Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (16): 4193-4203.doi: 10.12307/2026.720
Previous Articles Next Articles
Multidimensional target regulation of vascular endothelial growth factor A in articular cartilage development
Wang Zhengye, Liu Wanlin, Zhao Zhenqun
- Center for Pediatric Orthopedics, Second Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010090, Inner Mongolia Autonomous Region, China
-
Received:2025-06-06Accepted:2025-09-01Online:2026-06-08Published:2025-11-28 -
Contact:Liu Wanlin, MS, Professor, Center for Pediatric Orthopedics, Second Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010090, Inner Mongolia Autonomous Region, China Co-corresponding author: Zhao Zhenqun, PhD, Professor, Center for Pediatric Orthopedics, Second Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010090, Inner Mongolia Autonomous Region, China -
About author:Wang Zhengye, MS candidate, Center for Pediatric Orthopedics, Second Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010090, Inner Mongolia Autonomous Region, China -
Supported by:National Natural Science Foundation of China, Nos. 81960397 and 82260424 (both to LWL); National Natural Science Foundation of China, Nos. 82160414 and 81760391 (both to ZZQ); Inner Mongolia Autonomous Region Technology Transfer Project, No. CGZH2018146 (to LWL); Natural Science Foundation of Inner Mongolia Autonomous Region - Outstanding Young Scientist Fund Project, No. 2023SHZR1613 (to ZZQ)
CLC Number:
Cite this article
Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Multidimensional target regulation of vascular endothelial growth factor A in articular cartilage development[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4193-4203.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
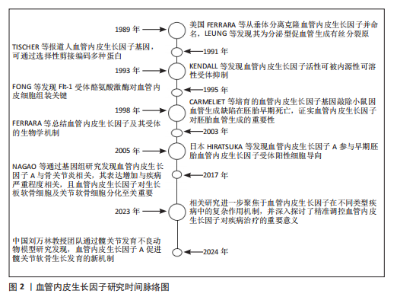
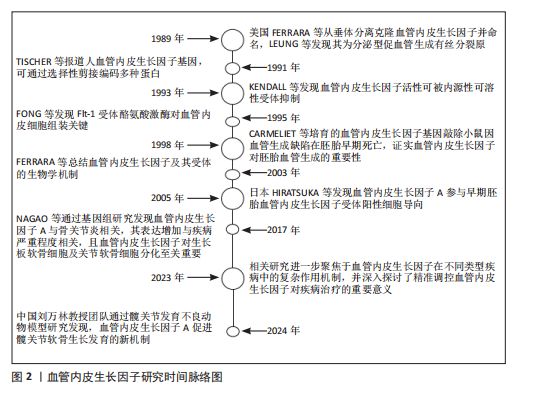
自1989年起,血管内皮生长因子的研究便不断推动着血管生理学与病理学领域的深入发展。美国科学家FERRARA等[24]于1989年从垂体组织中分离并克隆出血管内皮生长因子,同时将其正式统一命名为血管内皮生长因子。LEUNG等则发现血管内皮生长因子是一种具备分泌特性的促血管生成有丝分裂原,为后续对其生物学功能的深入探索奠定了基础。1991年,TISCHER等[25]对人血管内皮生长因子基因进行深入研究,揭示了其通过选择性剪接机制能够编码多种不同形式的蛋白,这一发现为理解血管内皮生长因子的多样性功能提供了关键线索。1993年,KENDALL等[26]通过一系列实验发现了血管内皮生长因子活性可被一种内源性编码的可溶性受体所抑制,这一成果为调控血管内皮生长因子信号通路提供了新的思路。1995年,FONG等[27]深入研究Flt-1受体酪氨酸激酶,发现其在血管内皮细胞组装过程中发挥着至关重要的作用,进一步完善了对血管内皮生长因子信号转导机制的认识。1998年,CARMELIET等[28]培育出血管内皮生长因子基因敲除小鼠,观察到其在胚胎早期因严重血管生成缺陷而死亡,这一突破性成果有力地证实了血管内皮生长因子在胚胎血管生成过程中扮演着不可或缺的角色。2003年,FERRARA等[29]对血管内皮生长因子及其受体的生物学机制进行了系统总结,为后续研究提供了全面且权威的理论支持。2005年,日本的HIRATSUKA等[30]在早期胚胎发育研究中发现血管内皮生长因子A参与了血管内皮生长因子受体阳性细胞向胚前部的导向过程,为理解血管内皮生长因子A在胚胎发育中的作用机制开辟了新方向。2017年,NAGAO等[31]通过基因组水平的深入研究,发现血管内皮生长因子A与骨关节炎的发病机制紧密相关,其表达水平的增加与疾病严重程度呈正相关关系。他们进一步揭示了血管内皮生长因子在发育过程中作为生长板软骨细胞的生存因子,对出生后关节软骨细胞分化以及骨骼区域骨质及时骨化具有至关重要的作用。在构建的手术诱导膝骨关节炎小鼠模型中,发现血管内皮生长因子表达增加与软骨细胞和滑膜细胞的分解代谢过程密切相关,而敲低血管内皮生长因子可有效减轻诱导产生的骨关节炎症状,关节内注射抗血管内皮生长因子抗体则能够抑制骨关节炎的进展,这些发现为骨关节炎的治疗提供了新的潜在靶点。至2023年,相关研究进一步聚焦于血管内皮生长因子在不同类型疾病中的复杂作用机制,并深入探讨了精准调控血管内皮生长因子对疾病治疗的重要意义。当前研究指出,在精准调控血管内皮生长因子具有促血管生成与促炎效应的双重作用,以及如何凭借新型递送系统实现靶向干预方面,仍面临着诸多亟待解决的挑战,这些难题成为了制约血管内皮生长因子相关疾病治疗进一步发展的关键因素[32]。而2024年,中国的刘万林教授团队的国家自然科学基金项目利用髋关节发育不良动物模型发现血管内皮生长因子A可以促进髋关节软骨的生长发育,为软骨疾病的治疗提供了新的研究方向[33],见图2。 2.1 血管内皮生长因子A信号通路 2.1.1 受体互作网络的时空特异性 血管内皮生长因子A的生物学效应高度依赖与不同受体(如血管内皮生长因子受体2、神经纤毛蛋白1)的组合激活模式。在胚胎发育早期,血管内皮生长因子受体2作为核心受体介导血管内皮细胞向软骨前体区域的定向迁移,促进软骨原基的血管化[2]。随着软骨成熟,血管内皮生长因子A信号通过激活血管内皮生长因子受体2与成纤维细胞生长因子信号通路的协同作用,诱导软骨细胞肥大化并启动钙化程序[3]。这一阶段中,血小板源性生长因子受体α信号的异常激活可通过抑制生长分化因子5表达,导致关节间区祖细胞向纤维软骨分化,提示血管内皮生长因子A与血小板源性生长因子受体α信号在时序上的拮抗关系[34]。此外,骨形态发生蛋白(bone morphogenetic protein,BMP)信号通路在Meckel软骨不同区域的活性差异(如前端高骨形态发生蛋白4表达与后端激活素受体样激酶3依赖性)进一步揭示了血管内皮生长因子A与其他发育信号通路的阶段性互作调控[35]。在成年期软骨稳态维持中,血管内皮生长因子A与粒细胞趋化蛋白2的时空表达分离现象尤为关键:胚胎期粒细胞趋化蛋白2仅在永久性关节软骨中表达,而生长板软骨中则无此配体,这种时间特异性分布避免了血管过早侵入终末分化软骨[36]。 在软骨发育的解剖学层面,血管内皮生长因子A受体网络呈现明显的区域化特征。例如,在生长板软骨的肥大区,血管内皮生长因子受体2与Notch信号通路的共定位激活促进血管生成素系统的整合,形成血管-软骨界面特异性信号模块;而在关节软骨表层,表皮生长因子受体与血管内皮生长因子受体2的共表达通过调控细胞外基质受体互作(如整合素β1-胶原结合),维持软骨机械稳定性并抑制血管侵入[37-38]。 单细胞转录组分析显示,颞下颌关节骨关节炎患者的髁突软骨中,血管内皮生长因子A信号与神经生长因子受体、成纤维细胞生长因子受体在特定细胞亚群中共表达,形成“血管-神经-炎症”三元互作网络,驱动病理状态下的异常血管生成和神经浸润[39]。此外,软骨下骨与软骨界面的细胞外基质受体互作(如Ⅰ型胶原蛋白α1链-整合素α2β1)通过机械信号传递调控血管内皮生长因子A的局部释放,进一步强化了空间特异性调控[40]。"
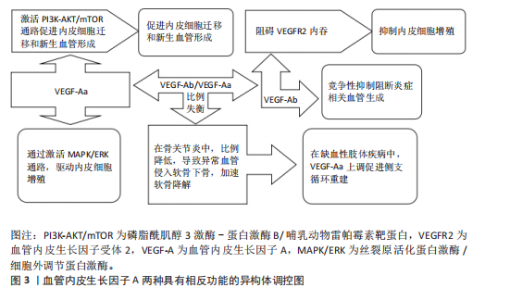
