家族成员4蛋白表达升高,谷胱甘肽过氧化物酶4蛋白表达下降;与模型组比较,预处理组酰基辅酶A合成酶长链家族成员4蛋白表达降低,谷胱甘肽过氧化物酶4蛋白表达升高,见图2C-E。模型组细胞内丙二醛和Fe2+含量明显高于对照组,谷胱甘肽含量低于对照组;预处理组细胞内丙二醛和Fe2+含量低于模型组,谷胱甘肽含量与模型组相比无明显差异,见图2F-H。 免疫荧光染色结果显示,与对照组比较,模型组酰基辅酶A合成酶长链家族成员4蛋白表达升高,"
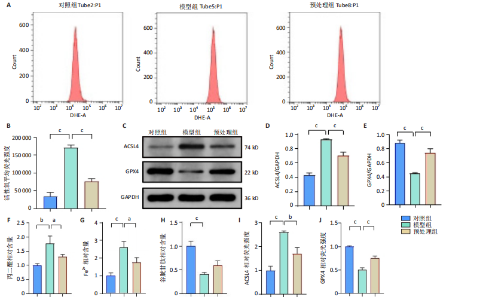
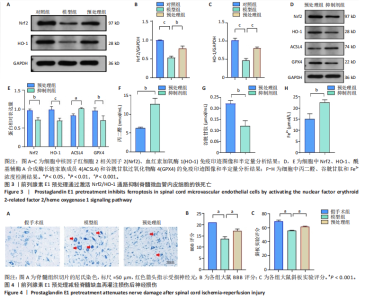
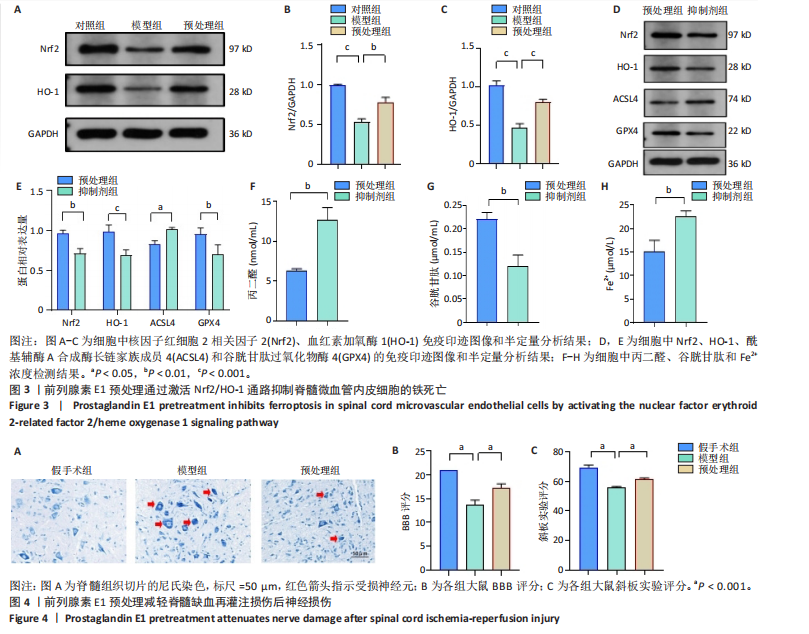
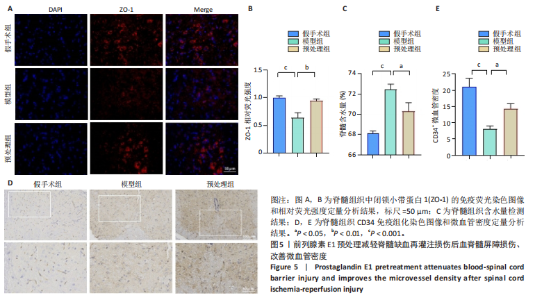
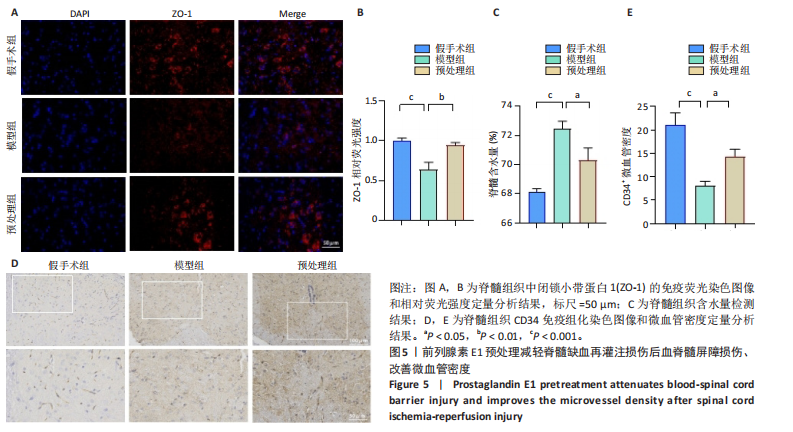
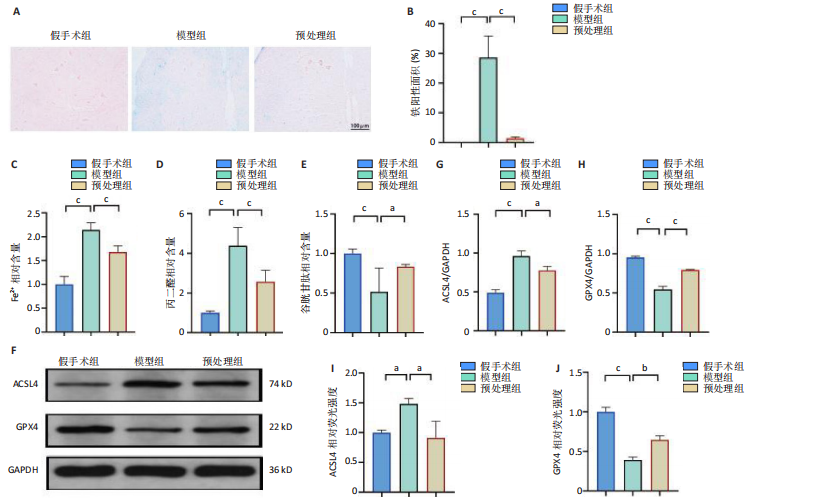
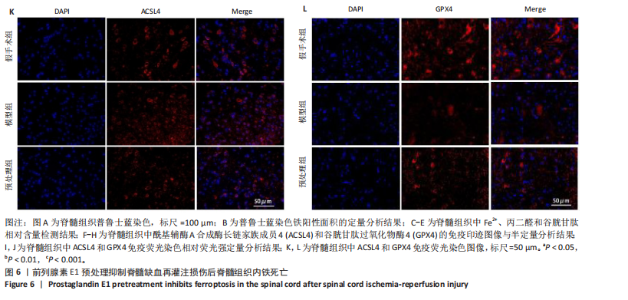
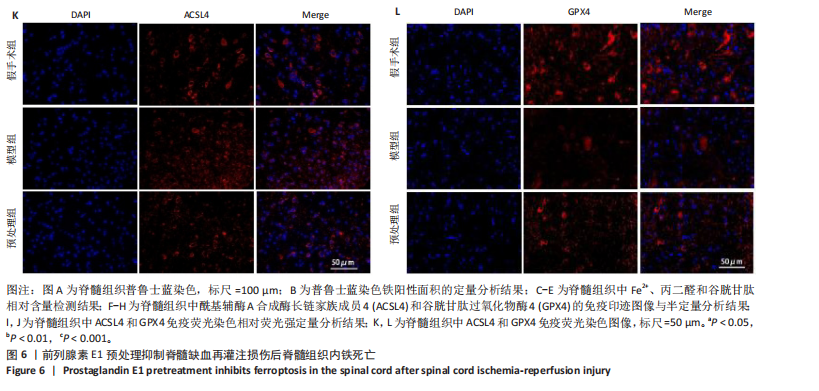
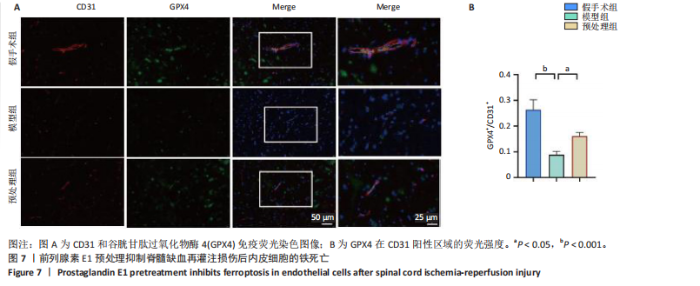
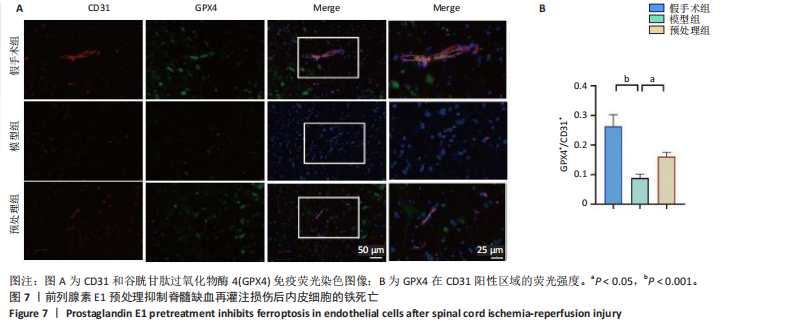
谷胱甘肽过氧化物酶4蛋白表达下降;与模型组比较,预处理组酰基辅酶A合成酶长链家族成员4蛋白表达降低,谷胱甘肽过氧化物酶4蛋白表达升高,见图2I-L。 这些结果表明氧-葡萄糖剥夺/复氧成功诱导脊髓微血管内皮细胞铁死亡,前列腺素E1预处理有效抑制氧-葡萄糖剥夺/复氧成功诱导的铁死亡。 2.1.3 前列腺素E1预处理通过激活Nrf2/HO-1通路抑制脊髓微血管内皮细胞的铁死亡 Western blot检测结果显示,模型组Nrf2、HO-1蛋白表达低于对照组,预处理组Nrf2、HO-1蛋白表达高于模型组,见图3A-C。Western blot检测结果显示,与预处理组比较,抑制剂组Nrf2、HO-1、谷胱甘肽过氧化物酶4蛋白表达降低,酰基辅酶A合成酶长链家族成员4蛋白表达升高,见图3D,E。抑制剂组细胞中丙二醛、Fe2+含量高于预处理组,谷胱甘肽含量低于预处理组,见图3F-H。 这些结果表明,Nrf2/HO-1通路参与前列腺素E1预处理对脊髓微血管内皮细胞铁死亡的抑制作用。 2.2 动物实验结果 2.2.1 实验动物数量分析 45只SD大鼠全部进入结果分析。 2.2.2 前列腺素E1预处理减轻脊髓缺血再灌注损伤后神经损伤 脊髓组织尼氏染色显示,假手术组神经元形态正常,核仁清晰;模型组神经元数量减少;预处理组神经元数量明显多于模型组,见图4A。模型组大鼠BBB评分低于假手术组,预处理组大鼠BBB评分高于模型组(图4B)。模型组大鼠斜板实验评分低于假手术组,预处理组大鼠平均斜板实验评分高于模型组(图4C)。这些结果提示前列腺素E1预处理减轻了脊髓缺血再灌注损伤后的神经元损伤,改善了损伤大鼠的运动功能。 2.2.3 前列腺素E1预处理减轻脊髓缺血再灌注损伤后血脊髓屏障损伤、改善微血管密度 闭锁小带蛋白1负责维持血脊髓屏障完整性[32]。免疫荧光染色结果显示,模型组脊髓组织中闭锁小带蛋白1表达低于假手术组,预处理组脊髓组织中闭锁小带蛋白1表达高于模型组,见图5A,B。 脊髓含水量是评价血脊髓屏障通透性的常用指标[33]。模型组脊髓组织含水量高于假手术组,预处理组脊髓组织含水量低于模型组,见图5C。 减少血管损伤有助于缓解缺血、缺氧诱导的炎症,是保护脊髓缺血再灌注损伤的关键策略[34-35]。此次研究采用CD34标记血管,CD34免疫组化染色结果显示,模型组微血管密度低于假手术组,预处理组微血管密度高于模型组,见图5D,E。 2.2.4 前列腺素E1预处理抑制脊髓缺血再灌注损伤后脊髓内铁死亡 普鲁士蓝染色结果显示,模型组脊髓组织中铁累积多于假手术组,预处理组脊髓组织中铁积累少于模型组(图6A,B)。模型组脊髓组织中Fe2+、丙二醛含量高于假手术组,谷胱甘肽含量低于假手术组;预处理组脊髓组织中Fe2+、丙二醛含量低于模型组,谷胱甘肽含量高于模型组,见图6C-E。 Western blot检测与免疫荧光染色结果显示,模型组脊髓组织中酰基辅酶A合成酶长链家族成员4蛋白表达高于假手术组,谷胱甘肽过氧化物酶4蛋白表达低于假手术组;预处理组脊髓组织中酰基辅酶A合成酶长链家族成员4蛋白表达低于模型组,谷胱甘肽过氧化物酶4蛋白表达高于模型组,见图6F-L。 2.2.5 前列腺素E1预处理抑制脊髓缺血再灌注损伤后内皮细胞的铁死亡 此次研究检测脊髓缺血再灌注损伤后内皮细胞是否发生了铁死亡,采用CD31抗体作为血管内皮细胞的标志物。免疫荧光染色结果显"
| [1] AWAD H, RAMADAN ME, EL SAYED HF, et al. Spinal Cord Injury after Thoracic Endovascular Aortic Aneurysm Repair. Can J Anaesth. 2017; 64(12):1218-1235. [2] ALZGHARI T, AN KR, HARIK L, et al. Spinal Cord Injury after Open and Endovascular Repair of Descending Thoracic Aneurysm and Thoracoabdominal Aortic Aneurysm: An Updated Systematic Review and Meta-Analysis. Ann Cardiothorac Sur. 2023;12(5):409-417. [3] MALINOVIC M, WALKER J, LEE F. Diffuse and Persistent Blood-Spinal Cord Barrier Disruption after Contusive Spinal Cord Injury Rapidly Recovers Following Intravenous Infusion of Bone Marrow Mesenchymal Stem Cells. Cureus. 2021;13(9):e18298. [4] 高煜,韩佳慧,葛新.脊髓缺血再灌注损伤后的免疫炎性微环境[J].中国组织工程研究,2023,8(27):1300-1305. [5] 艾琪勇,罗越,刘辉.脊髓缺血再灌注损伤的发病机制与治疗进展[J].实用医学杂志,2014,16(30):2678-2680. [6] RONG Y, FAN J, JI C, et al. USP11 Regulates Autophagy-Dependent Ferroptosis after Spinal Cord Ischemia-Reperfusion Injury by Deubiquitinating Beclin 1. Cell Death Differ. 2022;29(6):1164-1175. [7] LUAN X, CHEN P, MIAO L, et al. Ferroptosis in Organ Ischemia-Reperfusion Injuries: Recent Advancements and Strategies. Mol Cell Biochem. 2024;480(1):19-41. [8] DIXON SJ, LEMBERG KM, LAMPRECHT MR, et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell. 2012;149(5):1060-1072. [9] YU QJ, YANG Y. Function of SOD1, SOD2, and PI3K/AKT Signaling Pathways in the Protection of Propofol on Spinal Cord Ischemic Reperfusion Injury in a Rabbit Model. Life Sci. 2016;148:86-92. [10] DONG Y, AI C, CHEN Y, et al. Eph Receptor A4 Regulates Motor Neuron Ferroptosis in Spinal Cord Ischemia/Reperfusion Injury in Rats. Neural Regen Res. 2023;18(10):2219-2228. [11] GUO L, ZHANG D, REN X, et al. SYVN1 Attenuates Ferroptosis and Alleviates Spinal Cord Ischemia-Reperfusion Injury in Rats by Regulating the HMGB1/NRF2/HO-1 Axis. Int Immunopharmacol. 2023;123:110802. [12] LIU S, CHEN F, HAN J, et al. Ferrostatin-1 Improves Neurological Impairment Induced by Ischemia/Reperfusion Injury in the Spinal Cord through ERK1/2/SP1/GPX4. Exp Neurol. 