血管内皮生长因子A受体网络的时空特异性失衡与多种软骨发育异常密切相关。例如,血小板衍生生长因子受体α信号过度激活可通过抑制Wnt通路导致颅骨畸形,而表皮生长因子受体活性随年龄的梯度下降(表层早于深层)与骨关节炎中软骨退变的区域分布高度相关。在分子机制层面,缺氧诱导因子1α-血管内皮生长因子/血管内皮生长因子受体2轴与成纤维细胞生长因子信号的空间协同作用,通过调控SRY(性决定区Y)盒转录因子9(SRY-Box Transcription Factor 9,SOX9)转录活性决定软骨祖细胞向肥大或稳定表型分化[41]。 2.1.2 剪切异构体的功能分化 血管内皮生长因子A基因的选择性剪接产生具有相反功能的异构体,在调控血管稳态中发挥核心作用,见图3。促血管生成型异构体(如血管内皮生长因子Aa)含CDKPRR六肽序列,通过激活磷脂酰肌醇3激酶-蛋白激酶B/哺乳动物雷帕霉素靶蛋白通路促进内皮细胞迁移和新生血管形成[42-43]。抗血管生成型异构体(如血管内皮生长因子Ab)含SLTRKD序列,阻碍血管内皮生长因子受体2内吞,触发非经典信号通路抑制内皮细胞增殖[44]。仅6个氨基酸差异即可逆转血管内皮生长因子A的生物学效应,凸显剪接调控的精密性[45]。在信号传导机制方面,血管内皮生长因子Aa通过血管内皮生长因子受体2的酪氨酸激酶活性激活丝裂原活化蛋白激酶/细胞外调节蛋白激酶通路,驱动内皮细胞增殖,并协同增强血管通透性和基质重塑能力[46-47]。血管内皮生长因子Ab虽可结合血管内皮生长因子受体2,但阻碍受体二聚化,降低下游细胞外调节蛋白激酶1/2磷酸化水平,并通过竞争性抑制阻断炎症相关血管生成[48]。 剪接因子在血管内皮生长因子A异构体的表达调控中起关键作用。低氧环境下,丝氨酸/精氨酸富集剪接因子2(serine/arginine-rich splicing factor 2,SRSF2)促进抗血管生成型血管内皮生长因子A165b表达,而丝氨酸/精氨酸富集剪接因子1增强促血"
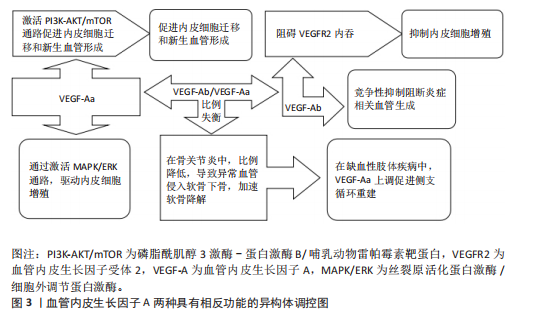
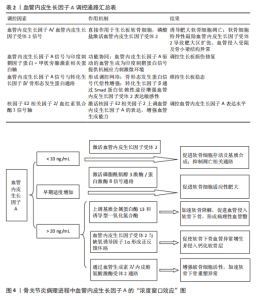
管生成型血管内皮生长因子A165a表达[49-50]。白细胞介素1β通过激活信号转导及转录激活因子3信号上调丝氨酸/精氨酸富集剪接因子2表达,形成炎症-血管稳态调控轴[51]。疾病状态下,剪接失衡与病理过程密切相关。在骨关节炎中,血管内皮生长因子Ab/血管内皮生长因子Aa比例降低,导致异常血管侵入软骨下骨,加速软骨降解[13]。在缺血性肢体疾病中,白细胞介素1β依赖性血管内皮生长因子Aa上调,促进侧支循环重建。 针对血管内皮生长因子A剪切异构体的治疗策略具有巨大潜力。重组血管内皮生长因子A165b已用于抑制实体瘤血管生成,疗效较传统抗血管内皮生长因子抗体提高[52-53]。反义寡核苷酸靶向抑制丝氨酸/精氨酸富集剪接因子2表达可逆转血管内皮生长因子Ab在糖尿病视网膜病变中的过度生成。利用纳米载体共递送血管内皮生长因子Aa/血管内皮生长因子Ab双表达质粒可在缺血区域实现时序性血管生成[54]。 然而,当前研究存在检测技术瓶颈和组织特异性调控机制不明的挑战。传统ELISA无法区分血管内皮生长因子Aa与血管内皮生长因子Ab,质谱联用免疫沉淀技术的灵敏度需提升[55]。软骨组织中的剪接调控因子是否参与血管内皮生长因子A异构体分化尚不明确,需单细胞测序进一步解析[56-57]。未来研究应聚焦于构建组织特异性剪接图谱和解析动态调控网络,为关节炎、缺血性疾病等提供精准治疗靶点。 2.1.3 信号转导的力学-生化耦合机制 血管内皮生长因子A信号通路在关节软骨发育与稳态中展现出显著的力学-生化耦合特性。在生理性负荷下,血管内皮生长因子A激活血管内皮生长因子受体2,抑制软骨下血管异常侵入,而在病理性负荷下,其过度表达促进血管内皮细胞向软骨内侵袭,加速软骨退变[12,58-59]。关节软骨的机械负荷通过分子传感器如瞬时受体电位香草酸亚型4离子通道转化为生化信号,进而调控血管内皮生长因子A通路活性[60-61],同时血管内皮生长因子A通过调控整合素β1的活性增强软骨细胞对基质刚度的感知,形成正反馈环路[38]。在胚胎期和成年软骨中,该通路分别通过细胞外基质重塑和抑制雷帕霉素靶蛋白复合体1信号维持低代谢稳态[37,62]。血管内皮生长因子A信号通路还与转化生长因子β、刺猬信号通路、Hippo-Yes相关蛋白等关键发育通路存在广泛的交叉对话,实现多途径的功能协同[63-67]。在骨关节炎中,血管内皮生长因子A信号通路的力学-生化耦合失衡表现为机械超负荷诱导血管内皮生长因子A过度表达,促进胶原网络降解[68-70],以及生化微环境紊乱破坏缺氧微环境,抑制自噬修复机制[61,71-72]。干预策略包括靶向血管内皮生长因子A的力学调控和多通路协同调控[6,17,73]。 2.2 发育动态中的精准调控网络 在关节软骨发育过程中,血管内皮生长因子A通过时空特异性的分子网络实现动态精准调控,其作用机制贯穿胚胎期软骨模板形成、出生后生长板调控及成年期稳态维持3个阶段。这种多维度调控网络不仅依赖于血管内皮生长因子A自身异构体的功能分化,更涉及与力学信号、表观遗传修饰及微环境因子的协同作用,形成复杂的正反馈与负反馈环路,见表2。 血管内皮生长因子A在生长板软骨重塑中发挥核心调控作用,与软骨细胞肥大化阶段紧密偶联,通过激活血管内皮生长因子受体2驱动血管内皮细胞迁移,形成功能性血管网络[2,74-75]。血管内皮生长因子A浓度梯度(0.1-100 ng/mL)对血管生成与骨形成协调至关重要,低剂量促进血管出芽,高剂量诱导骨基质矿化,可能通过调节Notch信号通路与血管生成素2交叉对话实现[76-77]。除经典血管生成作用外,血管内皮生长因子A/血管内皮生长因子受体2信号可直接作用于生长板软骨细胞。磷酸盐通过激活血管内皮生长因子受体2诱导肥大软骨细胞凋亡,软骨细胞特异性敲除血管内皮生长因子受体2会导致肥大区扩张、血管侵入受阻及骨小梁结构异常,表明血管内皮生长因子A信号通过血管依赖与非依赖途径双重调控生长板成熟[74]。血管内皮生长因子A信号与印度刺猬蛋白-甲状旁腺激素相关蛋白轴功能协同,在生长板损伤修复中,印度刺猬蛋白-甲状旁腺激素相关蛋白反馈环通过调节软骨细胞增殖分化状态影响血管内皮生长因子A表达模式,而血管内皮生长因子A驱动的血管生成为印度刺猬蛋白信号提供机械应力刺激微环境[78-79]。 血管内皮生长因子A信号与转化生长因子β/骨形态发生蛋白通路形成调控网络,在表皮生长因子受体缺陷模型中,骨形态发生蛋白2信号代偿性增强,提示血管内皮生长因子A可能通过调节表皮生长因子受体-骨形态发生蛋白轴平衡维持生长板稳态[80]。此外,转化生长因子β可通过Smad蛋白依赖性途径增强血管内皮生长因子受体2表达敏感性[81]。核因子E2相关因子2/血红素氧合酶1信号轴可调控血管内皮生长因子A表达水平,在缺氧微环境下,激活核因子E2相关因子2上调血管内皮生长因子A表达,增强血管生成能力,而胰岛素/胰岛素样生长因子1信号下调会减弱血管内皮生长因子A对软骨细胞分化的促进作用[46,82-84]。 在病理状态下,生长板损伤模型中血管内皮生长因子A表达呈现双相特征,初期由炎症因子驱动升高,后期由再生性软骨细胞自主分泌[85],外源性血管内皮生长因子A递送可加速修复进程,但需结合时空特异性载体避免异位骨化[86]。在糖尿病微环境中,晚期糖基化终末产物通过阻断血管内皮生长因子受体2酪氨酸激酶活性,导致生长板血管密度下降和软骨细胞成熟延迟,靶向血管内皮生长因子A/血管内皮生长因子受体2与晚期糖基化终末产物-晚期糖基化终末产物受体通路的双重抑制剂显示出改善生长板结构的潜力。 2.3 疾病关联 血管内皮生长因子A在关节软骨发育与稳态中的多效性,使它成为骨关节炎、骨骺发育不良等疾病的关键调控因子,然而,其作用机制解析与临床转化面临多重挑战。 2.3.1 骨关节炎中的“浓度窗口效应” 在骨关节炎病理进程中,血管内皮生长因子A展现出复杂的“浓度窗口效应”,其动态浓度依赖性作用揭示了在软骨代谢、血管生成及炎症调控中的双向调节特性,为骨关节炎治疗提供了关键理论依据,见图4。血管内皮生长因子A在关节软骨中的作用呈现明显剂量依赖性:低质量浓度血管"
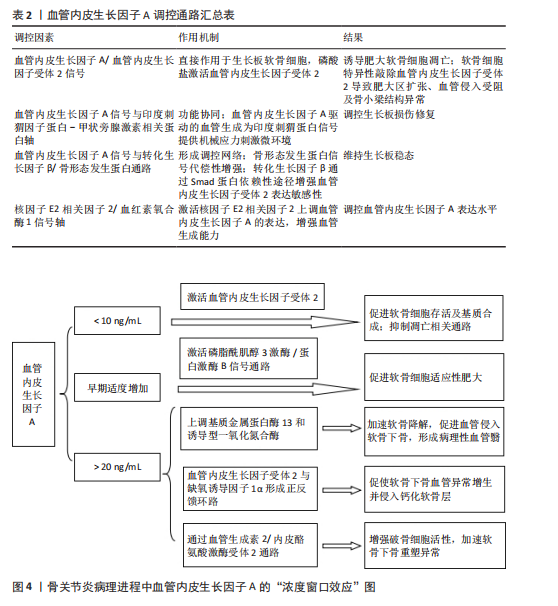

内皮生长因子A(< 10 ng/mL)通过激活血管内皮生长因子受体2促进软骨细胞存活及基质合成,抑制凋亡相关通路[12,87];然而,当局部质量浓度超过阈值(> 20 ng/mL)时,它通过上调基质金属蛋白酶13和诱导型一氧化氮合酶表达,加速软骨降解并促进血管侵入软骨下骨,形成病理性血管翳,这一现象可能与血管内皮生长因子受体1的竞争性结合有关[7,88-90]。在骨关节炎软骨中,血管内皮生长因子A表达呈现时空特异性升高,早期适度增加可激活磷脂酰肌醇3激酶/蛋白激酶B信号通路,促进软骨细胞适应性肥大[91],但随着病程进展,炎症微环境导致血管内皮生长因子A分泌失控,突破20 ng/mL临界值后,血管内皮生长因子受体2与缺氧诱导因子1α形成正反馈环路,促使软骨下骨血管异常增生并侵入钙化软骨层[92-93],破坏软骨-骨界面完整性,并通过血管生成素2/内皮酪氨酸激酶受体2通路增强破骨细胞活性,加速软骨下骨重塑异常[94-95]。 多项研究验证了精准调控血管内皮生长因子A浓度的治疗潜力。在兔骨关节炎模型中,关节腔内注射抗血管内皮生长因子抗体贝伐珠单抗显示剂量依赖性效应:5 mg剂量可有效抑制滑膜炎和骨赘形成,但20 mg高剂量反而加重软骨退变,提示过度抑制血管内皮生长因子A会破坏其生理性修复功能[14,96]。类似地,针对血管内皮生长因子受体2的小分子抑制剂在低浓度时可阻断病理性血管生成,而高浓度则抑制软骨细胞自噬,加剧基质降解[97],10-100 nmol/L有效浓度区间凸显精准给药的重要性。此外,骨关节炎患者的血管内皮生长因子A敏感性存在显著个体差异,与基因多态性密切相关,携带特定基因型的骨关节炎患者对血管内皮生长因子A抑制治疗反应更敏感。局部缺氧微环境通过缺氧诱导因子1α/血管内皮生长因子A/血管内皮生长因子受体2轴动态调节软骨细胞代谢状态:轻度缺氧可增强血管内皮生长因子A促修复作用,而重度缺氧则通过活性氧积累引发血管内皮生长因子A信号紊乱[98-99]。 血管内皮生长因子A的浓度窗口效应需在多尺度网络中解析,包括分子尺度上血管内皮生长因子A异构体的分泌比例影响受体激活模式、细胞尺度上软骨细胞-内皮细胞-滑膜细胞的旁分泌交互形成浓度梯度、组织尺度上关节腔液与软骨基质的扩散屏障共同塑造局部血管内皮生长因子A分布[6]。血管内皮生长因子A在骨关节炎中的“浓度窗口效应”揭示了它作为分子开关的双重角色:生理浓度下维持软骨稳态,超阈值浓度时驱动病理进程。未来治疗需结合基因分型、实时微环境监测及靶向递送技术,实现对血管内皮生长因子A浓度的动态精准调控,为开发基于血管内皮生长因子A信号的个体化骨关节炎疗法提供了新方向。 2.3.2 类风湿关节炎 类风湿关节炎是一种慢性自身免疫性疾病,其特征为滑膜增生、血管新生及关节软骨破坏。血管内皮生长因子A在该疾病进程中通过多种机制发挥作用,其通过激活血管内皮生长因子受体2/SRC酪氨酸激酶/磷脂酰肌醇3激酶/蛋白激酶B信号轴,最终形成过度血管化的“血管翳”结构[7,100]。这种血管化的滑膜组织不仅为炎症细胞的浸润提供了通道,还通过分泌金属蛋白酶直接侵蚀关节软骨及邻近骨质[101]。动物模型研究表明,血管内皮生长因子A表达水平与血管侵入软骨的深度呈正相关,阻断血管内皮生长因子A能够显著抑制软骨下骨的侵蚀以及关节变形[14]。类风湿关节炎滑膜中的巨噬细胞和成纤维样滑膜细胞在受到炎症因子刺激时,会大量分泌血管内皮生长因子A,而血管内皮生长因子A又能够进一步激活核因子κB等炎症通路,从而形成“炎症 - 血管生成”的恶性循环[102-103]。临床研究证实,类风湿关节炎患者滑液及血清中的血管内皮生长因子A水平明显高于骨关节炎患者及健康人群,且与疾病活动度(例如压痛关节数)呈正相关[104-105]。 基因多态性分析揭示了血管内皮生长因子A基因的特定位点(例如rs2010963、rs3025039、rs699947)与类风湿关节炎风险之间存在显著的相关性。其中,血管内皮生长因子A-634位点的G等位基因能够增强启动子活性,导致血管内皮生长因子A过表达,进而促进滑膜血管增生以及炎症浸润[106]。这些遗传变异或许能够解释类风湿关节炎患者对于血管内皮生长因子A靶向治疗反应存在的个体差异。 2.3.3 先天性髋关节发育不良 在先天性髋关节发育不良的进程中,血管内皮生长因子A起着至关重要的作用。研究表明,血管内皮生长因子A表达异常会导致生长板软骨的血管化过程紊乱,具体表现为血管过早或延迟侵入肥大软骨区,从而破坏软骨基质正常的矿化节奏,最终引发髋臼覆盖不足和关节对合异常[2-3,107]。在先天性髋关节发育不良病理状态下,异常的机械应力能够诱导软骨细胞中血管内皮生长因子A的表达失调,这种失调表现为早期过度分泌或后期分泌不足。先天性髋关节发育不良患者的软骨处于异常生物力学环境中,这一环境能够激活诸如核因子κB等炎症通路,进而进一步上调血管内皮生长因子A的表达水平[108]。随之而来的是血管加速侵入未成熟的软骨区域,导致软骨下骨过早发生硬化以及关节面出现形态异常。然而,在先天性髋关节发育不良中,成纤维细胞生长因子受体信号通路的异常激活会导致血管内皮生长因子A/血管内皮生长因子受体2轴过度活跃,加速肥大软骨细胞的凋亡过程,破坏生长板软骨的层状结构[109]。近期研究揭示,先天性髋关节发育不良的软骨下骨异常血管化与血管内皮生长因子A介导的血管-成骨偶联机制失调有着密切关联。正常发育过程中,血管内皮生长因子A通过促进CD31+Emcn+型血管的生成,维持软骨下骨的骨重塑平衡[95]。 然而在先天性髋关节发育不良疾病中,血管内皮生长因子A的异常波动导致功能性血管减少,与此同时促炎性血管增生,从而引发软骨下骨硬化以及关节间隙狭窄。针对先天性髋关节发育不良的血管内皮生长因子A靶向干预已经展现出潜在的应用价值。动物实验显示,关节腔内注射抗血管内皮生长因子抗体能够显著抑制病理性血管侵入软骨,延缓先天性髋关节发育不良继发的骨关节炎发展进程[14,110],不过需要精准掌控干预时机。临床研究也证实,通过髋臼周围截骨术纠正生物力学异常后,关节软骨中的血管内皮生长因子A表达模式能够部分恢复常态,这暗示了力学矫正与局部血管内皮生长因子A调控相结合可能成为未来的治疗方向[111-112]。 尽管血管内皮生长因子A在先天性髋关节发育不良中的作用已得到初步证实,但其调控网络的时空特异性仍有待深入探究。例如,SOX9等软骨发育核心转录因子如何与血管内皮生长因子A协同调控先天性髋关节发育不良进程,以及不同血管内皮生长因子亚型在髋关节三维形态构建中的互补作用,均为亟待突破的研究难点[37,113-115]。此外,借助单细胞测序技术揭示先天性髋关节发育不良软骨中血管内皮生长因子A表达的细胞异质性,将为精准干预提供新的靶点[116]。动物实验成功模拟了先天性髋关节发育不良模型[33],研究发现,在稳定“同心”复位的机械应力刺激下,联合血管内皮生长因子A三维微载体治疗可进一步促进股骨近端次级骨化中心的发育,而次级骨化中心骨化延迟可能导致先天性髋关节发育不良后续发展中出现股骨头缺血坏死及髋关节残余发育不良。研究得出结论:先天性髋关节发育不良早期复位对预后有积极效应;联合血管内皮生长因子A三维微载体治疗能促进次级骨化中心发育;次级骨化中心骨化延迟与股骨头缺血坏死及髋关节残余发育不良发生相关。"

| [1] 王正业,刘万林,赵振群.血管内皮生长因子A靶向调控血管化治疗激素性股骨头坏死的机制[J].中国组织工程研究, 2026,30(3):671-679. [2] MENG X, MENG X, HE Z, et al. Selenium Deficiency Can Promote the Expression of VEGF and Inflammatory Factors in Cartilage Differentiation and Mediates Cartilage Injury. Biol Trace Elem Res. 2024; 202(9):4170-4179. [3] RIBATTI D, D’AMATI A. Bone angiocrine factors. Front Cell Dev Biol. 2023;11:1244372. [4] ZHANG C, ZHAO R, DONG Z, et al. IHH-GLI-1-HIF-2α signalling influences hypertrophic chondrocytes to exacerbate osteoarthritis progression. J Orthop Translat. 2024;49: 207-217. [5] 李正南.RAD54L调控HIF-1α/VEGF信号通路影响骨关节炎的作用及机制研究[D].南昌:南昌大学,2024. [6] CHEN Y, CHEN W, REN Y, et al. Lipid nanoparticle-encapsulated VEGFa siRNA facilitates cartilage formation by suppressing angiogenesis. Int J Biol Macromol. 2022; 221:1313-1324. [7] AHMED I, JOHN P, BHATTI A. Association analysis of Vascular Endothelial Growth Factor-A (VEGF-A) polymorphism in rheumatoid arthritis using computational approaches. Sci Rep. 2023;13(1):21957. [8] MA K, SINGH G, WANG J, et al. Targeting Vascular Endothelial Growth Factor Receptors as a Therapeutic Strategy for Osteoarthritis and Associated Pain. Int J Biol Sci. 2023;19(2):675-690. [9] CHEN Z, BO Q, WANG C, et al. Single BMSC-derived cartilage organoids for gradient heterogeneous osteochondral regeneration by leveraging native vascular microenvironment. J Nanobiotechnology. 2025;23(1):325. [10] PUEYO MOLINER A, ITO K, ZAUCKE F, et al. Restoring articular cartilage: insights from structure, composition and development. Nat Rev Rheumatol. 2025;21(5):291-308. [11] NOSSIN Y, FARRELL E, KOEVOET WJLM, et al. The Releasate of Avascular Cartilage Demonstrates Inherent Pro-Angiogenic Properties In Vitro and In Vivo. Cartilage. 2021;13(2_suppl):559S-570S. [12] QIAN JJ, XU Q, XU WM, et al. Expression of VEGF-A Signaling Pathway in Cartilage of ACLT-induced Osteoarthritis Mouse Model. J Orthop Surg Res. 2021;16(1):379. [13] MORA-PEREIRA M, BOONE L, NASKOU M, et al. Horse serum or equine platelet lysate increases total vascular endothelial growth factor A concentrations and correlates with vascular growth in an equine facial arterial ring assay. Am J Vet Res. 2023;84(7): ajvr.23.02.0023. [14] VADALÀ G, AMBROSIO L, CATTANI C, et al. Bevacizumab Arrests Osteoarthritis Progression in a Rabbit Model: A Dose-Escalation Study. J Clin Med. 2021;10(13): 2825. [15] SOTOZAWA M, KUMAGAI K, ISHIKAWA K, et al. Bevacizumab suppressed degenerative changes in articular cartilage explants from patients with osteoarthritis of the knee. J Orthop Surg Res. 2023;18(1):25. [16] SOGO Y, TOYODA E, NAGAI T, et