2024;373:114659. [13] WANG P, REN Q, SHI M, et al. Overexpression of Mitochondrial Ferritin Enhances Blood–Brain Barrier Integrity Following Ischemic Stroke in Mice by Maintaining Iron Homeostasis in Endothelial Cells. Antioxidants. 2022;11(7):1257. [14] ZHOU R, LI J, WANG R, et al. The Neurovascular Unit in Healthy and Injured Spinal Cord. J Cereb Blood Flow Metab. 2023;43(9):1437-1455. [15] YOU Z, GAO X, KANG X, et al. Microvascular Endothelial Cells Derived from Spinal Cord Promote Spinal Cord Injury Repair. Bioact Mater. 2023;29:36-49. [16] LIU L, ZHANG H, SHI Y, et al. Prostaglandin E1 Improves Cerebral Microcirculation Through Activation of Endothelial NOS and GRPCH1. J Mol Neurosci. 2020;70(12):2041-2048. [17] XIE X, LU W, CHEN Y, et al. Prostaglandin E1 Alleviates Cognitive Dysfunction in Chronic Cerebral Hypoperfusion Rats by Improving Hemodynamics. Front Neurosci. 2019;13:549. [18] SHEN J, CAO MS, ZHOU T, et al. PGE1 Triggers Nrf2/HO-1 Signal Pathway to Resist Hemin-Induced Toxicity in Mouse Cortical Neurons. Ann Transl Med. 2021;9(8):634. [19] QIN Z, KONG B, ZHENG J, et al. Alprostadil Injection Attenuates Coronary Microembolization-Induced Myocardial Injury Through GSK-3β/Nrf2/HO-1 Signaling-Mediated Apoptosis Inhibition. Drug Des Devel Ther. 2020;14:4407-4422. [20] DODSON M, CASTRO-PORTUGUEZ R, ZHANG DD. NRF2 Plays a Critical Role in Mitigating Lipid Peroxidation and Ferroptosis. Redox Biol. 2019;23:101107. [21] LIU J, ZHANG S, FAN X, et al. Dexmedetomidine Preconditioning Ameliorates Inflammation and Blood–Spinal Cord Barrier Damage After Spinal Cord Ischemia-Reperfusion Injury by Down-Regulation High Mobility Group Box 1-Toll-Like Receptor 4-Nuclear Factor κB Signaling Pathway. Spine. 2019;44(2):E74-E81. [22] YE J, LI F, HUANG S, et al. Effects of Ginsenoside Rb1 on Spinal Cord Ischemia-Reperfusion Injury in Rats. J Orthop Surg Res. 2019;14(1):259. [23] SMITH PD, PUSKAS F, MENG X, et al. The Evolution of Chemokine Release Supports a Bimodal Mechanism of Spinal Cord Ischemia and Reperfusion Injury. Circulation. 