al. Disease-Modifying Effects of Lenvatinib, a Multiple Receptor Tyrosine Kinase Inhibitor, on Posttraumatic Osteoarthritis of the Knee. Int J Mol Sci. 2024;25(12):6514. [17] LEE JS, GUO P, KLETT K, et al. VEGF-attenuated platelet-rich plasma improves therapeutic effect on cartilage repair. Biomater Sci. 2022;10(9):2172-2181. [18] MENG N, XIE HX, HOU JR, et al. Design and semisynthesis of oleanolic acid derivatives as VEGF inhibitors: Inhibition of VEGF-induced proliferation, angiogenesis, and VEGFR2 activation in HUVECs. Chin J Nat Med. 2022;20(3):229-240. [19] SONG J, GUAN Z, SONG C, et al. Apatinib suppresses the migration, invasion and angiogenesis of hepatocellular carcinoma cells by blocking VEGF and PI3K/AKT signaling pathways. Mol Med Rep. 2021; 23(6):429. [20] LI J, CHEN H, ZHANG D, et al. The role of stromal cell-derived factor 1 on cartilage development and disease. Osteoarthritis Cartilage. 2021;29(3):313-322. [21] ZHAO Z, SUN X, TU P, et al. Mechanisms of vascular invasion after cartilage injury and potential engineering cartilage treatment strategies. FASEB J. 2024;38(6):e23559. [22] WEI Y, XIE C, WEI Y, et al. SVF Cell Sheets as a New Multicellular Material-Based Strategy for Promoting Angiogenesis and Regeneration in Diced Cartilage Grafts. J Craniofac Surg. 2025. doi: 10.1097/SCS.0000000000011358. [23] XIE W, JIANG L, HUANG X, et al. Hsa_circ_0004662 Accelerates the Progression of Osteoarthritis via the microRNA-424-5p/VEGFA Axis. Curr Mol Med. 2024;24(2):217-225. [24] FERRARA N, HENZEL WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989; 161(2):851-858. [25] TISCHER E, MITCHELL R, HARTMAN T, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266(18):11947-11954. [26] KENDALL RL, THOMAS KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90(22):10705-10709. [27] FONG GH, ROSSANT J, GERTSENSTEIN M, et al. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376(6535):66-70. [28] CARMELIET P, MOONS L, COLLEN D. Mouse models of angiogenesis, arterial stenosis, atherosclerosis and hemostasis. Cardiovasc Res. 1998;39(1):8-33. [29] FERRARA N, GERBER HP, LECOUTER J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669-676. [30] HIRATSUKA S, KATAOKA Y, NAKAO K, et al. Vascular endothelial growth factor A (VEGF-A) is involved in guidance of VEGF receptor-positive cells to the anterior portion of early embryos. Mol Cell Biol. 2005;25(1):355-363. [31] NAGAO M, HAMILTON JL, KC R, et al. Vascular Endothelial Growth Factor in Cartilage Development and Osteoarthritis. Sci Rep. 2017;7(1):13027. [32] PÉREZ-GUTIÉRREZ L, FERRARA N. Biology and therapeutic targeting of vascular endothelial growth factor A. Nat Rev Mol Cell Biol. 2023;24(11):816-834. [33] 杨宇飞.VEGF-A微载体靶向调控血管化促进DDH中股骨近端发育的机制研究[D].呼和浩特:内蒙古医科大学,2024. [34] BARTOLETTI G, DONG C, UMAR M, et al. Pdgfra regulates multipotent cell differentiation towards chondrocytes via inhibiting Wnt9a/beta-catenin pathway during chondrocranial cartilage development. Dev Biol. 2020;466(1-2):36-46. [35] ZHANG J, LIN C, SONG Y, et al. BMP4/ALK3 deficiency leads to Meckel’s cartilage truncation mimicking the mandible Tessier 30 cleft. Oral Dis. 2022;28(4):1215-1227. [36] CAXARIA S, KOUVATSOS N, ELDRIDGE SE, et al. Disease modification and symptom relief in osteoarthritis using a mutated GCP-2/CXCL6 chemokine. EMBO Mol Med. 2023;15(1):e16218. [37] WEI Y, MA X, SUN H, et al. EGFR Signaling Is Required for Maintaining Adult Cartilage Homeostasis and Attenuating Osteoarthritis Progression. J Bone Miner Res. 2022;37(5):1012-1023. [38] FARAHAT M, HARA ES, ANADA R, et al. Mechanotransductive Mechanisms of Biomimetic Hydrogel Cues Modulating Meckel’s Cartilage Degeneration. Adv Biol (Weinh). 2022;6(6):e2101315. [39] QIN W, ZHANG Z, YAN J, et al. Interaction of Neurovascular Signals in the Degraded Condylar Cartilage. Front Bioeng Biotechnol. 2022;10:901749. [40] WANG X, LU X, TIAN D, et al. Transcriptomic integration and ligand-receptor crosstalk reveal the underlying molecular mechanisms between hip cartilage and subchondral bone in osteonecrosis of femoral head. Gene. 2025;939:149179. [41] XUE M, HUANG N, LUO Y, et al. Combined Transcriptomics and Metabolomics Identify Regulatory Mechanisms of Porcine Vertebral Chondrocyte Development In Vitro. Int J Mol Sci. 2024;25(2):1189. [42] STAR E, STEVENS M, GOODING C, et al. A drug-repositioning screen using splicing-sensitive fluorescent reporters identifies novel modulators of VEGF-A splicing with anti-angiogenic properties. Oncogenesis. 2021;10(5):36. [43] RAJA A, GANTA V. Synthetic Antiangiogenic Vascular Endothelial Growth Factor-A Splice Variant Revascularizes Ischemic Muscle in Peripheral Artery Disease. J Am Heart Assoc. 2024;13(20):e034304. [44] MONTEMAGNO C, DURIVAULT J, GASTALDI C, et al. A group of novel VEGF splice variants as alternative therapeutic targets in renal cell carcinoma. Mol Oncol. 2023; 17(7):1379-1401. [45] GANTA VC, ANNEX BH. Peripheral vascular disease: preclinical models and emerging therapeutic targeting of the vascular endothelial growth factor ligand-receptor system. Expert Opin Ther Targets. 2021;25(5):381-391. [46] XU Y, YANG Y, HUANG Y, et al. Inhibition of Nrf2/HO-1 signaling pathway by Dextran Sulfate suppresses angiogenesis of Gastric Cancer. J Cancer. 2021;12(4):1042-1060. [47] SONG S, ZHANG G, CHEN X, et al. HIF-1α increases the osteogenic capacity of ADSCs by coupling angiogenesis and osteogenesis via the HIF-1α/VEGF/AKT/mTOR signaling pathway. J Nanobiotechnology. 2023; 21(1):257. [48] MANTSOUNGA CS, LEE C, NEVERSON J, et al. Macrophage IL-1β promotes arteriogenesis by autocrine STAT3- and NF-κB-mediated transcription of pro-angiogenic VEGF-A. Cell Rep. 2022;38(5):110309. [49] YADAV P, PANDEY A, KAKANI P, et al. Hypoxia-induced loss of SRSF2-dependent DNA methylation promotes CTCF-mediated alternative splicing of VEGFA in breast cancer. iScience. 2023;26(6):106804. [50] BELALI T, WODI C, CLARK B, et al. WT1 activates transcription of the splice factor kinase SRPK1 gene in PC3 and K562 cancer cells in the absence of corepressor BASP1. Biochim Biophys Acta Gene Regul Mech. 2020;1863(12):194642. [51] AL KAWAS H, SAAID I, JANK P, et al. How VEGF-A and its splice variants affect breast cancer development - clinical implications. Cell Oncol (Dordr). 2022;45(2):227-239. [52] MABETA P, STEENKAMP V. The VEGF/VEGFR Axis Revisited: Implications for Cancer Therapy. Int J Mol Sci. 2022;23(24):15585. [53] AMEYA KP, ASHIKHA SHIRIN USMAN PP, SEKAR D. Navigating the tumor landscape: VEGF, MicroRNAs, and the future of cancer treatment. Biochim Biophys Acta Gene Regul Mech. 2025;1868(2):195091. [54] SHI Z, KUAI M, LI B, et al. The role of VEGF in Cancer angiogenesis and tumorigenesis: Insights for anti-VEGF therapy. Cytokine. 2025;189:156908. [55] YAMAKUCHI M, OKAWA M, TAKENOUCHI K, et al. VEGF-A165 is the predominant VEGF-A isoform in platelets, while VEGF-A121 is abundant in serum and plasma from healthy individuals. PLoS One. 2023;18(4):e0284131. [56] MAMER SB, WITTENKELLER A, IMOUKHUEDE PI. VEGF-A splice variants bind VEGFRs with differential affinities. Sci Rep. 2020;10(1):14413. [57] AULICINO F, PEDONE E, SOTTILE F, et al. Canonical Wnt Pathway Controls mESC Self-Renewal Through Inhibition of Spontaneous Differentiation via β-Catenin/TCF/LEF Functions. Stem Cell Reports. 2020; 15(3):646-661. [58] HUSSAIN N, MUMTAZ M, ADIL M, et al. Investigation of VEGF (rs 699947) polymorphism in the progression of Rheumatoid Arthritis (RA) and in-silico nanoparticle drug delivery of potential phytochemicals to cure RA. Acta Biochim Pol. 2023;70(3):591-598. [59] PEI YA, CHEN S, PEI M. The essential anti-angiogenic strategies in cartilage engineering and osteoarthritic cartilage repair. Cell Mol Life Sci. 2022;79(1):71. [60] TROMPETER N, GARDINIER JD, DEBARROS V, et al. Insulin-like growth factor-1 regulates the mechanosensitivity of chondrocytes by modulating TRPV4. Cell Calcium. 2021; 99:102467. [61] RAHMAN MM, WATTON PN, NEU CP, et al. A chemo-mechano-biological modeling framework for cartilage evolving in health, disease, injury, and treatment. Comput Methods Programs Biomed. 2023;231: 107419. [62] QIAN Z, GAO X, JIN X, et al. Cartilage-specific deficiency of clock gene Bmal1 accelerated articular cartilage degeneration in osteoarthritis by up-regulation of mTORC1 signaling. Int Immunopharmacol. 2023;115:109692. [63] CHE X, JIN X, PARK NR, et al. Cbfβ Is a Novel Modulator against Osteoarthritis by Maintaining Articular Cartilage Homeostasis through TGF-β Signaling. Cells. 2023;12(7):1064. [64] MA G, YANG Y, CHEN Y, et al. Blockade of TRPM7 Alleviates Chondrocyte Apoptosis and Articular Cartilage Damage in the Adjuvant Arthritis Rat Model Through Regulation of the Indian Hedgehog Signaling Pathway. Front Pharmacol. 2021;12:655551. [65] DENG H, XIAO X, CHILUFYA MM, et al. Altered Expression of the Hedgehog Pathway Proteins BMP2, BMP4, SHH, and IHH Involved in Knee Cartilage Damage of Patients With Osteoarthritis and Kashin-Beck Disease. Cartilage. 