2012;126(11 Suppl 1):S110-117. [24] COHEN J. Statistical Power Analysis for the Behavioral Sciences. New York: Routledge, 2013:567. [25] YANG H, MAJNO P, MOREL P, et al. Prostaglandin E(1) Protects Human Liver Sinusoidal Endothelial Cell from Apoptosis Induced by Hypoxia Reoxygenation. Microvasc Res. 2002;64(1):94-103. [26] YANG M, FAN Z, ZHANG Z, et al. MitoQ Protects against High Glucose-Induced Brain Microvascular Endothelial Cells Injury via the Nrf2/HO-1 Pathway. J Pharmacol Sci. 2021;145(1):105-114. [27] LI W, ZHAO X, ZHANG R, et al. Ferroptosis Inhibition Protects Vascular Endothelial Cells and Maintains Integrity of the Blood-Spinal Cord Barrier after Spinal Cord Injury. Neural Regen Res. 2023;18(11):2474-2481. [28] 潘玉,赵刃峰,李兴平.铁过载诱导成骨前体细胞铁死亡并抑制成骨分化[J].中国组织工程研究,2025,29(30):6381-6390. [29] CEMIL B, GOKCE EC, KAHVECI R, et al. Aged Garlic Extract Attenuates Neuronal Injury in a Rat Model of Spinal Cord Ischemia/Reperfusion Injury. J Med Food. 2016;19(6):601-606. [30] 王荣荣,黄玉珊,李湘淼,等.创伤性脊髓损伤急性期前列腺素E1对血管相关因子的调节和微循环功能的保护[J].中国组织工程研究,2025,29(5):958-967. [31] BAI T, LI M, LIU Y, et al. Inhibition of Ferroptosis Alleviates Atherosclerosis through Attenuating Lipid Peroxidation and Endothelial Dysfunction in Mouse Aortic Endothelial Cell. Free Radical Biol Med. 2020;160:92-102. [32] HU J, YU Q, XIE L, et al. Targeting the Blood-Spinal Cord Barrier: A Therapeutic Approach to Spinal Cord Protection against Ischemia-Reperfusion Injury. Life Sci. 2016;158:1-6. [33] YING X, XIE Q, YU X, et al. Water Treadmill Training Protects the Integrity of the Blood-Spinal Cord Barrier Following SCI via the BDNF/TrkB-CREB Signalling Pathway. Neurochem Int. 2021;143:104945. [34] SHEN N, WANG L, WU Y, et al. Adeno-Associated Virus Packaged TRPC5 Gene Therapy Alleviated Spinal Cord Ischemic Reperfusion Injury in Rats. Neuroreport. 2020;31(1):29. [35] REN ZX, XU JH, CHENG X, et al. Pathophysiological Mechanisms of Chronic Compressive Spinal Cord Injury Due to Vascular Events. Neural Regen Res. 2023;18(4):790-796. [36] MATSUSHITA T, LANKFORD KL, ARROYO EJ, et al. Diffuse and Persistent Blood-Spinal Cord Barrier Disruption after Contusive Spinal Cord Injury Rapidly Recovers Following Intravenous Infusion of Bone Marrow Mesenchymal Stem Cells. Exp Neurol. 