2022;13(1):19476035221087706. [66] LI M, ZHANG FJ, BAI RJ. The Hippo-YAP Signaling Pathway in Osteoarthritis and Rheumatoid Arthritis. J Inflamm Res. 2024; 17:1105-1120. [67] ZHANG Y, ZUO T, MCVICAR A, et al. Runx1 is a key regulator of articular cartilage homeostasis by orchestrating YAP, TGFβ, and Wnt signaling in articular cartilage formation and osteoarthritis. Bone Res. 2022;10(1):63. [68] YUAN LB, JIN T, YAO L, et al. The role and mechanism of biological collagen membranes in repairing cartilage injury through the p38MAPK signaling pathway. J Orthop Surg Res. 2023;18(1):837. [69] LIN W, KANG H, DAI Y, et al. Early patellofemoral articular cartilage degeneration in a rat model of patellar instability is associated with activation of the NF-κB signaling pathway. BMC Musculoskelet Disord. 2021;22(1):90. [70] ZHANG W, ZHENG X, GONG Y, et al. VX-11e protects articular cartilage and subchondral bone in osteoarthritis by inhibiting the RIP1/RIP3/MLKL and MAPK signaling pathways. Bioorg Chem. 2022;120:105632. [71] QI F, CUI SL, ZHANG B, et al. T-2 toxin-induced damage to articular cartilage in rats coincided with impaired autophagy linked to the HIF-1α/AMPK signaling axis. Toxicon. 2024;243:107735. [72] SIDDIQ MAB, JAHAN I, RASKER JJ. Statin in Clinical and Preclinical Knee Osteoarthritis-What E vidence Exists for Future Clinical Use?-A Literature Review. Curr Rheumatol Rev. 2023;19(3):270-280. [73] SUN X, LONG R, CHEN Q, et al. miR-378a-3p Regulates the BMP2-Smad Pathway to Promote Chondrogenic Differentiation of Synovium-Derived Mesenchymal Stem Cells. Cell Biochem Biophys. 2025;83(1):1277-1288. [74] YADAV PS, PAPAIOANNOU G, KOBELSKI MM, et al. Phosphate-induced activation of VEGFR2 leads to caspase-9-mediated apoptosis of hypertrophic chondrocytes. iScience. 2023;26(9):107548. [75] MCKENZIE JA, GALBREATH IM, COELLO AF, et al. VEGFA from osteoblasts is not required for lamellar bone formation following tibial loading. Bone. 2022;163:116502. [76] MCCANN M, LI Y, BACCOUCHE B, et al. VEGF Induces Expression of Genes That Either Promote or Limit Relaxation of the Retinal Endothelial Barrier. Int J Mol Sci. 2023;24(7):6402. [77] GROSSO A, LUNGER A, BURGER MG, et al. VEGF dose controls the coupling of angiogenesis and osteogenesis in engineered bone. NPJ Regen Med. 2023; 8(1):15. [78] ETSCHMAIER V, ÜÇAL M, LOHBERGER B, et al. Ex vivo organotypic bone slice culture reveals preferential chondrogenesis after sustained growth plate injury. Cells Dev. 2024;179:203927. [79] DI MAGGIO N, BANFI A. The osteo-angiogenic signaling crosstalk for bone regeneration: harmony out of complexity. Curr Opin Biotechnol. 2022;76:102750. [80] LEES-SHEPARD JB, FLINT K, FISHER M, et al. Cross-talk between EGFR and BMP signals regulates chondrocyte maturation during endochondral ossification. Dev Dyn. 2022;251(1):75-94. [81] SINHA A, IYENGAR PV, TEN DIJKE P. E3 Ubiquitin Ligases: Key Regulators of TGFβ Signaling in Cancer Progression. Int J Mol Sci. 2021;22(2):476. [82] HE Q, CHEN Z, LI J, et al. SENP6-Mediated deSUMOylation of VEGFR2 Enhances Its Cell Membrane Transport in Angiogenesis. Int J Mol Sci. 2023;24(3):2544. [83] QIU J, FAN X, DING H, et al. Antenatal dexamethasone retarded fetal long bones growth and development by down-regulating of insulin-like growth factor 1 signaling in fetal rats. Hum Exp Toxicol. 2022;41:9603271211072870. [84] HUYGENS C, RIBEIRO LOPES M, GAGET K, et al. Evolutionary diversification of insulin-related peptides (IRPs) in aphids and spatiotemporal distribution in Acyrthosiphon pisum. Insect Biochem Mol Biol. 2022;141:103670. [85] TAO D, ZHANG L, DING Y, et al. Primary cilia support cartilage regeneration after injury. Int J Oral Sci. 2023;15(1):22. [86] DREYER CH, KJAERGAARD K, DING M, et al. Vascular endothelial growth factor for in vivo bone formation: A systematic review. J Orthop Translat. 2020;24:46-57. [87] SAETAN N, HONSAWEK S, TANAVALEE A, et al. Association between Common Variants in VEGFA Gene and the Susceptibility of Primary Knee Osteoarthritis. Cartilage. 2022;13(4):66-76. [88] MA L, ZHAO X, LIU Y, et al. Dihydroartemisinin attenuates osteoarthritis by inhibiting abnormal bone remodeling and angiogenesis in subchondral bone. Int J Mol Med. 2021;47(3):04855. [89] HORVÁTH E, SÓLYOM Á, SZÉKELY J, et al. Inflammatory and Metabolic Signaling Interfaces of the Hypertrophic and Senescent Chondrocyte Phenotypes Associated with Osteoarthritis. Int J Mol Sci. 2023;24(22):16468. [90] 陈德胜,张学森,张晨,等.MMP-9和VEGF在膝关节骨性关节炎软骨中的表达及意义[J].