2015;267:152-164. [37] XU J, LI P, LU F, et al. Domino Reaction of Neurovascular Unit in Neuropathic Pain after Spinal Cord Injury. Exp Neurol. 2023;359: 114273. [38] LUO Y, YAO F, HU X, et al. M1 Macrophages Impair Tight Junctions between Endothelial Cells after Spinal Cord Injury. Brain Res Bull. 2022;180:59-72. [39] LIU Q, SONG T, CHEN B, et al. Ferroptosis of Brain Microvascular Endothelial Cells Contributes to Hypoxia-Induced Blood-Brain Barrier Injury. FASEB J. 2023;37(5):e22874. [40] LI C, CHEN X, DU Z, et al. Inhibiting Ferroptosis in Brain Microvascular Endothelial Cells: A Potential Strategy to Mitigate Polystyrene Nanoplastics‒induced Blood‒brain Barrier Dysfunction. Environ Res. 2024;250:118506. [41] JIA B, LI J, SONG Y, et al. ACSL4-Mediated Ferroptosis and Its Potential Role in Central Nervous System Diseases and Injuries. Int J Mol Sci. 2023;24(12):10021. [42] SEIBT TM, PRONETH B, CONRAD M. Role of GPX4 in Ferroptosis and Its Pharmacological Implication. Free Radical Bio Med. 2019; 133:144-152. [43] WANG D, FANG B, WANG Z, et al. Sevoflurane Pretreatment Regulates Abnormal Expression of MicroRNAs Associated with Spinal Cord Ischemia/Reperfusion Injury in Rats. Ann Transl Med. 2021;9(9):752-752. [44] LI X, CAO X, WANG J, et al. Sevoflurane Preconditioning Ameliorates Neuronal Deficits by Inhibiting Microglial MMP-9 Expression after Spinal Cord Ischemia/Reperfusion in Rats. Mol Brain. 2014;7(1):69. [45] GÜR FM, BILGIÇ S. A Synthetic Prostaglandin E1 Analogue, Misoprostol, Ameliorates Paclitaxel-Induced Oxidative Damage in Rat Brain. Prostag Oth Lipid M. 2022;162:106663. [46] AYALA A, MUÑOZ MF, ARGÜELLES S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid Med Cell Longev. 2014; 2014:360438. [47] XU Y, LI Y, LI J, et al. Ethyl Carbamate Triggers Ferroptosis in Liver through Inhibiting GSH Synthesis and Suppressing Nrf2 Activation. Redox Biol. 2022;53:102349. [48] CHEN L, HUANG J, YAO ZM, et al. Procyanidins Alleviated Cerebral Ischemia/Reperfusion Injury by Inhibiting Ferroptosis via the Nrf2/HO-1 Signaling Pathway. Molecules. 2023;28(8):3582. [49] LI R, ZHANG J, ZHOU Y, et al. Transcriptome Investigation and In Vitro Verification of Curcumin-Induced HO-1 as a Feature of Ferroptosis in Breast Cancer Cells. Oxid Med Cell Longev. 2020;2020:e3469840. [50] GE H, XUE X, XIAN J, et al. Ferrostatin-1 Alleviates White Matter Injury Via Decreasing Ferroptosis Following Spinal Cord Injury. Mol Neurobiol. 2022;59(1):161-176. [51] SHI J, XUE X, YUAN L, et al. Amelioration of White Matter Injury Through Mitigating Ferroptosis Following Hepcidin Treatment After Spinal Cord Injury. Mol Neurobiol. 2023;60(6):3365-3378. |
| [1] | Lyu Guoqing, Aizimaitijiang·Rouzi, Xiong Daohai. Irisin inhibits ferroptosis in human articular chondrocytes: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1359-1367. |
| [2] | Wen Fan, Xiang Yang, Zhu Huan, Tuo Yanfang, Li Feng. Exercise improves microvascular function in patients with type 2 diabetes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1225-1235. |
| [3] | Zou Rongji, Yu Fangfang, Wang Maolin, Jia Zhuopeng. Triptolide inhibits ferroptosis and improves cerebral ischemia-reperfusion injury in a rat model of cerebral artery occlusion/reperfusion [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 873-881. |
| [4] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [5] | Zheng Peng, Jia Xiaoning, Tao Jingwei, Fan Xiao. Tetramethylpyrazine improves iron metabolism disorders in a rat model of spinal cord injury via the Keap-1/Nrf2 signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 6134-6141. |
| [6] | Zhou Wen, Yang Hongwei. Molecular mechanism and natural drug screening for ferroptosis-targeted therapy in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 6051-6061. |