宁夏医科大学学报,2020, 42(7):678-682 [91] ZHANG H, HU S, SANCHES JGP, et al. Sorcin promotes proliferation of hepatocellular carcinoma by regulating VEGFA/B via PI3K pathway. J Physiol Biochem. 2024;80(2): 381-392. [92] LI J, ZHANG W, LIU X, et al. Endothelial Stat3 activation promotes osteoarthritis development. Cell Prolif. 2023;56(12): e13518. [93] XIAO H, LIU S, FANG B, et al. Panax notoginseng saponins promotes angiogenesis after cerebral ischemia-reperfusion injury. J Ginseng Res. 2024; 48(6):592-602. [94] ZHAO Y, FU B, CHEN P, et al. Activated mesangial cells induce glomerular endothelial cells proliferation in rat anti-Thy-1 nephritis through VEGFA/VEGFR2 and Angpt2/Tie2 pathway. Cell Prolif. 2021; 54(6):e13055. [95] XIONG G, YANG Y, GUO M. Effect of resveratrol on abnormal bone remodeling and angiogenesis of subchondral bone in osteoarthritis. Int J Clin Exp Pathol. 2021; 14(4):417-425. [96] ABBADESSA A, NUÑEZ BERNAL P, BUTTITTA G, et al. Biofunctionalization of 3D printed collagen with bevacizumab-loaded microparticles targeting pathological angiogenesis. J Control Release. 2023;360: 747-758. [97] MA K, PHAM T, WANG J, et al. Nanoparticle-based inhibition of vascular endothelial growth factor receptors alleviates osteoarthritis pain and cartilage damage. Sci Adv. 2024;10(7):eadi5501. [98] VASUDEVAN MT, RANGARAJ K, RAMESH R, et al. Inhibitory effects of Gracilaria edulis and Gracilaria salicornia against the MGMT and VEGFA biomarkers involved in the onset and advancement of glioblastoma using in silico and in vitro approaches. Biotechnol Appl Biochem. 2025;72(1):207-224. [99] ABDELGALIL AA, MONIR R, ELMETWALLY M, et al. The Relation of VEGFA, VEGFR2, VEGI, and HIF1A Genetic Variants and Their Serum Protein Levels with Breast Cancer in Egyptian Patients. Biochem Genet. 2024; 62(1):547-573. [100] MAO X, YAN X, LI C, et al. Extensive preclinical evaluation of combined mangiferin and glycyrrhizic acid for restricting synovial neovascularization in rheumatoid arthritis. Chin Med. 2023; 18(1):156. [101] HANLON MM, CANAVAN M, BARKER BE, et al. Metabolites as drivers and targets in rheumatoid arthritis. Clin Exp Immunol. 2022;208(2):167-180. [102] SCURUCHI M, ALIQUÒ F, AVENOSO A, et al. Endocan Knockdown Down-Regulates the Expression of Angiogenesis-Associated Genes in Il-1ß Activated Chondrocytes. Biomolecules. 2023;13(5):851. [103] ZHANG Z, WANG G, ZHANG Z, et al. Locally administered liposomal drug depot enhances rheumatoid arthritis treatment by inhibiting inflammation and promoting cartilage repair. J Nanobiotechnology. 2025;23(1):69. [104] ACHUDHAN D, LIU SC, LIN YY, et al. Antcin K inhibits VEGF-dependent angiogenesis in human rheumatoid arthritis synovial fibroblasts. J Food Biochem. 2022;46(1): e14022. [105] YILDIRIM A, ÖNDER ME, ÖZKAN D. Ultrasonographic evaluation of distal femoral and talar cartilage thicknesses in patients with early rheumatoid arthritis and their relationship with disease activity. Clin Rheumatol. 2022;41(7):2001-2007. [106] LI K, WANG Y, HUANG P. Association of Four VEGFA Gene Variants with Rheumatoid Arthritis Risk: A Meta-analysis and Trial Sequential Analysis. Biochem Genet. 2025; 63(2):984-1013. [107] 韦宜山,刘万林,丁良甲,等.发育性髋脱位髋臼软骨细胞病理学改变的实验研究[J].内蒙古医学院学报,2012,34(6):447-452. [108] WANG CL, ZUO B, LI D, et al. The long noncoding RNA H19 attenuates force-driven cartilage degeneration via miR-483-5p/Dusp5. Biochem Biophys Res Commun. 2020;529(2):210-217. [109] FLORENCIO-SILVA R, SASSO GRDS, SASSO-CERRI E, et al. Relationship between autophagy and NLRP3 inflammasome during articular cartilage degradation in oestrogen-deficient rats with streptozotocin-induced diabetes. Ann Anat. 2025;257:152318. [110] UTSUNOMIYA H, GAO X, CHENG H, et al. Intra-articular Injection of Bevacizumab Enhances Bone Marrow Stimulation-Mediated Cartilage Repair in a Rabbit Osteochondral Defect Model. Am J Sports Med. 2021;49(7):1871-1882. [111] ALTER T, FITCH A, BAILEY TERHUNE E, et al. The economics of patients undergoing periacetabular osteotomy for hip dysplasia: the financial relationship between physicians and hospitals. J Hip Preserv Surg. 2022;9(4):225-231. [112] KIM YJ. Hip Osteoarthritis: Bench to Bedside Perspective. Adv Exp Med Biol. 2023;1402:125-133. [113] HASEEB A, KC R, ANGELOZZI M, et al. SOX9 keeps growth plates and articular cartilage healthy by inhibiting chondrocyte dedifferentiation/osteoblastic redifferentiation. Proc Natl Acad Sci U S A. 2021;118(8):e2019152118. [114] LIN X, BELL RD, CATHELINE SE, et al. Targeting Synovial Lymphatic Function as a Novel Therapeutic Intervention for Age-Related Osteoarthritis in Mice. Arthritis Rheumatol. 