| [7] | Tao Xiangyu, Wang Shuang, Li Yuhan, Cao Jimin, Sun Teng. Effects of piRNA CFAPIR in doxorubicin-induced ferroptosis models of rat and human cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5756-5769. |
| [8] | Liao Xingzhuan, Li Guangdi, Wu Yabin, Liu Xingyu, Wan Jiajia. Molecular mechanisms underlying non-coding RNA regulation of ferroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4713-4725. |
| [9] | Yang Chong, Wu Yuci, Yang Han, Wang Meiting, Liu Lei. Promoting effect of acupuncture combined with rehabilitation training on the reconstruction of damaged neurological function in rats with cerebral infarction [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4347-4356. |
| [10] | Yu Le, Nan Songhua, Shi Zijian, He Qiqi, Li Zhenjia, Cui Yinglin. Mechanisms underlying mitophagy, ferroptosis, cuproptosis, and disulfidptosis in Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4446-4456. |
| [11] | Liu Annan, Li Jianhui, Gao Wei, Li Xue, Song Jing, Xing Liping, Li Honglin. Bibliometric analysis of ferroptosis and Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4278-4288. |
| [12] | Chen Xinlong, Meng Tao, Wang Yaomin, Zhang Kefan, Li Jian, Shi Hui, Zhang Chenchen. Ferroptosis inhibitors in the treatment of osteoarthritis: diversity and multitarget characteristics [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4166-4179. |
| [13] | Zan Yongfeng, Song Keguan, Liu Yuda. Glutamine regulates the effect of hormones on the apoptosis of bone microvascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(12): 2965-2974. |
| [14] | Wu Jiazhou, Qian Tao, Liu Zexian, Wu Yanbin, He Ying, Li Yazhou, Peng Jiang. Three-dimensional culture of stromal vascular fraction self-assembles into complex vascularized osteogenic organoids [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2681-2690. |
| [15] | Yao Shunhua, Huang Caiding, Zhang Mengyu, Zhang Kexin, Yin Changjiang, Yang Kunbao. Huidouba inhibits ferroptosis in high glucose-cultured HK-2 cells to attenuate cell fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2774-2783. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||