2023;75(6):923-936. [115] FUCHS B, BIRT A, MOELLHOFF N, et al. The use of commercial fibrin glue in dermal replacement material reduces angiogenic and lymphangiogenic gene and protein expression in vitro. J Biomater Appl. 2023; 37(10):1858-1873. [116] LIU Y, DA W, XU MJ, et al. Single-cell transcriptomics reveals novel chondrocyte and osteoblast subtypes and their role in knee osteoarthritis pathogenesis. Signal Transduct Target Ther. 2025;10(1):40. [117] HOUTMAN E, TUERLINGS M, RIECHELMAN J, et al. Elucidating mechano-pathology of osteoarthritis: transcriptome-wide differences in mechanically stressed aged human cartilage explants. Arthritis Res Ther. 2021;23(1):215. |
| [1] | Zhang Nan, Meng Qinghua, Bao Chunyu. Characteristics and clinical application of ankle joint finite element models [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2343-2349. |
| [2] | Chen Qiuhan, Yang Long, Yuan Daizhu, Wu Zhanyu, Zou Zihao, Ye Chuan. Peri-knee osteotomy for treatment of knee osteoarthritis: optimization of treatment strategies [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2303-2312. |
| [3] | Zhang Zizheng, Luo Wang, Liu Changlu. Application value of finite element analysis on unicompartmental knee arthroplasty for medial knee compartmental osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2313-2322. |
| [4] | Li Qingbin, Lin Jianhui, Huang Wenjie, Wang Mingshuang, Du Jiankai, Lao Yongqiang. Bone cement filling after enlarged curettage of giant cell tumor around the knee joint: a comparison of subchondral bone grafting and non-grafting [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1896-1902. |
| [5] | Li Linzhen, Jiao Hongzhuo, Chen Weinan, Zhang Mingzhe, Wang Jianlong, Zhang Juntao. Effect of icariin-containing serum on lipopolysaccharide-induced inflammatory damage in human chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1368-1374. |
| [6] | Chen Ju, Zheng Jinchang, Liang Zhen, Huang Chengshuo, Lin Hao, Zeng Li. Effect and mechanism of beta-caryophyllene in mice with osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1341-1347. |
| [7] | Lyu Guoqing, Aizimaitijiang·Rouzi, Xiong Daohai. Irisin inhibits ferroptosis in human articular chondrocytes: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1359-1367. |
| [8] | Wu Zhilin, , He Qin, Wang Pingxi, Shi Xian, Yuan Song, Zhang Jun, Wang Hao . DYRK2: a novel therapeutic target for rheumatoid arthritis combined with osteoporosis based on East Asian and European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1569-1579. |
| [9] | Li Hao, Tao Hongcheng, Zeng Ping, Liu Jinfu, Ding Qiang, Niu Chicheng, Huang Kai, Kang Hongyu. Mitogen-activated protein kinase signaling pathway regulates the development of osteoarthritis: guiding targeted therapy with traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1476-1485. |
| [10] | Guo Ying, Tian Feng, Wang Chunfang. Potential drug targets for the treatment of rheumatoid arthritis: large sample analysis from European databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1549-1557. |
| [11] | Bu Yangyang, Ning Xinli, Zhao Chen. Intra-articular injections for the treatment of osteoarthritis of the temporomandibular joint: different drugs with multiple combined treatment options [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1215-1224. |
| [12] | Zheng Yin, Wu Zhenhua, Zhang Cheng, Ruan Kexin, Gang Xiaolin, Ji Hong. Safety and efficacy of immunoadsorption therapy for rheumatoid arthritis: a network meta-analysis and systematic review [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1260-1268. |
| [13] | Liu Fengzhi, Dong Yuna, Tian Wenyi, Wang Chunlei, Liang Xiaodong, Bao Lin. Gene-predicted associations between 731 immune cell phenotypes and rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1311-1319. |
| [14] | Zhang Qian, Huang Dongfeng. Weighted gene co-expression network analysis combined with machine learning to screen and validate biomarkers for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1096-1105. |
| [15] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||